Oral Anticoagulation Therapy: An Update on Usage and Costs in the Endemic COVID-19 Era
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hirsh, J.; de Vries, T.A.C.; Eikelboom, J.W.; Bhagirath, V.; Chan, N.C. Clinical Studies with Anticoagulants that Have Changed Clinical Practice. Semin. Thromb. Hemost. 2023, 49, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Mattiuzzi, C.; Favaloro, E.J. Letter to the Editor: 10-Year Evolution in Worldwide Usage of Anticoagulant Drugs. Semin. Thromb. Hemost. 2023, 49, 314–316. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Gosselin, R.; Favaloro, E.J. Current and Emerging Direct Oral Anticoagulants: State-of-the-Art. Semin. Thromb. Hemost. 2019, 45, 490–501. [Google Scholar] [CrossRef]
- Piccini, J.P.; Patel, M.R.; Steffel, J.; Ferdinand, K.; Van Gelder, I.C.; Russo, A.M.; Ma, C.S.; Goodman, S.G.; Oldgren, J.; Hammett, C.; et al. Asundexian versus Apixaban in Patients with Atrial Fibrillation. N. Engl. J. Med. 2025, 392, 23–32. [Google Scholar] [CrossRef]
- Barnes, G.D. New targets for antithrombotic medications: Seeking to decouple thrombosis from hemostasis. J. Thromb. Haemost. 2024, 23, 1146–1159. [Google Scholar] [CrossRef]
- Weitz, J.I.; Strony, J.; Ageno, W.; Gailani, D.; Hylek, E.M.; Lassen, M.R.; Mahaffey, K.W.; Notani, R.S.; Roberts, R.; Segers, A.; et al. Milvexian for the Prevention of Venous Thromboembolism. N. Engl. J. Med. 2021, 385, 2161–2172. [Google Scholar] [CrossRef]
- Favaloro, E.J.; Pasalic, L.; Lippi, G. Replacing warfarin therapy with the newer direct oral anticoagulants, or simply a growth in anticoagulation therapy? Implications for Pathology testing. Pathology 2017, 49, 639–643. [Google Scholar] [CrossRef]
- Favaloro, E.J.; Pasalic, L.; Lippi, G. Oral anticoagulation therapy: An update on usage, costs and associated risks. Pathology 2020, 52, 736–741. [Google Scholar] [CrossRef]
- Douxfils, J.; Adcock, D.M.; Bates, S.M.; Favaloro, E.J.; Gouin-Thibault, I.; Guillermo, C.; Kawai, Y.; Lindhoff-Last, E.; Kitchen, S.; Gosselin, R.C. 2021 Update of the International Council for Standardization in Haematology Recommendations for Laboratory Measurement of Direct Oral Anticoagulants. Thromb. Haemost. 2021, 121, 1008–1020. [Google Scholar] [CrossRef]
- Favaloro, E.J.; Arunachalam, S.; Chapman, K.; Pasalic, L. Continued Harmonization of the International Normalized Ratio (INR) across a large laboratory network: Evidence of sustained low inter-laboratory variation and bias after a change in instrumentation. Am. J. Clin. Pathol. 2025, 163, 28–41. [Google Scholar] [CrossRef]
- Lippi, G.; Sanchis-Gomar, F.; Cervellin, G. Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge. Int. J. Stroke 2021, 16, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Sanchis-Gomar, F.; Favaloro, E.J.; Lavie, C.J.; Henry, B.M. Coronavirus Disease 2019-Associated Coagulopathy. Mayo Clin. Proc. 2021, 96, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Bikdeli, B.; Madhavan, M.V.; Jimenez, D.; Chuich, T.; Dreyfus, I.; Driggin, E.; Nigoghossian, C.D.; Ageno, W.; Madjid, M.; Guo, Y.; et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-up. JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 2950–2973. [Google Scholar] [CrossRef]
- Bikdeli, B.; Madhavan, M.V.; Gupta, A.; Jimenez, D.; Burton, J.R.; Der Nigoghossian, C.; Chuich, T.; Nouri, S.N.; Dreyfus, I.; Driggin, E.; et al. Pharmacological Agents Targeting Thromboinflammation in COVID-19: Review and Implications for Future Research. Thromb. Haemost. 2020, 120, 1004–1024. [Google Scholar]
- Hashemi, A.; Madhavan, M.V.; Bikdeli, B. Pharmacotherapy for Prevention and Management of Thrombosis in COVID-19. Semin. Thromb. Hemost. 2020, 46, 789–795. [Google Scholar] [CrossRef]
- Ortega-Paz, L.; Talasaz, A.H.; Sadeghipour, P.; Potpara, T.S.; Aronow, H.D.; Jara-Palomares, L.; Sholzberg, M.; Angiolillo, D.J.; Lip, G.Y.H.; Bikdeli, B. COVID-19-Associated Pulmonary Embolism: Review of the Pathophysiology, Epidemiology, Prevention, Diagnosis, and Treatment. Semin. Thromb. Hemost. 2023, 49, 816–832. [Google Scholar] [CrossRef]
- Rizk, J.G.; Gupta, A.; Lazo, J.G., Jr.; Sardar, P.; Henry, B.M.; Lavie, C.J.; Effron, M.B. To Anticoagulate or Not to Anticoagulate in COVID-19: Lessons after 2 Years. Semin. Thromb. Hemost. 2023, 49, 62–72. [Google Scholar] [CrossRef]
- Candeloro, M.; Schulman, S. Arterial Thrombotic Events in Hospitalized COVID-19 Patients: A Short Review and Meta-Analysis. Semin. Thromb. Hemost. 2023, 49, 47–54. [Google Scholar] [CrossRef]
- Di Minno, A.; Ambrosino, P.; Calcaterra, I.; Di Minno, M.N.D. COVID-19 and Venous Thromboembolism: A Meta-analysis of Literature Studies. Semin. Thromb. Hemost. 2020, 46, 763–771. [Google Scholar] [CrossRef]
- Alkhameys, S.; Barrett, R. Impact of the COVID-19 pandemic on England’s national prescriptions of oral vitamin K antagonist (VKA) and direct-acting oral anticoagulants (DOACs): An interrupted time series analysis (January 2019–February 2021). Curr. Med. Res. Opin. 2022, 38, 1081–1092. [Google Scholar] [CrossRef]
- Curtis, H.J.; MacKenna, B.; Walker, A.J.; Croker, R.; Mehrkar, A.; Morton, C.; Bacon, S.; Hickman, G.; Inglesby, P.; Bates, C.; et al. OpenSAFELY: Impact of national guidance on switching anticoagulant therapy during COVID-19 pandemic. Open Heart 2021, 8, e001784. [Google Scholar]
- Dale, C.E.; Takhar, R.; Carragher, R.; Katsoulis, M.; Torabi, F.; Duffield, S.; Kent, S.; Mueller, T.; Kurdi, A.; Le Anh, T.N.; et al. The impact of the COVID-19 pandemic on cardiovascular disease prevention and management. Nat. Med. 2023, 29, 219–225. [Google Scholar] [CrossRef]
- Pharmaceutical Benefits Scheme (PBS). Available online: https://www.pbs.gov.au/pbs/home (accessed on 5 February 2024).
- Pharmaceutical Benefits Schedule Item Reports. Available online: http://medicarestatistics.humanservices.gov.au/statistics/pbs_item.jsp (accessed on 5 February 2024).
- National Notifiable Disease Surveillance System. Available online: https://nindss.health.gov.au/pbi-dashboard/ (accessed on 5 February 2024).
- Australian Bureau of Statistics. Available online: https://www.abs.gov.au/statistics/people/population/national-state-and-territory-population/latest-release (accessed on 5 February 2024).
- Ageno, W.; Gallus, A.S.; Wittkowsky, A.; Crowther, M.; Hylek, E.M.; Palareti, G. Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141 (Suppl. S2), e44S–e88S. [Google Scholar] [CrossRef]
- Bezabhe, W.M.; Bereznicki, L.R.; Radford, J.; Wimmer, B.C.; Curtain, C.; Salahudeen, M.S.; Peterson, G.M. Ten-Year Trends in the Use of Oral Anticoagulants in Australian General Practice Patients With Atrial Fibrillation. Front. Pharmacol. 2021, 12, 586370. [Google Scholar] [CrossRef]
- Navar, A.M.; Kolkailah, A.A.; Overton, R.; Shah, N.P.; Rousseau, J.F.; Flaker, G.C.; Pignone, M.P.; Peterson, E.D. Trends in Oral Anticoagulant Use Among 436,864 Patients with Atrial Fibrillation in Community Practice, 2011 to 2020. J. Am. Heart Assoc. 2022, 11, e026723. [Google Scholar] [CrossRef] [PubMed]
- Lavalle, C.; Pierucci, N.; Mariani, M.V.; Piro, A.; Borrelli, A.; Grimaldi, M.; Rossillo, A.; Notarstefano, P.; Compagnucci, P.; Dello Russo, A.; et al. Italian Registry in the Setting of Atrial Fibrillation Ablation with Rivaroxaban—IRIS. Minerva Cardiol. Angiol. 2024, 72, 625–637. [Google Scholar] [CrossRef]
- Favaloro, E.J.; Pasalic, L. COVID-19 vaccine induced (immune) thrombotic thrombocytopenia (VITT)/thrombosis with thrombocytopenia syndrome (TTS): An update. Aust. J. Med. Sci. 2021, 42, 86–93. [Google Scholar]
- Hafeez, M.U.; Ikram, M.; Shafiq, Z.; Sarfraz, A.; Sarfraz, Z.; Jaiswal, V.; Sarfraz, M.; Chérrez-Ojeda, I. COVID-19 Vaccine-Associated Thrombosis with Thrombocytopenia Syndrome (TTS): A Systematic Review and Post Hoc Analysis. Clin. Appl. Thromb. Hemost. 2021, 27, 10760296211048815. [Google Scholar] [CrossRef]
- Selvadurai, M.V.; Favaloro, E.J.; Chen, V.M. Mechanisms of Thrombosis in Heparin-Induced Thrombocytopenia and Vaccine-Induced Immune Thrombotic Thrombocytopenia. Semin. Thromb. Hemost. 2023, 49, 444–452. [Google Scholar] [CrossRef]
- Favaloro, E.J.; Clifford, J.; Leitinger, E.; Parker, M.; Sung, P.; Chunilal, S.; Tran, H.; Kershaw, G.; Fu, S.; Passam, F.; et al. Assessment of immunological anti-platelet factor 4 antibodies for vaccine-induced thrombotic thrombocytopenia (VITT) in a large Australian cohort: A multicentre study comprising 1284 patients. J. Thromb. Haemost. 2022, 20, 2896–2908. [Google Scholar] [CrossRef]
- Lawal, O.D.; Aronow, H.D.; Shobayo, F.; Hume, A.L.; Taveira, T.H.; Matson, K.L.; Zhang, Y.; Wen, X. Comparative Effectiveness and Safety of Direct Oral Anticoagulants and Warfarin in Patients with Atrial Fibrillation and Chronic Liver Disease: A Nationwide Cohort Study. Circulation 2023, 147, 782–794. [Google Scholar] [CrossRef] [PubMed]
- Talasaz, A.H.; McGonagle, B.; HajiQasemi, M.; Ghelichkhan, Z.A.; Sadeghipour, P.; Rashedi, S.; Cuker, A.; Lech, T.; Goldhaber, S.Z.; Jennings, D.L.; et al. Pharmacokinetic and Pharmacodynamic Interactions between Food or Herbal Products and Oral Anticoagulants: Evidence Review, Practical Recommendations, and Knowledge Gaps. Semin. Thromb. Hemost. 2024. [Google Scholar] [CrossRef] [PubMed]
- Mar, P.L.; Gopinathannair, R.; Gengler, B.E.; Chung, M.K.; Perez, A.; Dukes, J.; Ezekowitz, M.D.; Lakkireddy, D.; Lip, G.Y.H.; Miletello, M.; et al. Drug Interactions Affecting Oral Anticoagulant Use. Circ. Arrhythmia Electrophysiol. 2022, 15, e007956. [Google Scholar] [CrossRef] [PubMed]
- Fredenburgh, J.C.; Weitz, J.I. New anticoagulants: Moving beyond the direct oral anticoagulants. J. Thromb. Haemost. 2021, 19, 20–29. [Google Scholar] [CrossRef]
- Lippi, G.; Favaloro, E.J. Pearls and Pitfalls in the Measurement of Direct Oral Anticoagulants. Semin. Thromb. Hemost. 2024, 50, 1114–1122. [Google Scholar] [CrossRef]
- Favaloro, E.J.; Mohammed, S.; Curnow, J.; Pasalic, L. Laboratory testing for lupus anticoagulant (LA) in patients taking direct oral anticoagulants (DOACs): Potential for false positives and false negatives. Pathology 2019, 51, 292–300. [Google Scholar] [CrossRef]
- Favaloro, E.J. Danger of false negative (exclusion) or false positive (diagnosis) for ‘congenital thrombophilia’ in the age of anticoagulants. Clin. Chem. Lab. Med. 2019, 57, 873–882. [Google Scholar] [CrossRef]
- Favaloro, E.J.; Lippi, G. Interference of direct oral anticoagulants in haemostasis assays: High potential for diagnostic false positives and false negatives. Blood Transfus. 2017, 15, 491–494. [Google Scholar]
- Favaloro, E.J.; Pasalic, L. Innovative Diagnostic Solutions in Hemostasis. Diagnostics 2024, 14, 2521. [Google Scholar] [CrossRef]
- Brennan, Y.; Favaloro, E.J.; Pasalic, L.; Keenan, H.; Curnow, J. Lessons learnt from local real-life experience with idarucizumab for the reversal of dabigatran. Intern. Med. J. 2019, 49, 59–65. [Google Scholar] [CrossRef]
- Kalathottukaren, M.T.; Creagh, A.L.; Abbina, S.; Lu, G.; Karbarz, M.J.; Pandey, A.; Conley, P.B.; Kizhakkedathu, J.N.; Haynes, C. Comparison of reversal activity and mechanism of action of UHRA, andexanet, and PER977 on heparin and oral FXa inhibitors. Blood Adv. 2018, 2, 2104–2114. [Google Scholar] [CrossRef]
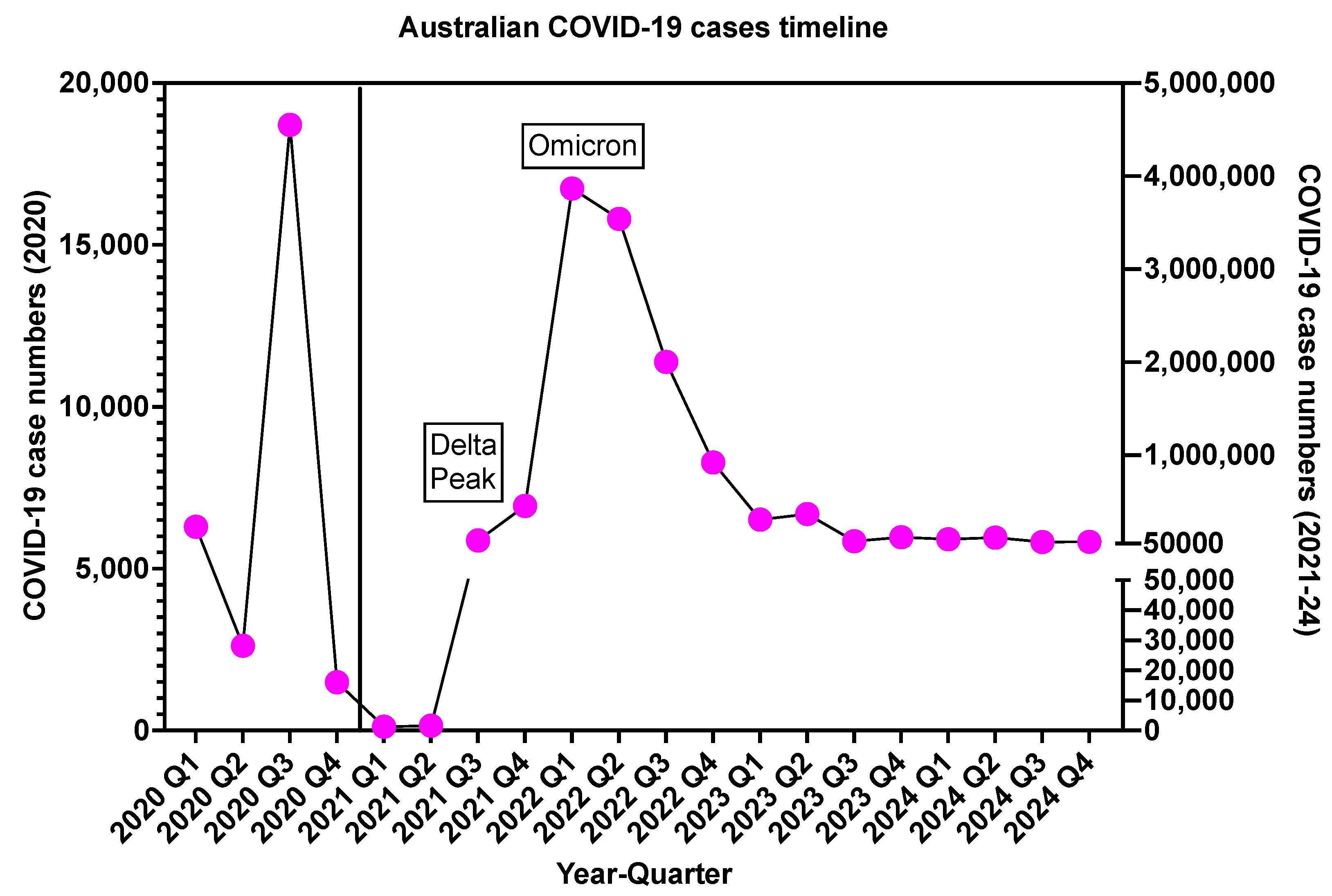
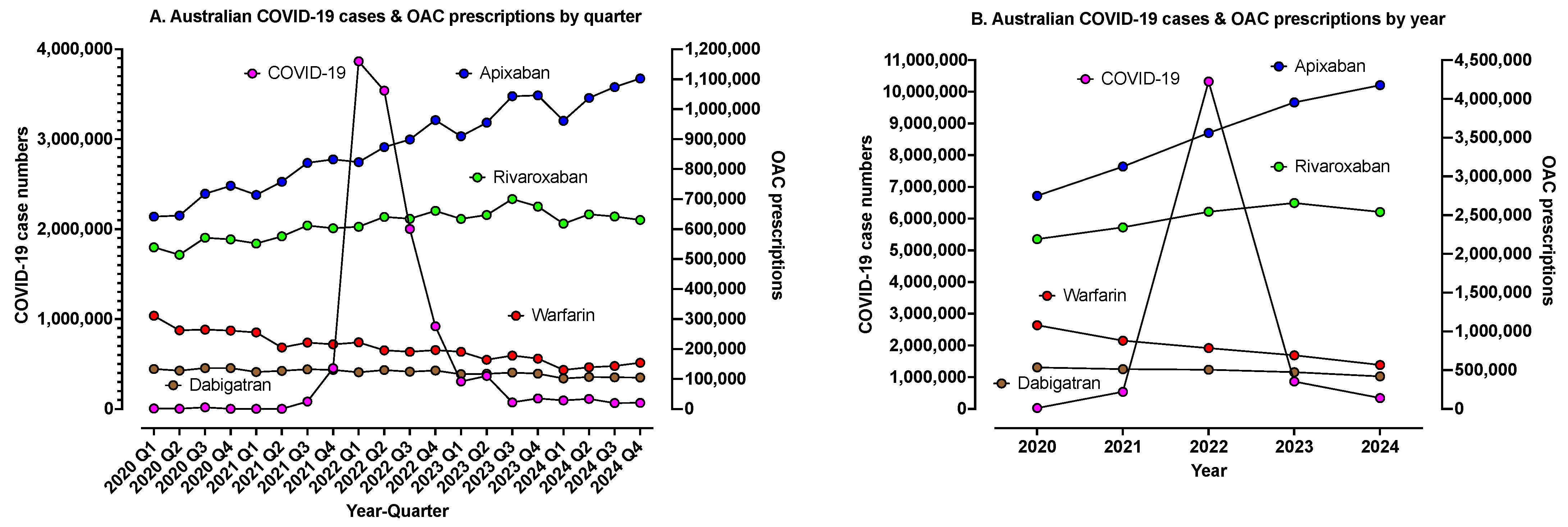
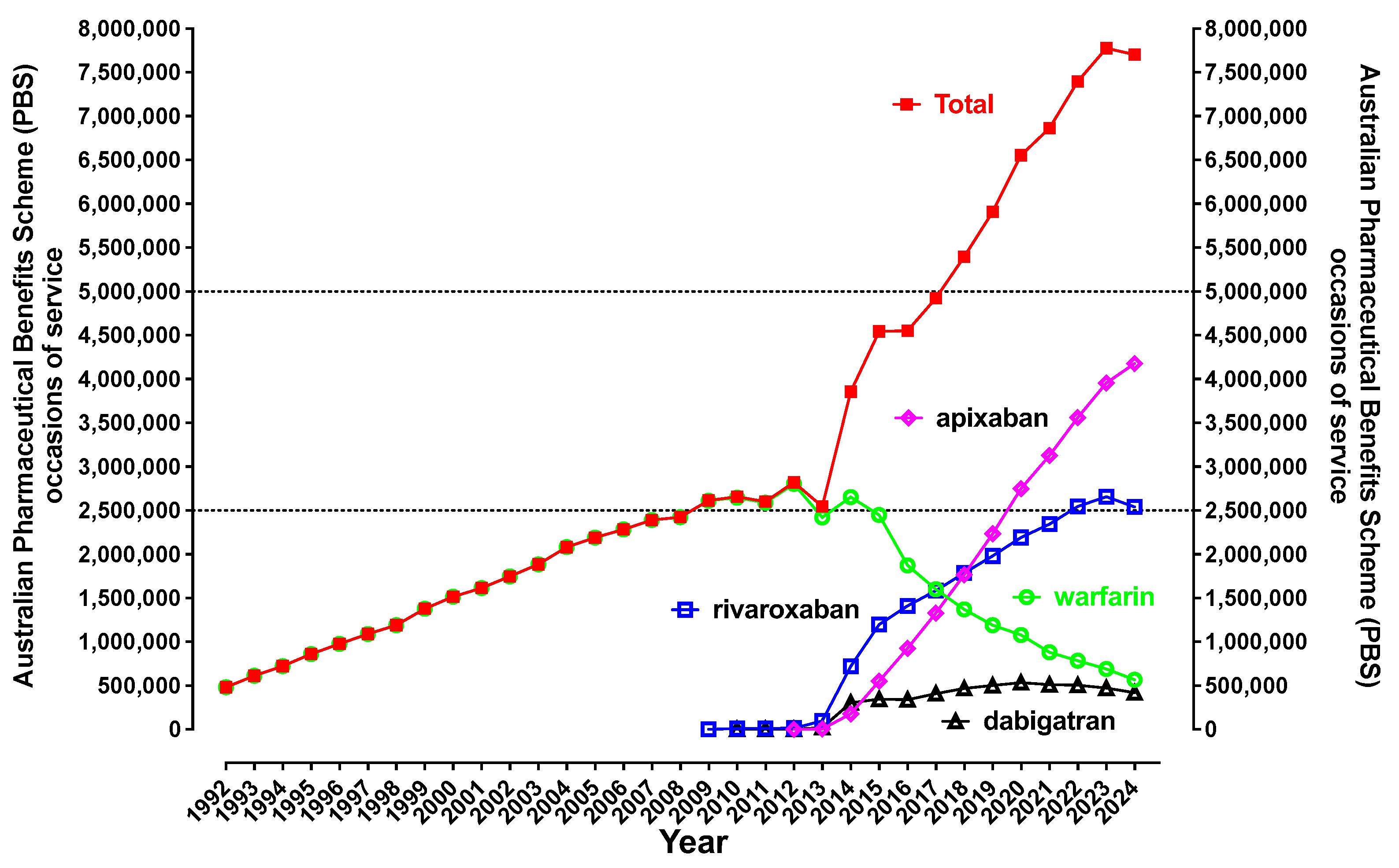
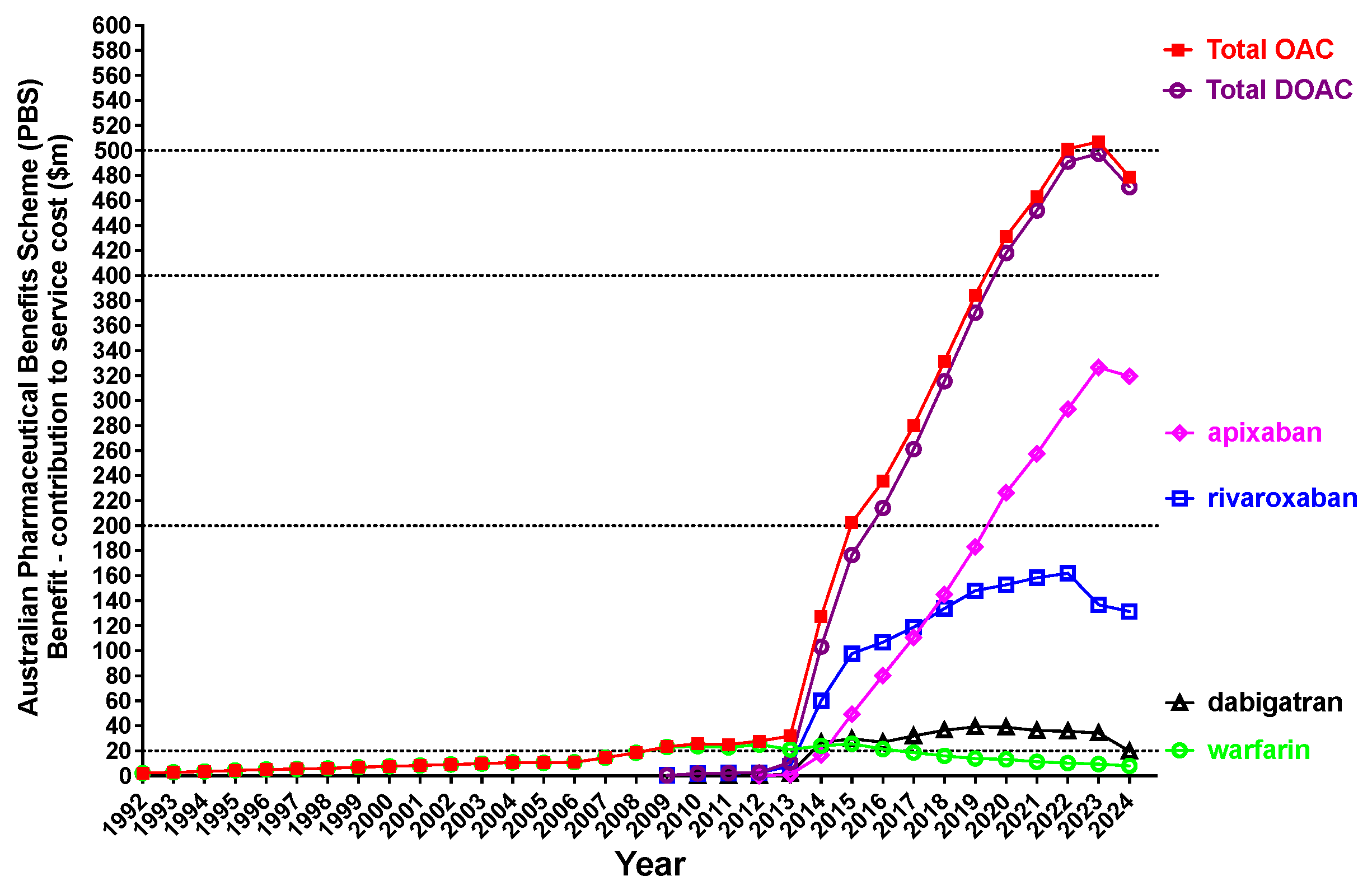

| Oral Anticoagulant, Available Brands and PBS Codes | Presentation (Dose, Pack Size) | Clinical Indication(s) |
|---|---|---|
| Warfarin sodium (Coumadin, Marevan) | ||
| 2843P | 1 mg tablet, 50 | Prevention of stroke or systemic embolism |
| 2209G | 2 mg tablet, 50 | Continuing treatment of DVT/PE |
| 2844Q | 3 mg tablet, 50 | Prevention of recurrent VTE |
| 2211J | 5 mg tablet, 50 | Coronary occlusion adjunctive treatment |
| Dabigatran etexilate (Pradaxa; newer generics: ARX-Dabigatran, Dabigatran Sandoz, PHARMACOR DABIGATRAN) | ||
| 9318K | 75 mg capsule, 10 | Prevention of VTE after total hip replacement |
| 9322P | 75 mg capsule, 10 | Prevention of VTE after total knee replacement |
| 9319L | 110 mg capsule, 10 | Prevention of VTE after total hip replacement |
| 9323Q | 110 mg capsule, 10 | Prevention of VTE after total knee replacement |
| 13489Y, 2769R, 13523R, 2753X | 150 mg capsule, 60 | Prevention of stroke or systemic embolism |
| 9321N | 110 mg capsule, 60 | Prevention of VTE after total hip replacement |
| 9320M | 75 mg capsule, 60 | Prevention of VTE after total hip replacement |
| Rivaroxaban (Xarelto; newer generics: RIVOXA, Rivaroxaban-Teva, iXarola, Rivaroxaban Sandoz) | ||
| 12192Q, 12197Y, 13366L | 2.5 mg tablet, 60 | Chronic stable atherosclerotic disease |
| 11633G, 13521P | 10 mg tablet, 30 | Prevention of recurrent VTE |
| 9467G | 10 mg tablet, 30 | Prevention of VTE after total hip replacement |
| 9466F | 10 mg tablet, 15 | Prevention of VTE after hip replacement |
| 9469J | 10 mg tablet, 15 | Prevention of VTE after knee replacement |
| 2160Q | 15 mg tablet, 42 | Initial treatment of DVT/PE |
| 13462M, 2268J | 20 mg tablet, 28 | Continuing treatment of DVT/PE; prevention of recurrent VTE, Prevention of stroke or systemic embolism |
| 13463N, 2691P | 15 mg tablet, 28 | Prevention of stroke or systemic embolism |
| Apixaban (Eliquis) | ||
| 13525W | 5 mg tablet, 60 | Prevention of stroke or systemic embolism |
| 2735Y | 5 mg tablet, 60 | Continuing treatment of DVT/PE; prevention of stroke or systemic embolism |
| 5500L | 2.5 mg tablet, 20 | Prevention of VTE after total knee or hip replacement |
| 5054B | 2.5 mg tablet, 30 | Prevention of VTE after total knee replacement |
| 13464P | 2.5 mg tablet, 60 | Prevention of stroke or systemic embolism |
| 2744K | 2.5 mg tablet, 60 | Prevention of recurrent VTE; prevention of stroke or systemic embolism |
| 5061J | 2.5 mg tablet, 60 | Prevention of VTE after total hip replacement |
| 10414D | 5 mg tablet, 28 | Initial treatment of DVT/PE |
| Oral Anticoagulant, PBS Codes/Generics | Pricing in 2020 | Pricing in 2024 (Original Item) | Pricing in 2024 (Generic Items) | Comment (2024 vs. 2020) |
|---|---|---|---|---|
| Warfarin sodium (Coumadin, Marevan) | ||||
| 2843P | 16.16 | 16.16 | NA | No change |
| 2209G | 16.35 | 16.35 | NA | No change |
| 2844Q | 16.42 | 16.42 | NA | No change |
| 2211J | 16.86 | 16.86 | NA | No change |
| Dabigatran etexilate (Pradaxa; newer generics: ARX-Dabigatran, Dabigatran Sandoz, PHARMACOR DABIGATRAN) | ||||
| 9318K | 44.39 | 31.25 | NA | Reduced |
| 9322P | NA | 35.21 | NA | NA |
| 9319L | 37.25 | 26.31 | NA | Reduced |
| 9323Q | NA | 26.31 | NA | NA |
| 13489Y | NA | 102.17 | 84.31 | Generics cheaper |
| 2769R | 88.77 | 57.81 | 48.88 | Reduced, generics cheaper |
| 13523R | NA | 102.69 | 84.83 | Generics cheaper |
| 2753X | 88.77 | 58.07 | 49.14 | Reduced, generics cheaper |
| 9321N | NA | 58.07 | 49.14 | Generics cheaper |
| 9320M | 110.18 | 79.63 | 79.63 | Reduced |
| Rivaroxaban (Xarelto; newer generics: RIVOXA, Rivaroxaban-Teva, iXarola, Rivaroxaban Sandoz) | ||||
| 12192Q, 12197Y | NA | 60.12 | 60.12 | NA |
| 13366L | NA | 84.15 | 84.15 | NA |
| 11633G | 92.99 | 66.36 | 66.36 | Reduced |
| 13521P | NA | 93.55 | 93.55 | NA |
| 9467G | NA | 53.50 | 53.50 | NA |
| 9466F | 52.24 | 39.68 | 39.68 | Reduced |
| 9469J | NA | 39.68 | 39.68 | NA |
| 2160Q | 125.6 | 86.85 | 86.85 | Reduced |
| 13462M | NA | 110.23 | 110.23 | NA |
| 2268J | 87.56 | 61.61 | 61.61 | Reduced |
| 13463N | NA | 111.45 | 111.45 | NA |
| 2691P | 87.56 | 62.22 | 62.22 | Reduced |
| Apixaban (Eliquis) | ||||
| 13525W | NA | 171.25 | NA | NA |
| 2735Y | 93.31 | 93.73 | NA | Small increase |
| 5500L | 38.76 | 38.90 | NA | Small increase |
| 5054B | 52.4 | 51.86 | NA | Small reduction |
| 13464P | NA | 171.25 | NA | NA |
| 2744K | 93.31 | 90.73 | NA | Reduced |
| 5061J | NA | 69.13 | NA | NA |
| 10414D | 49.67 | 49.27 | NA | Small reduction |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Favaloro, E.J.; Pasalic, L.; Lippi, G. Oral Anticoagulation Therapy: An Update on Usage and Costs in the Endemic COVID-19 Era. J. Clin. Med. 2025, 14, 2591. https://doi.org/10.3390/jcm14082591
Favaloro EJ, Pasalic L, Lippi G. Oral Anticoagulation Therapy: An Update on Usage and Costs in the Endemic COVID-19 Era. Journal of Clinical Medicine. 2025; 14(8):2591. https://doi.org/10.3390/jcm14082591
Chicago/Turabian StyleFavaloro, Emmanuel J., Leonardo Pasalic, and Giuseppe Lippi. 2025. "Oral Anticoagulation Therapy: An Update on Usage and Costs in the Endemic COVID-19 Era" Journal of Clinical Medicine 14, no. 8: 2591. https://doi.org/10.3390/jcm14082591
APA StyleFavaloro, E. J., Pasalic, L., & Lippi, G. (2025). Oral Anticoagulation Therapy: An Update on Usage and Costs in the Endemic COVID-19 Era. Journal of Clinical Medicine, 14(8), 2591. https://doi.org/10.3390/jcm14082591








