Optimization of the Tacrolimus Concentration-to-Dose Ratio Cut-Off Value to Define Metabolism Groups
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Statistics
3. Results
3.1. Patients’ Characteristics
3.2. C/D Ratio Cut-Off Calculation
3.3. Tac Doses, Trough Level, and C/D Ratios
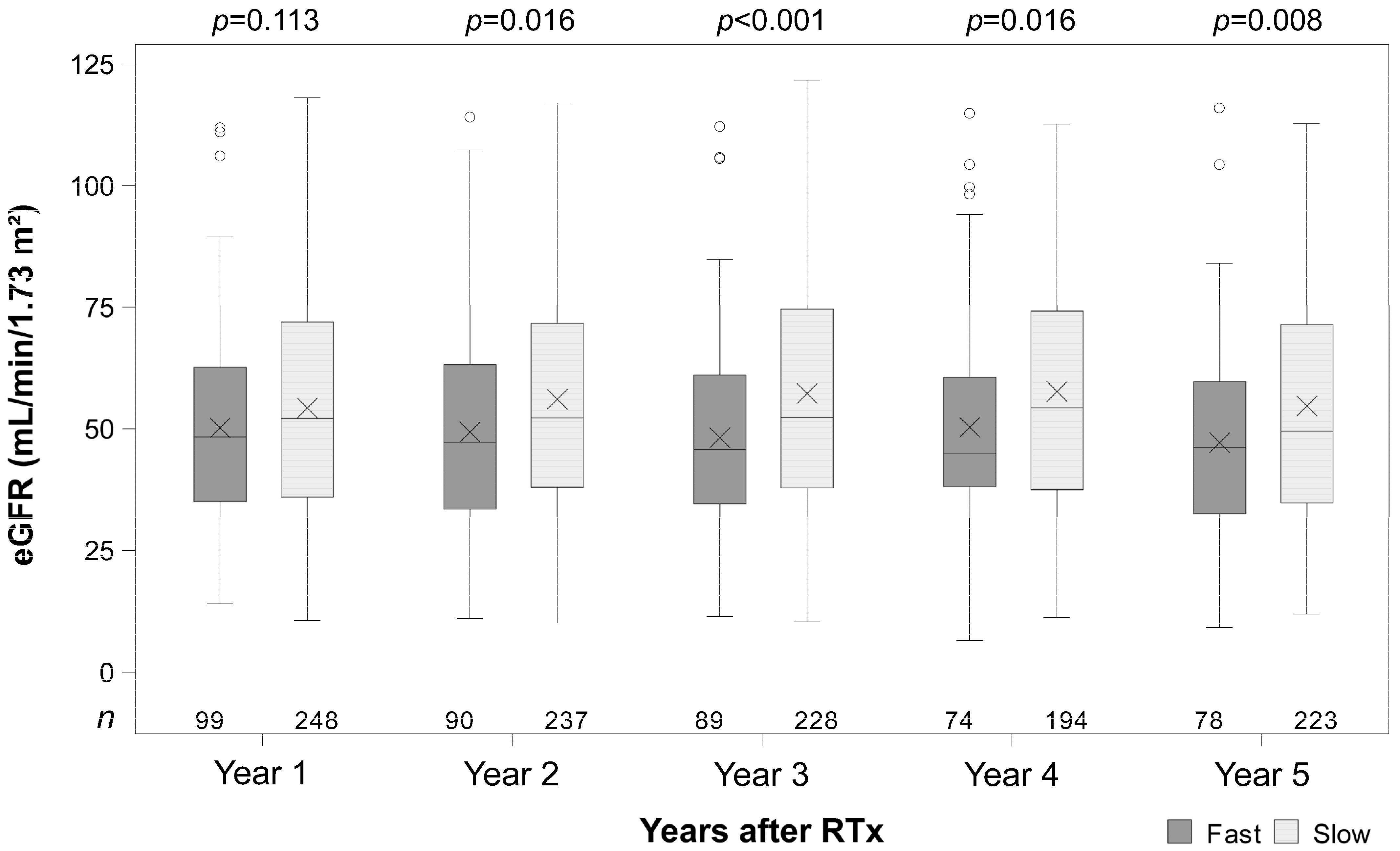
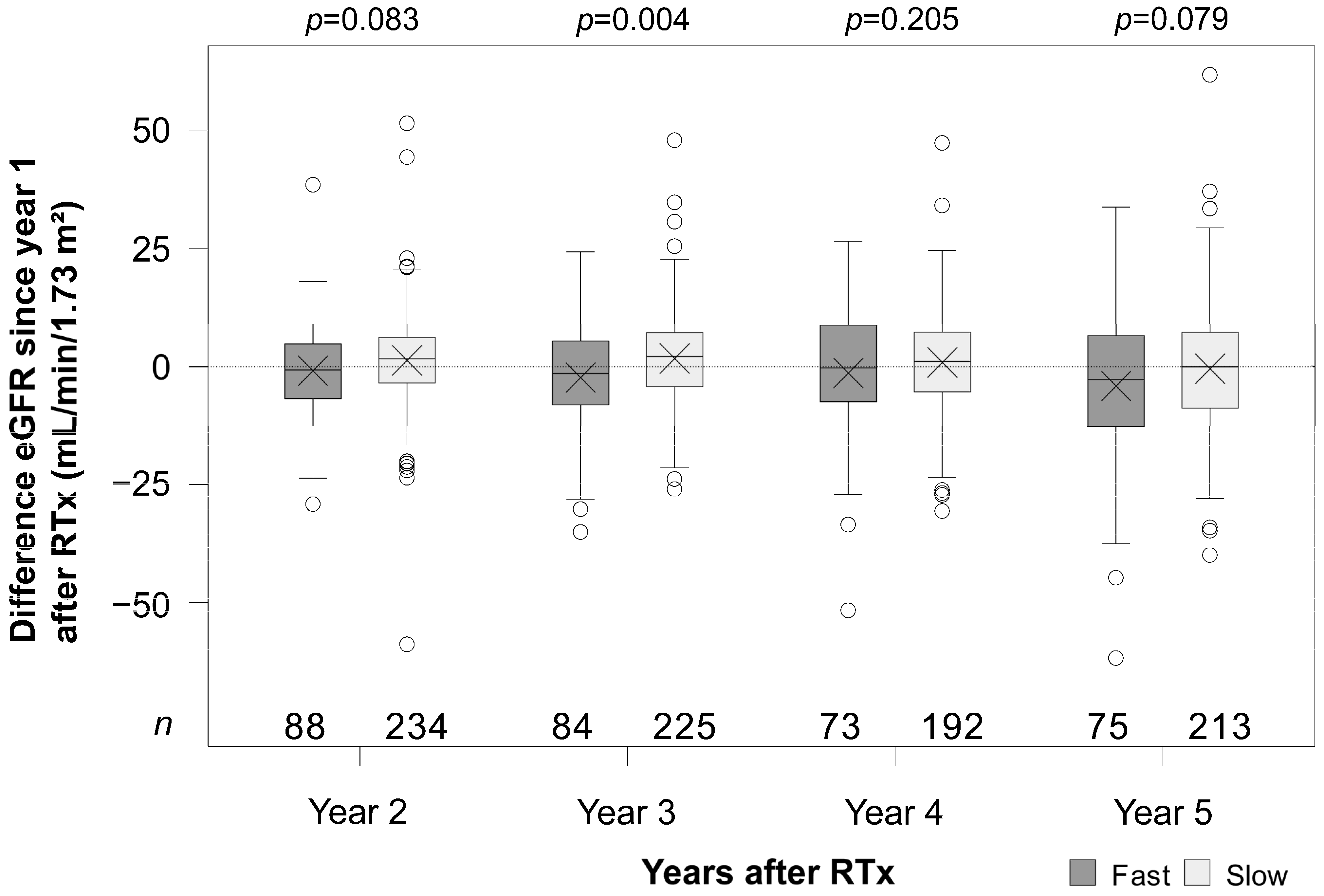
3.4. Renal Function
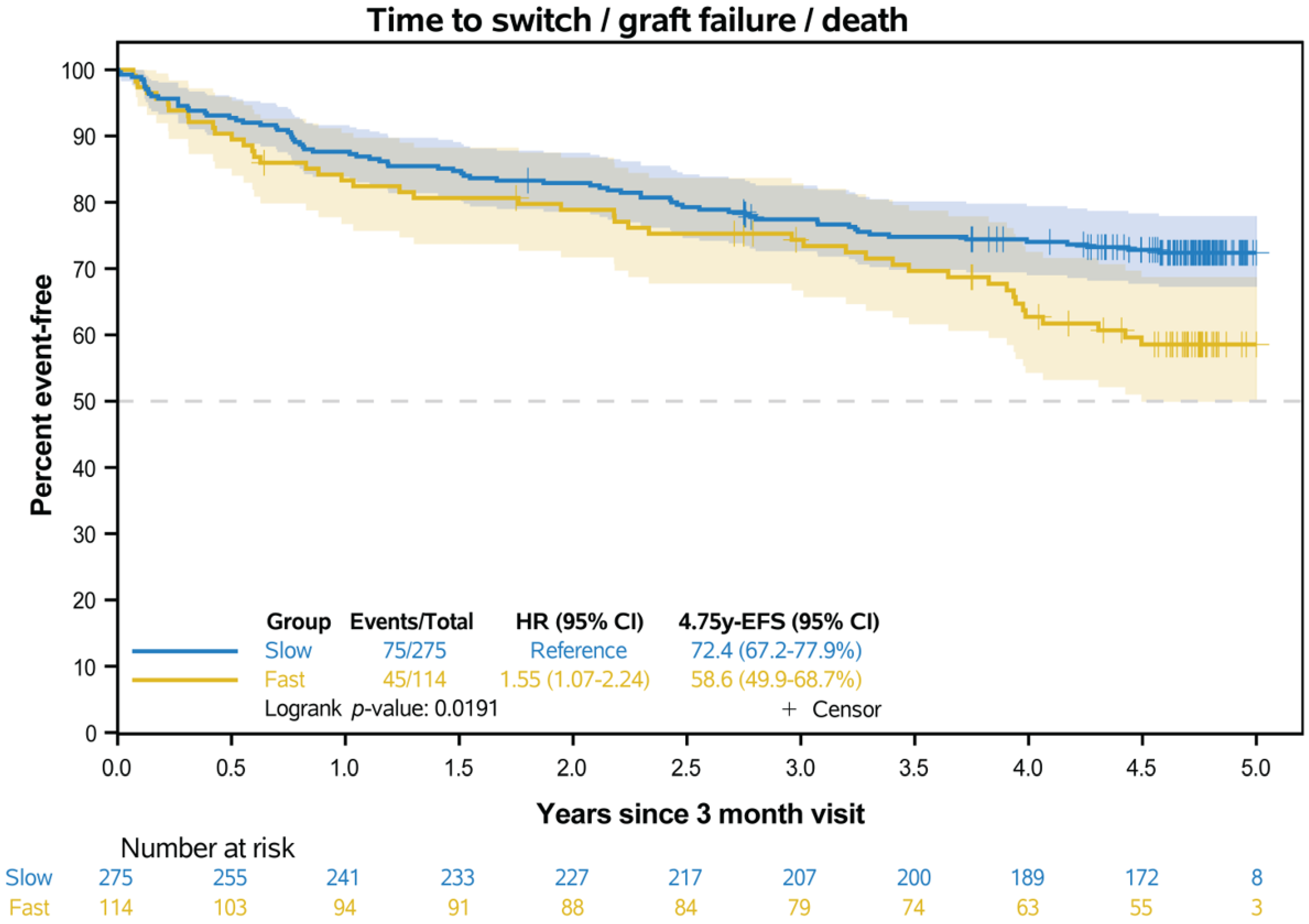
3.5. Event-Free Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| C/D ratio | concentration-to-dose ratio |
| RTx | renal transplantation |
| IR-Tac | immediate-release tacrolimus |
| AR | acute rejection |
| Tac | tacrolimus |
| CNI | calcineurin-inhibitor |
| CNIT | calcineurin inhibitor nephrotoxicity |
| eGFR | estimated glomerular filtration rate |
| IQR | interquartile range |
| CI | confidence interval |
| ESP | European Senior Program |
| EFS | event-free survival |
| HR | hazard ratios |
| sub-HR | subdistribution hazard ratios |
| cs-HR | cause-specific hazard |
| CIF est | cumulative incidence function estimate |
| BMI | body mass index |
| ABO-i | ABO incompatible transplantation |
| DGF | delayed graft function |
| HLA MM | human leucocyte antigen mismatch |
| PRA | panel reactive antibodies |
| ESRD | end-stage renal disease |
References
- Evans, I.M. Individualizing therapy, customizing clinical science. J. Behav. Ther. Exp. Psychiatry 1996, 27, 99–105. [Google Scholar] [CrossRef]
- Abubakar, M.B.; Gan, S.H. Molecular Targets in Advanced Therapeutics of Cancers: The Role of Pharmacogenetics. Oncology 2016, 91, 3–12. [Google Scholar] [CrossRef]
- Andrews, L.M.; Hesselink, D.A.; van Schaik, R.H.N.; van Gelder, T.; de Fijter, J.W.; Lloberas, N.; Elens, L.; Moes, D.; de Winter, B.C.M. A population pharmacokinetic model to predict the individual starting dose of tacrolimus in adult renal transplant recipients. Br. J. Clin. Pharmacol. 2019, 85, 601–615. [Google Scholar] [CrossRef]
- Yu, M.; Liu, M.; Zhang, W.; Ming, Y. Pharmacokinetics, Pharmacodynamics and Pharmacogenetics of Tacrolimus in Kidney Transplantation. Curr. Drug Metab. 2018, 19, 513–522. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am. J. Transplant. 2009, 9 (Suppl. S3), S1–S155. [Google Scholar] [CrossRef]
- Ekberg, H.; Tedesco-Silva, H.; Demirbas, A.; Vitko, S.; Nashan, B.; Gurkan, A.; Margreiter, R.; Hugo, C.; Grinyo, J.M.; Frei, U.; et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N. Engl. J. Med. 2007, 357, 2562–2575. [Google Scholar] [CrossRef]
- Oberbauer, R.; Bestard, O.; Furian, L.; Maggiore, U.; Pascual, J.; Rostaing, L.; Budde, K. Optimization of tacrolimus in kidney transplantation: New pharmacokinetic perspectives. Transplant. Rev. 2020, 34, 100531. [Google Scholar] [CrossRef]
- Tholking, G.; Schutte-Nutgen, K.; Schmitz, J.; Rovas, A.; Dahmen, M.; Bautz, J.; Jehn, U.; Pavenstadt, H.; Heitplatz, B.; Van Marck, V.; et al. A Low Tacrolimus Concentration/Dose Ratio Increases the Risk for the Development of Acute Calcineurin Inhibitor-Induced Nephrotoxicity. J. Clin. Med. 2019, 8, 1586. [Google Scholar] [CrossRef]
- Tholking, G.; Fortmann, C.; Koch, R.; Gerth, H.U.; Pabst, D.; Pavenstadt, H.; Kabar, I.; Husing, A.; Wolters, H.; Reuter, S.; et al. The tacrolimus metabolism rate influences renal function after kidney transplantation. PLoS ONE 2014, 9, e111128. [Google Scholar] [CrossRef]
- Schutte-Nutgen, K.; Tholking, G.; Steinke, J.; Pavenstadt, H.; Schmidt, R.; Suwelack, B.; Reuter, S. Fast Tac Metabolizers at Risk (-) It is Time for a C/D Ratio Calculation. J. Clin. Med. 2019, 8, 587. [Google Scholar] [CrossRef]
- Jouve, T.; Fonrose, X.; Noble, J.; Janbon, B.; Fiard, G.; Malvezzi, P.; Stanke-Labesque, F.; Rostaing, L. The TOMATO study (TacrOlimus MetabolizAtion in kidney TransplantatiOn): Impact of the concentration-dose ratio on death-censored graft survival. Transplantation 2019, 104, 1263–1271. [Google Scholar] [CrossRef]
- Nowicka, M.; Gorska, M.; Nowicka, Z.; Edyko, K.; Edyko, P.; Wislicki, S.; Zawiasa-Bryszewska, A.; Strzelczyk, J.; Matych, J.; Kurnatowska, I. Tacrolimus: Influence of the Posttransplant Concentration/Dose Ratio on Kidney Graft Function in a Two-Year Follow-Up. Kidney Blood Press. Res. 2019, 44, 1075–1088. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowska, E.; Kwiatkowski, S.; Wahler, F.; Gryczman, M.; Domanki, L.; Marchelk-Mysliwiec, M.; Ciechanowski, K.; Drozd-Dabrowska, M. C/D Ratio in Long-Term Renal Function. Transplant. Proc. 2019, 51, 3265–3270. [Google Scholar] [CrossRef] [PubMed]
- Debska-Slizien, A.; Kuzmiuk-Glembin, I.; Hozejowski, R.; Kaminska, D.; Krajewska, M.; Zawiasa-Bryszewska, A.; Kurnatowska, I.; Smykal-Jankowiak, K.; Glyda, M.; Koziol, L.; et al. Renal Allograft Function and the Tacrolimus C/D Ratio: Insights from a Prospective Study on MeltDose Tacrolimus. J. Clin. Med. 2024, 13, 6241. [Google Scholar] [CrossRef]
- Stratta, P.; Quaglia, M.; Cena, T.; Antoniotti, R.; Fenoglio, R.; Menegotto, A.; Ferrante, D.; Genazzani, A.; Terrazzino, S.; Magnani, C. The interactions of age, sex, body mass index, genetics, and steroid weight-based doses on tacrolimus dosing requirement after adult kidney transplantation. Eur. J. Clin. Pharmacol. 2012, 68, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Kuypers, D.R.; Naesens, M.; de Jonge, H.; Lerut, E.; Verbeke, K.; Vanrenterghem, Y. Tacrolimus dose requirements and CYP3A5 genotype and the development of calcineurin inhibitor-associated nephrotoxicity in renal allograft recipients. Ther. Drug Monit. 2010, 32, 394–404. [Google Scholar] [CrossRef]
- Egeland, E.J.; Reisaeter, A.V.; Robertsen, I.; Midtvedt, K.; Strom, E.H.; Holdaas, H.; Hartmann, A.; Asberg, A. High tacrolimus clearance—A risk factor for development of interstitial fibrosis and tubular atrophy in the transplanted kidney: A retrospective single-center cohort study. Transpl. Int. 2019, 32, 257–269. [Google Scholar] [CrossRef]
- Egeland, E.J.; Robertsen, I.; Hermann, M.; Midtvedt, K.; Storset, E.; Gustavsen, M.T.; Reisaeter, A.V.; Klaasen, R.; Bergan, S.; Holdaas, H.; et al. High Tacrolimus Clearance Is a Risk Factor for Acute Rejection in the Early Phase After Renal Transplantation. Transplantation 2017, 101, e273–e279. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Hothorn, T.; Lausen, B. On the exact distribution of maximally selected rank statistics. Comput. Stat. Data Anal. 2003, 43, 121–137. [Google Scholar]
- Fine, J.P.; Gray, R.J. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J. Am. Stat. Assoc. 1999, 94, 496–509. [Google Scholar] [CrossRef]
- Aalen, O.O.; Johansen, S. An Empirical Transition Matrix for Non-Homogeneous Markov Chains Based on Censored Observations. Scand. J. Stat. 1978, 5, 141–150. [Google Scholar]
- Gray, R.J. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. Ann. Stat. 1988, 16, 1141–1154. [Google Scholar]
- Prentice, R.L.; Kalbfleisch, J.D.; Peterson, A.V.; Flournoy, N.; Farewell, V.T.; Breslow, N.E. The Analysis of Failure Times in the Presence of Competing Risks. Biometrics 1978, 34, 541–554. [Google Scholar] [CrossRef]
- Suwelack, B.; Bunnapradist, S.; Meier-Kriesche, U.; Stevens, D.R.; Procaccianti, C.; Morganti, R.; Budde, K. Effect of Concentration/Dose Ratio in De Novo Kidney Transplant Recipients Receiving LCP-Tacrolimus or Immediate-Release Tacrolimus: Post Hoc Analysis of a Phase 3 Clinical Trial. Ann. Transplant. 2020, 25, e923278. [Google Scholar] [CrossRef]
- Ro, H.; Jeong, J.C.; Kong, J.M.; Min, J.W.; Park, S.K.; Lee, J.; Koo, T.Y.; Yang, J.; Kim, M.S.; Hwang, S.; et al. The tacrolimus metabolism affect post-transplant outcome mediating acute rejection and delayed graft function: Analysis from Korean Organ Transplantation Registry data. Transpl. Int. 2021, 34, 163–174. [Google Scholar] [CrossRef]
- Bartlett, F.E.; Carthon, C.E.; Hagopian, J.C.; Horwedel, T.A.; January, S.E.; Malone, A. Tacrolimus Concentration-to-Dose Ratios in Kidney Transplant Recipients and Relationship to Clinical Outcomes. Pharmacotherapy 2019, 39, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Rostaing, L.; Bunnapradist, S.; Grinyo, J.M.; Ciechanowski, K.; Denny, J.E.; Silva, H.T., Jr.; Budde, K.; Kulkarni, S.; Hricik, D.; Bresnahan, B.A.; et al. Novel Once-Daily Extended-Release Tacrolimus Versus Twice-Daily Tacrolimus in De Novo Kidney Transplant Recipients: Two-Year Results of Phase 3, Double-Blind, Randomized Trial. Am. J. Kidney Dis. 2016, 67, 648–659. [Google Scholar] [CrossRef]
- von Samson-Himmelstjerna, F.A.; Messtorff, M.L.; Kakavand, N.; Eisenberger, U.; Korth, J.; Lange, U.; Kolbrink, B.; Aldag, L.; Schulze Dieckhoff, T.; Feldkamp, T.; et al. The Tacrolimus Concentration/Dose Ratio Does Not Predict Early Complications After Kidney Transplantation. Transpl. Int. 2023, 36, 11027. [Google Scholar] [CrossRef]
- Lemaitre, F.; Budde, K.; Van Gelder, T.; Bergan, S.; Lawson, R.; Noceti, O.; Venkataramanan, R.; Elens, L.; Moes, D.; Hesselink, D.A.; et al. Therapeutic drug monitoring and dosage adjustments of immunosuppressive drugs when combined with nirmatrelvir/ritonavir in patients with COVID-19. Ther. Drug Monit. 2022, 45, 191–199. [Google Scholar] [CrossRef]
- Anglicheau, D.; Flamant, M.; Schlageter, M.H.; Martinez, F.; Cassinat, B.; Beaune, P.; Legendre, C.; Thervet, E. Pharmacokinetic interaction between corticosteroids and tacrolimus after renal transplantation. Nephrol. Dial. Transplant. 2003, 18, 2409–2414. [Google Scholar] [CrossRef] [PubMed]
- Sellares, J.; de Freitas, D.G.; Mengel, M.; Reeve, J.; Einecke, G.; Sis, B.; Hidalgo, L.G.; Famulski, K.; Matas, A.; Halloran, P.F. Understanding the causes of kidney transplant failure: The dominant role of antibody-mediated rejection and nonadherence. Am. J. Transplant. 2012, 12, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Tomizawa, M.; Hori, S.; Inoue, K.; Nishimura, N.; Nakai, Y.; Miyake, M.; Yoneda, T.; Fujimoto, K. A Low Tacrolimus Concentration-to-Dose Ratio Increases Calcineurin Inhibitor Nephrotoxicity and Cytomegalovirus Infection Risks in Kidney Transplant Recipients: A Single-Center Study in Japan. Transplant. Proc. 2023, 55, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowska, E.; Ciechanowski, K.; Domanski, L.; Dziedziejko, V.; Przybycinski, J.; Pawlik, A. Intrapatient Variability (IPV) and the Blood Concentration Normalized by the Dose (C/D Ratio) of Tacrolimus-Their Correlations and Effects on Long-Term Renal Allograft Function. Biomedicines 2022, 10, 2860. [Google Scholar] [CrossRef]

| Fast Metabolizers (n = 114) | Slow Metabolizers (n = 275) | p-Value | |
|---|---|---|---|
| age (years) | 50.1 ± 13.7 | 52.7 ± 13.9 | 0.099 a |
| sex (m/f), n (%) | 60 (52.6%)/54 (47.4%) | 167 (60.7%)/108 (39.3%) | 0.144 b |
| BMI (kg/m2) | 24.8 ± 3.9 | 25.4 ± 4.1 | 0.205 a |
| living donor transplantation | 36 (31.6%) | 63 (22.9%) | 0.096 b |
| ABO-i | 1 (0.9%) | 11 (4%) | 0.193 b |
| ESP transplantation | 23 (20.2%) | 58 (21.1%) | 0.892 b |
| time on waiting list (months) | 49 (20–88) | 60 (26–91) | 0.133 c |
| DGF | 17/113 (16%) | 41/274 (16%) | 1 b |
| cold ischemic time (h) | 8.2 ± 5.5 | 8.6 ± 4.9 | 0.565 a |
| warm ischemic time (min) | 30 (27–35) | 30 (28–35) | 0.984 c |
| prior kidney transplantation | |||
| 0 | 99 (86.8%) | 234 (85.1%) | 0.962 b |
| 1 | 14 (12.3%) | 37 (13.5%) | |
| 2 | 1 (0.9%) | 31 (1.1%) | |
| 3 | 0 | 1 (0.4%) | |
| HLA MM | |||
| 0–3 | 76 (67.3%) | 185 (67.5%) | 1 b |
| 4–6 | 37 (32.7%) | 89 (32.5%) | |
| PRA > 20% | 2 (1.8%) | 6 (2.2%) | 1 b |
| donor characteristics | |||
| donor age (years) | 55.0 ± 13.2 | 51.6 ± 16.5 | 0.367 a |
| donor sex (m/f), n (%) | 49 (43%)/65 (57%) | 130 (47.3%)/145 (52.7%) | 0.503 b |
| diagnosis for ESRD | |||
| benign nephrosclerosis | 8 (7.0%) | 23 (8.4%) | 0.516 b |
| diabetic nephropathy | 1 (0.9%) | 11 (4.0%) | |
| polycystic kidney disease | 17 (14.9%) | 41 (14.9%) | |
| obstructive nephropathy | 11 (9.6%) | 22 (8.0%) | |
| glomerulonephritis | 48 (42.1%) | 112 (40.7%) | |
| vasculitis | 1 (0.9%) | 5 (1.8%) | |
| interstitial nephritis | 1 (0.9%) | 5 (1.8%) | |
| other | 27 (23.7%) | 55 (20.0%) | |
| comorbidities before transplantation | |||
| arterial hypertension | 108 (95.6%) | 260 (94.5%) | 0.804 b |
| diabetes mellitus | 12 (10.6%) | 37 (13.5%) | 0.504 b |
| Fast Metabolizers | Slow Metabolizers | p-Value | |
|---|---|---|---|
| n = 114 | n = 275 | ||
| Tac C/D ratio (ng/mL·1/mg) | 0.67 (0.17–0.94) | 1.66 (0.95–6.33) | * |
| Tac dose (mg) | 10.0 (7.5–13.0) | 5.0 (3.5–6.5) | <0.001 |
| Tac trough levels (ng/mL) | 6.6 (4.7–7.9) | 8.2 (6.7–10.1) | <0.001 |
| Model-Based Estimates of eGFR (mL/min/1.73 m2) | |||||
|---|---|---|---|---|---|
| Dependent Variables and Contrasts | Estimate | Lower 95% Confidence Limit | Upper 95% Confidence Limit | p-Value | |
| ESP transplantation | yes vs. no | −13.3 | −19.4 | −7.2 | <0.001 |
| Living donor transplantation | yes vs. no | 6.8 | 2.13 | 11.4 | 0.004 |
| Age at RTx | x vs. x-10 years | −1.8 | −3.7 | 0.4 | 0.055 |
| Sex | female vs. male | 15.0 | 10.9 | 19.0 | <0.001 |
| Diabetes | yes vs. no | −2.1 | −8.9 | 4.7 | 0.546 |
| Difference of metabolism over all time points | slow vs. fast | 9.0 | 4.7 | 13.1 | <0.001 |
| Effect of time combined over both metabolism groups | 0.004 | ||||
| Interaction term of metabolism groups × time points | 0.039 | ||||
| Covariate adjusted least square mean differences between fast and slow metabolizer by time points (combination of main and interaction effects of tacrolimus metabolism group and time points) | |||||
| Year 1 after RTx | slow vs. fast | 6.1 | 1.9 | 10.2 | 0.004 |
| Year 2 after RTx | slow vs. fast | 8.2 | 3.7 | 12.7 | <0.001 |
| Year 3 after RTx | slow vs. fast | 10.3 | 5.8 | 14.8 | <0.001 |
| Year 4 after RTx | slow vs. fast | 9.6 | 4.8 | 14.4 | <0.001 |
| Year 5 after RTx | slow vs. fast | 10.7 | 5.8 | 15.5 | <0.001 |
| Covariate adjusted least square means of the mean change between the time points (Δ) within metabolism group (combination of main and interaction effects of tacrolimus metabolism group and time points) | |||||
| fast metabolizer | Δ year 2 vs. year 1 | −1.0 | −3.1 | 1.1 | 0.358 |
| Δ year 3 vs. year 1 | −2.5 | −4.8 | −0.1 | 0.038 | |
| Δ year 4 vs. year 1 | −2.6 | −5.5 | 0.4 | 0.086 | |
| Δ year 5 vs. year 1 | −5.2 | −8.6 | −1.7 | 0.003 | |
| slow metabolizer | Δ year 2 vs. year 1 | 1.2 | −0.2 | 2.5 | 0.090 |
| Δ year 3 vs. year 1 | 1.8 | 0.5 | 3.1 | 0.007 | |
| Δ year 4 vs. year 1 | 0.9 | −0.6 | 2.5 | 0.231 | |
| Δ year 5 vs. year 1 | −0.5 | −2.3 | 1.2 | 0.548 | |
| Least square mean differences in the change between time points (Δ) compared between metabolism groups (combination of main and interaction effects of tacrolimus metabolism group and time points) | |||||
| Δ year 2 vs. year 1 | fast vs. slow | 2.1 | −0.3 | 4.6 | 0.091 |
| Δ year 3 vs. year 1 | fast vs. slow | 2.1 | −0.4 | 4.6 | 0.093 |
| Δ year 4 vs. year 1 | fast vs. slow | −0.7 | −3.0 | 1.5 | 0.524 |
| Δ year 5 vs. year 1 | fast vs. slow | 1.1 | −1.4 | 3.5 | 0.381 |
| Metabolizer Groups | p-Value | ||
|---|---|---|---|
| Fast (n = 114) | Slow (n = 275) | ||
| Event-free survival | |||
| Number of events, n | 45 | 75 | - |
| HR (95% CI) | 1.55 (1.07–2.24) | Reference | 0.019 * |
| KM est of EFS at 5 years after RTx, % (95% CI) | 59% (50–69) | 72% (67–78) | |
| Competing risk analysis of event-free survival | |||
| Switch from IR-Tac | |||
| Number of events, n | 26 | 53 | - |
| sub-HR (95% CI) | 1.22 (0.76–1.94) | Reference | 0.406 ** |
| CIF est 5 years after RTx, % (95% CI) | 24% (17–33) | 19% (15–25) | |
| cs-HR (95% CI) | 1.26 (0.79–2.01) | Reference | 0.338 *** |
| Graft Failure | |||
| Number of events, n | 9 | 10 | - |
| sub-HR (95% CI) | 2.22 (0.90–5.47) | Reference | 0.073 ** |
| CIF est 5 years after RTx, % (95% CI) | 8.5% (4–16) | 4% (2–7) | |
| cs-HR (95% CI) | 2.36 (0.96–5.82) | 0.061 *** | |
| Death (without prior switch or graft failure) | |||
| Number of events, n | 10 | 12 | - |
| sub-HR (95% CI) | 2.09 (0.91–4.83) | Reference | 0.077 ** |
| CIF est 5 years after RTx, % (95% CI) | 9% (5–16.5) | 4.5% (3–8) | |
| cs-HR (95% CI) | 2.18 (0.96–5.05) | Reference | 0.069 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thölking, G.; Hüls, S.; Schütte-Nütgen, K.; Jehn, U.; Pavenstädt, H.; Reuter, S.; Koch, R. Optimization of the Tacrolimus Concentration-to-Dose Ratio Cut-Off Value to Define Metabolism Groups. J. Clin. Med. 2025, 14, 2542. https://doi.org/10.3390/jcm14082542
Thölking G, Hüls S, Schütte-Nütgen K, Jehn U, Pavenstädt H, Reuter S, Koch R. Optimization of the Tacrolimus Concentration-to-Dose Ratio Cut-Off Value to Define Metabolism Groups. Journal of Clinical Medicine. 2025; 14(8):2542. https://doi.org/10.3390/jcm14082542
Chicago/Turabian StyleThölking, Gerold, Sophia Hüls, Katharina Schütte-Nütgen, Ulrich Jehn, Hermann Pavenstädt, Stefan Reuter, and Raphael Koch. 2025. "Optimization of the Tacrolimus Concentration-to-Dose Ratio Cut-Off Value to Define Metabolism Groups" Journal of Clinical Medicine 14, no. 8: 2542. https://doi.org/10.3390/jcm14082542
APA StyleThölking, G., Hüls, S., Schütte-Nütgen, K., Jehn, U., Pavenstädt, H., Reuter, S., & Koch, R. (2025). Optimization of the Tacrolimus Concentration-to-Dose Ratio Cut-Off Value to Define Metabolism Groups. Journal of Clinical Medicine, 14(8), 2542. https://doi.org/10.3390/jcm14082542





