Rethinking the Assessment of Arthrogenic Muscle Inhibition After ACL Reconstruction: Implications for Return-to-Sport Decision-Making—A Narrative Review
Abstract
:1. Introduction
2. Methodology
2.1. Databases and Search Strategy
- (“arthrogenic muscle inhibition” OR “AMI”) AND (“ACL reconstruction” OR “anterior cruciate ligament”);
- (“quadriceps inhibition” OR “central activation failure”) AND (“neuromuscular control” OR “corticospinal excitability”);
- (“return to sport” OR “return to running”) AND (“biomechanics” OR “gait analysis”);
- (“functional performance” OR “rehabilitation outcomes”) AND (“ACL” OR “AMI”).
2.2. Inclusion and Exclusion Criteria
- Peer-reviewed articles;
- Works focused on AMI mechanisms, assessment, or rehabilitation following ACL injury or reconstruction;
- Studies reporting neuromuscular, biomechanical, or functional outcomes;
- Studies in English, published between 2010 and 2024, with a focus on recent developments (2022–2024).
- Conference abstracts without full-text availability;
- Case reports and expert opinion pieces lacking empirical data;
- Non-English publications.
2.3. Study Selection and Relevance
3. Neurophysiological Mechanisms of AMI
3.1. Central and Peripheral Contributions to AMI
3.2. Muscle Groups Affected
3.3. Factors Influencing AMI Persistence
4. Clinical and Functional Manifestations
4.1. Neuromuscular Deficits Affecting RTR and RTS
4.2. Gait Alterations Due to AMI
4.3. Clinical and Functional Tests
4.4. Correlation Between Persistent AMI and Reinjury Risk
5. Assessment of AMI
5.1. Electromyography Analysis
5.2. Isokinetic and Isometric Strength Testing
5.3. Reflex and Corticospinal Excitability Tests
5.4. Joint Effusion and Sensory Deficit Assessment
5.5. Functional and Performance-Based Evaluations
5.5.1. Single-Leg Hop and Drop-Jump Tests
5.5.2. Gait and Running Biomechanics’ Analysis
5.5.3. Fatigue-Resistant Strength and Activation Tests
5.5.4. Patient-Reported Outcome Measures (PROMs)
5.5.5. Cognitive–Motor Integration Tests
5.6. Empirical Evidence Supporting AMI Assessment
6. Rehabilitation Strategies and Clinical Implications
6.1. Need for Individualized Assessment Protocols
6.2. Advancements in Technology for AMI Assessment
6.3. Challenges in AMI Research and Clinical Translation
6.4. Practical Recommendations for Clinicians and Researchers
6.5. Structured Rehabilitation Framework Targeting AMI
6.6. Alignment with International Guidelines on Return to Sport
7. Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Griffin, L.Y.; Agel, J.; Albohm, M.J.; Arendt, E.A.; Dick, R.W.; Garrett, W.E.; Garrick, J.G.; Hewett, T.E.; Huston, L.; Ireland, M.L.; et al. Noncontact Anterior Cruciate Ligament Injuries: Risk Factors and Prevention Strategies. J. Am. Acad. Orthop. Surg. 2000, 8, 141–150. [Google Scholar] [CrossRef]
- Forelli, F.; Riera, J.; Mazeas, J.; Coulondre, C.; Putnis, S.; Neri, T.; Rambaud, A. Ligament Healing After Anterior Cruciate Ligament Rupture: An Important New Patient Pathway? Int. J. Sports Phys. Ther. 2023, 18, 1032–1035. [Google Scholar] [CrossRef] [PubMed]
- Frobell, R.B.; Roos, E.M.; Roos, H.P.; Ranstam, J.; Lohmander, L.S. A Randomized Trial of Treatment for Acute Anterior Cruciate Ligament Tears. N. Engl. J. Med. 2010, 363, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Arendt, E.; Dick, R. Knee Injury Patterns Among Men and Women in Collegiate Basketball and Soccer: NCAA Data and Review of Literature. Am. J. Sports Med. 1995, 23, 694–701. [Google Scholar] [CrossRef]
- Benjaminse, A.; Gokeler, A.; Van Der Schans, C.P. Clinical Diagnosis of an Anterior Cruciate Ligament Rupture: A Meta-Analysis. J. Orthop. Sports Phys. Ther. 2006, 36, 267–288. [Google Scholar] [CrossRef] [PubMed]
- Bucher, C.; Lamy, D.; Debaty, G.; Pailhé, R.; Saragaglia, D. Validity of the Lever Sign Test for the Clinical Diagnosis of Anterior Cruciate Ligament Tears: Assessments in Ski Resorts. Orthop. Traumatol. Surg. Res. 2022, 108, 103254. [Google Scholar] [CrossRef]
- Hesmerg, M.K.; Oostenbroek, M.H.W.; Van Der List, J.P. Lever Sign Test Shows High Diagnostic Accuracy for Anterior Cruciate Ligament Injuries: A Systematic Review and Meta-Analysis of 3299 Observations. Knee 2024, 47, 81–91. [Google Scholar] [CrossRef]
- Krakowski, P.; Nogalski, A.; Jurkiewicz, A.; Karpiński, R.; Maciejewski, R.; Jonak, J. Comparison of Diagnostic Accuracy of Physical Examination and MRI in the Most Common Knee Injuries. Appl. Sci. 2019, 9, 4102. [Google Scholar] [CrossRef]
- Makhmalbaf, H.; Moradi, A.; Ganji, S.; Omidi-Kashani, F. Accuracy of Lachman and Anterior Drawer Tests for Anterior Cruciate Ligament Injuries. Arch. Bone Jt. Surg. 2013, 1, 94–97. [Google Scholar]
- Ardern, C.L.; Taylor, N.F.; Feller, J.A.; Webster, K.E. Fifty-Five per Cent Return to Competitive Sport Following Anterior Cruciate Ligament Reconstruction Surgery: An Updated Systematic Review and Meta-Analysis Including Aspects of Physical Functioning and Contextual Factors. Br. J. Sports Med. 2014, 48, 1543–1552. [Google Scholar] [CrossRef]
- Gokeler, A.; Dingenen, B.; Hewett, T.E. Rehabilitation and Return to Sport Testing After Anterior Cruciate Ligament Reconstruction: Where Are We in 2022? Arthrosc. Sports Med. Rehabil. 2022, 4, e77–e82. [Google Scholar] [CrossRef] [PubMed]
- Rice, D.A.; McNair, P.J. Quadriceps Arthrogenic Muscle Inhibition: Neural Mechanisms and Treatment Perspectives. Semin. Arthritis Rheum. 2010, 40, 250–266. [Google Scholar] [CrossRef]
- Moiroux-Sahraoui, A.; Forelli, F.; Mazeas, J.; Rambaud, A.J.; Bjerregaard, A.; Riera, J. Quadriceps Activation After Anterior Cruciate Ligament Reconstruction: The Early Bird Gets the Worm! Int. J. Sports Phys. Ther. 2024, 19, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Palmieri-Smith, R.M.; Thomas, A.C.; Wojtys, E.M. Maximizing Quadriceps Strength After ACL Reconstruction. Clin. Sports Med. 2008, 27, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Sonnery-Cottet, B.; Hopper, G.P.; Gousopoulos, L.; Vieira, T.D.; Thaunat, M.; Fayard, J.-M.; Freychet, B.; Ouanezar, H.; Cavaignac, E.; Saithna, A. Arthrogenic Muscle Inhibition Following Knee Injury or Surgery: Pathophysiology, Classification, and Treatment. Video J. Sports Med. 2022, 2, 263502542210862. [Google Scholar] [CrossRef]
- Sonnery-Cottet, B.; Saithna, A.; Quelard, B.; Daggett, M.; Borade, A.; Ouanezar, H.; Thaunat, M.; Blakeney, W.G. Arthrogenic Muscle Inhibition after ACL Reconstruction: A Scoping Review of the Efficacy of Interventions. Br. J. Sports Med. 2019, 53, 289–298. [Google Scholar] [CrossRef]
- Hart, J.M.; Pietrosimone, B.; Hertel, J.; Ingersoll, C.D. Quadriceps Activation Following Knee Injuries: A Systematic Review. J. Athl. Train. 2010, 45, 87–97. [Google Scholar] [CrossRef]
- Kuenze, C.M.; Hertel, J.; Weltman, A.; Diduch, D.; Saliba, S.A.; Hart, J.M. Persistent Neuromuscular and Corticomotor Quadriceps Asymmetry After Anterior Cruciate Ligament Reconstruction. J. Athl. Train. 2015, 50, 303–312. [Google Scholar] [CrossRef]
- Hopkins, J.T.; Ingersoll, C.D. Arthrogenic Muscle Inhibition: A Limiting Factor in Joint Rehabilitation. J. Sport Rehabil. 2000, 9, 135–159. [Google Scholar] [CrossRef]
- Lepley, L.K.; Palmieri-Smith, R.M. Quadriceps Strength, Muscle Activation Failure, and Patient-Reported Function at the Time of Return to Activity in Patients Following Anterior Cruciate Ligament Reconstruction: A Cross-Sectional Study. J. Orthop. Sports Phys. Ther. 2015, 45, 1017–1025. [Google Scholar] [CrossRef]
- McPherson, A.L.; Schilaty, N.D.; Anderson, S.; Nagai, T.; Bates, N.A. Arthrogenic Muscle Inhibition After Anterior Cruciate Ligament Injury: Injured and Uninjured Limb Recovery over Time. Front. Sports Act. Living 2023, 5, 1143376. [Google Scholar] [CrossRef] [PubMed]
- Dingenen, B.; Gokeler, A. Optimization of the Return-to-Sport Paradigm After Anterior Cruciate Ligament Reconstruction: A Critical Step Back to Move Forward. Sports Med. 2017, 47, 1487–1500. [Google Scholar] [CrossRef] [PubMed]
- Forelli, F.; Le Coroller, N.; Gaspar, M.; Memain, G.; Kakavas, G.; Miraglia, N.; Marine, P.; Maille, P.; Hewett, T.E.; Rambaud, A.J. Ecological and Specific Evidence-Based Safe Return to Play After Anterior Cruciate Ligament Reconstruction in Soccer Players: A New International Paradigm. Int. J. Sports Phys. Ther. 2023, 18, 526–540. [Google Scholar] [CrossRef]
- Schmitt, L.C.; Paterno, M.V.; Ford, K.R.; Myer, G.D.; Hewett, T.E. Strength Asymmetry and Landing Mechanics at Return to Sport after Anterior Cruciate Ligament Reconstruction. Med. Sci. Sports Exerc. 2015, 47, 1426–1434. [Google Scholar] [CrossRef] [PubMed]
- Paterno, M.V.; Rauh, M.J.; Schmitt, L.C.; Ford, K.R.; Hewett, T.E. Incidence of Second ACL Injuries 2 Years After Primary ACL Reconstruction and Return to Sport. Am. J. Sports Med. 2014, 42, 1567–1573. [Google Scholar] [CrossRef]
- Forelli, F.; Traulle, M.; Bechaud, N.; Sansonnet, C.; Marine, P.; Vandebrouck, A.; Duffiet, P.; Mazeas, J. Predict Anterior Cruciate Ligament Injury in Elite Male Soccer Players? Focus on the Five Factors Maximum Model. Int. J. Physiother. 2021, 8, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Grooms, D.R.; Page, S.J.; Nichols-Larsen, D.S.; Chaudhari, A.M.W.; White, S.E.; Onate, J.A. Neuroplasticity Associated with Anterior Cruciate Ligament Reconstruction. J. Orthop. Sports Phys. Ther. 2017, 47, 180–189. [Google Scholar] [CrossRef]
- Grindem, H.; Snyder-Mackler, L.; Moksnes, H.; Engebretsen, L.; Risberg, M.A. Simple Decision Rules Can Reduce Reinjury Risk by 84% after ACL Reconstruction: The Delaware-Oslo ACL Cohort Study. Br. J. Sports Med. 2016, 50, 804–808. [Google Scholar] [CrossRef]
- Biały, M.; Wilczyński, B.; Forelli, F.; Hewett, T.E.; Gnat, R. Functional Deficits in Non-Elite Soccer (Football) Players: A Strength, Balance, and Movement Quality Assessment After Anterior Cruciate Ligament Reconstruction. Cureus 2024, 16, e75846. [Google Scholar] [CrossRef]
- Needle, A.R.; Lepley, A.S.; Grooms, D.R. Central Nervous System Adaptation After Ligamentous Injury: A Summary of Theories, Evidence, and Clinical Interpretation. Sports Med. 2017, 47, 1271–1288. [Google Scholar] [CrossRef]
- Schilaty, N.D.; McPherson, A.L.; Nagai, T.; Bates, N.A. Arthrogenic Muscle Inhibition Manifests in Thigh Musculature Motor Unit Characteristics After Anterior Cruciate Ligament Injury. Eur. J. Sport Sci. 2023, 23, 840–850. [Google Scholar] [CrossRef] [PubMed]
- Lepley, A.S.; Lepley, L.K. Mechanisms of Arthrogenic Muscle Inhibition. J. Sport Rehabil. 2022, 31, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Norte, G.; Rush, J.; Sherman, D. Arthrogenic Muscle Inhibition: Best Evidence, Mechanisms, and Theory for Treating the Unseen in Clinical Rehabilitation. J. Sport Rehabil. 2022, 31, 717–735. [Google Scholar] [CrossRef]
- Lepley, A.S.; Gribble, P.A.; Thomas, A.C.; Tevald, M.A.; Sohn, D.H.; Pietrosimone, B.G. Quadriceps Neural Alterations in Anterior Cruciate Ligament Reconstructed Patients: A 6-month Longitudinal Investigation. Scand. Med. Sci. Sports 2015, 25, 828–839. [Google Scholar] [CrossRef] [PubMed]
- Pietrosimone, B.G.; Lepley, A.S.; Ericksen, H.M.; Gribble, P.A.; Levine, J. Quadriceps Strength and Corticospinal Excitability as Predictors of Disability After Anterior Cruciate Ligament Reconstruction. J. Sport Rehabil. 2013, 22, 1–6. [Google Scholar] [CrossRef]
- Zunzarren, G.; Garet, B.; Vinciguerra, B.; Murgier, J. Persistence of Neuromuscular Activation Deficit in the Lower Limb at 3-Years of Follow-up After ACL Reconstruction Surgery. Knee 2023, 43, 97–105. [Google Scholar] [CrossRef]
- Kiapour, A.M.; Flannery, S.W.; Murray, M.M.; Miller, P.E.; BEAR Trial Team; Proffen, B.L.; Sant, N.; Portilla, G.; Sanborn, R.; Freiberger, C.; et al. Regional Differences in Anterior Cruciate Ligament Signal Intensity After Surgical Treatment. Am. J. Sports Med. 2021, 49, 3833–3841. [Google Scholar] [CrossRef]
- Moiroux-Sahraoui, A.; Mazeas, J.; Gold, M.; Kakavas, G.; Forelli, F. Neuromuscular Control Deficits After Anterior Cruciate Ligament Reconstruction: A Pilot Study Using Single-Leg Functional Tests and Electromyography. J. Funct. Morphol. Kinesiol. 2025, 10, 98. [Google Scholar] [CrossRef]
- Grooms, D.; Appelbaum, G.; Onate, J. Neuroplasticity Following Anterior Cruciate Ligament Injury: A Framework for Visual-Motor Training Approaches in Rehabilitation. J. Orthop. Sports Phys. Ther. 2015, 45, 381–393. [Google Scholar] [CrossRef]
- Sonnery-Cottet, B.; Hopper, G.P.; Gousopoulos, L.; Pioger, C.; Vieira, T.D.; Thaunat, M.; Fayard, J.-M.; Freychet, B.; Cavaignac, E.; Saithna, A. Incidence of and Risk Factors for Arthrogenic Muscle Inhibition in Acute Anterior Cruciate Ligament Injuries: A Cross-Sectional Study and Analysis of Associated Factors from the SANTI Study Group. Am. J. Sports Med. 2024, 52, 60–68. [Google Scholar] [CrossRef]
- Palmieri-Smith, R.M.; Thomas, A.C. A Neuromuscular Mechanism of Posttraumatic Osteoarthritis Associated with ACL Injury. Exerc. Sport Sci. Rev. 2009, 37, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Baumeister, J.; Reinecke, K.; Schubert, M.; Weiß, M. Altered Electrocortical Brain Activity after ACL Reconstruction During Force Control. J. Orthop. Res. 2011, 29, 1383–1389. [Google Scholar] [CrossRef]
- Büttner, C.; Lisee, C.; Favoreto, N.; Bjornsen, E.; Buck, A.; Creighton, A.; Kamath, G.; Spang, J.; Blackburn, T.; Pietrosimone, B. Bilateral Gait Differs from Controls Preoperatively to 4 Months Following Anterior Cruciate Ligament Reconstruction: 1394. Med. Sci. Sports Exerc. 2024, 56, 467–468. [Google Scholar] [CrossRef]
- Pietrosimone, B.; Lepley, A.S.; Kuenze, C.; Harkey, M.S.; Hart, J.M.; Blackburn, J.T.; Norte, G. Arthrogenic Muscle Inhibition Following Anterior Cruciate Ligament Injury. J. Sport Rehabil. 2022, 31, 694–706. [Google Scholar] [CrossRef] [PubMed]
- Lepley, L.K.; Wojtys, E.M.; Palmieri-Smith, R.M. Combination of Eccentric Exercise and Neuromuscular Electrical Stimulation to Improve Quadriceps Function Post-ACL Reconstruction. Knee 2015, 22, 270–277. [Google Scholar] [CrossRef]
- Kram, R.; Griffin, T.M.; Donelan, J.M.; Chang, Y.H. Force Treadmill for Measuring Vertical and Horizontal Ground Reaction Forces. J. Appl. Physiol. 1998, 85, 764–769. [Google Scholar] [CrossRef]
- Martinez, C.; Garbett, S.; Hiromasa, K.; Jackson, R.; Miya, E.; Miya, M.; White, J.D.; Baum, B.S.; Reinking, M.F. Comparison of 2-D and 3-D Analysis of Running Kinematics and Actual Versus Predicted Running Kinetics. Int. J. Sports Phys. Ther. 2022, 17, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Pamukoff, D.N.; Montgomery, M.M.; Choe, K.H.; Moffit, T.J.; Garcia, S.A.; Vakula, M.N. Bilateral Alterations in Running Mechanics and Quadriceps Function Fol Lowing Unilateral Anterior Cruciate Ligament Reconstruction. J. Orthop. Sports Phys. Ther. 2018, 48, 960–967. [Google Scholar] [CrossRef]
- Pietrosimone, B.G.; Lepley, A.S.; Ericksen, H.M.; Clements, A.; Sohn, D.H.; Gribble, P.A. Neural Excitability Alterations After Anterior Cruciate Ligament Reconstruction. J. Athl. Train. 2015, 50, 665–674. [Google Scholar] [CrossRef]
- Palmieri-Smith, R.M.; Lepley, L.K. Quadriceps Strength Asymmetry After Anterior Cruciate Ligament Reconstruction Alters Knee Joint Biomechanics and Functional Performance at Time of Return to Activity. Am. J. Sports Med. 2015, 43, 1662–1669. [Google Scholar] [CrossRef]
- Paço, M.; Peysson, M.; Dumont, E.; Correia, M.; Quialheiro, A.; Chaves, P. The Effect of Physiotherapy on Arthrogenic Muscle Inhibition After ACL Injury or Reconstruction: A Systematic Review. Life 2024, 14, 1586. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.C.; Sylvester, C.; Whife, C.; D’Alessandro, P.; Rio, E.K.; Vallence, A.-M. Anodal Transcranial Direct Current Stimulation (tDCS) Modulates Quadriceps Motor Cortex Inhibition and Facilitation During Rehabilitation Following Anterior Cruciate Ligament (ACL) Reconstruction: A Triple-Blind, Randomised Controlled Proof of Concept Trial. BMJ Open Sport. Exerc. Med. 2024, 10, e002080. [Google Scholar] [CrossRef] [PubMed]
- Meredith, S.J.; Rauer, T.; Chmielewski, T.L.; Fink, C.; Diermeier, T.; Rothrauff, B.B.; Svantesson, E.; Hamrin Senorski, E.; Hewett, T.E.; Sherman, S.L.; et al. Return to Sport After Anterior Cruciate Ligament Injury: Panther Symposium ACL Injury Return to Sport Consensus Group. Orthop. J. Sports Med. 2020, 8, 2325967120930829. [Google Scholar] [CrossRef] [PubMed]
- Wright, R.W.; Haas, A.K.; Anderson, J.; Calabrese, G.; Cavanaugh, J.; Hewett, T.E.; Lorring, D.; McKenzie, C.; Preston, E.; Williams, G.; et al. Anterior Cruciate Ligament Reconstruction Rehabilitation: MOON Guidelines. Sports Health Multidiscip. Approach 2015, 7, 239–243. [Google Scholar] [CrossRef]
- Xu, S.; Cheema, S.; Tarakemeh, A.; Randall, J.; Bechtold, M.; Mullen, S.; Schroeppel, P.; Mulcahey, M.; Vopat, B. Return to Sport After Primary Anterior Cruciate Ligament (ACL) Reconstruction: A Survey of the American Orthopaedic Society for Sports Medicine. Kans. J. Med. 2023, 16, 105–109. [Google Scholar] [CrossRef]
- Van Melick, N.; Van Cingel, R.E.H.; Brooijmans, F.; Neeter, C.; Van Tienen, T.; Hullegie, W.; Nijhuis-van Der Sanden, M.W.G. Evidence-Based Clinical Practice Update: Practice Guidelines for Anterior Cruciate Ligament Rehabilitation Based on a Systematic Review and Multidisciplinary Consensus. Br. J. Sports Med. 2016, 50, 1506–1515. [Google Scholar] [CrossRef]
- Chona, D.; Eriksson, K.; Young, S.W.; Denti, M.; Sancheti, P.K.; Safran, M.; Sherman, S. Return to Sport Following Anterior Cruciate Ligament Reconstruction: The Argument for a Multimodal Approach to Optimise Decision-Making: Current Concepts. J. ISAKOS 2021, 6, 344–348. [Google Scholar] [CrossRef]
- Van Melick, N.; Senorski, E.H.; Królikowska, A.; Prill, R. Anterior Cruciate Ligament Reconstruction Rehabilitation: Decades of Change. Knee Surg. Sports Traumatol. Arthrosc. 2025, 33, 1178–1182. [Google Scholar] [CrossRef]
| Study | Assessment Tool | Main Findings | Quantitative Data | Statistical Data |
|---|---|---|---|---|
| Lepley and Palmieri-Smith (2015) [20] | EMG—CAR | Reduced voluntary quadricep activation | CAR: 0.82 (injured) vs. 0.94 (uninvolved) | p = 0.002 |
| Kuenze et al. (2015) [18] | Isokinetic dynamometry | Quadricep torque asymmetry at 6 months | Symmetry index ~73% | p < 0.001; Cohen’s d = 1.02 |
| Pietrosimone et al. (2013) [35] | TMS | ↑ intracortical inhibition (SICI), ↓ excitability | ↓ MEP, ↑ SICI | p < 0.05; Cohen’s d = 0.80 |
| Pamukoff et al. (2018) [48] | 3D gait + force plate | ↓ knee flexion angle, ↓ GRF | 7–12° ↓ flexion; ↓ GRF ~10–15% | p < 0.01 |
| Hart et al. (2010) [17] | EMG | Quadricep inhibition across conditions | 18–25% ↓ EMG amplitude | Not quantified (review) |
| Pietrosimone et al. (2015) [49] | TMS + strength | Corticospinal predictors of quad strength | Positive correlation (r = 0.47) | p = 0.006 |
| Baumeister et al. (2011) [42] | EEG—force control | Altered electrocortical patterns | Delayed theta-band response | p < 0.05 |
| Büttner et al. (2024) [43] | Bilateral gait analysis | Asymmetry in loading and stride | ↓ step length and GRF on involved side | p < 0.05 |
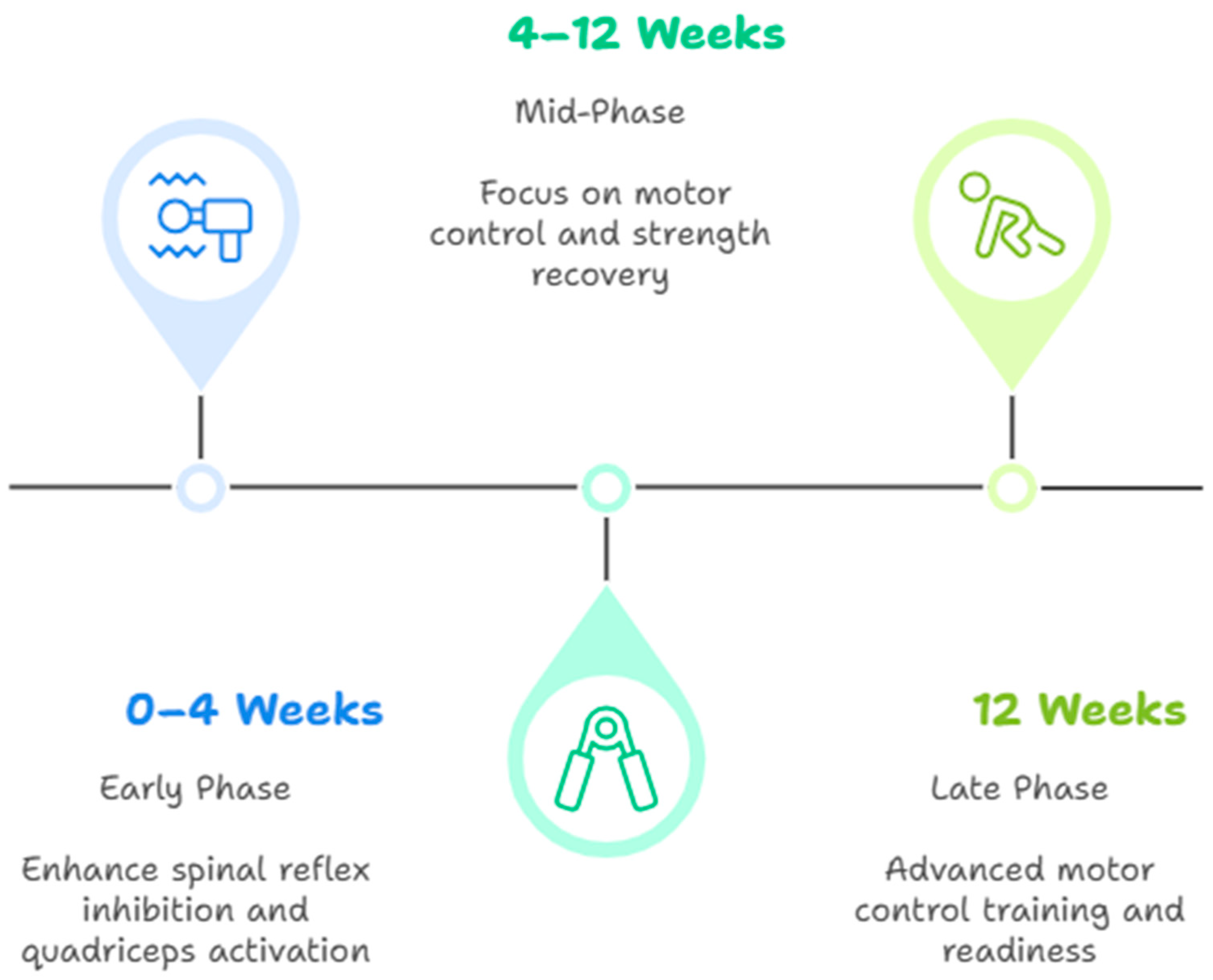
| Phase | Objective | Interventions | Frequency/Duration | Evidence/Effectiveness |
|---|---|---|---|---|
| Early (0–4 weeks) | Reactivate quadriceps, reduce reflex inhibition | NMES, cryotherapy, joint mobilization, and visual feedback | NMES: 5×/week, 20 min, 50–75 Hz; ice: 15 min post-ex | ↑ quadricep activation [11], ↓ pain [5] |
| Mid (4–12 weeks) | Improve volitional contraction, restore neuromuscular control | Eccentric exercise, blood flow restriction, and motor imagery | BFR: 2–3×/week, 30/15/15/15 reps; eccentric: 3×/week | ↑ strength gains [5], ↑ EMG [28] |
| Late (>12 weeks) | Reintegrate cognitive-motor control, prep for RTS | Dual-task training, perturbation, and sport-specific drills | 2–3×/week, 30–60 min | ↑ cortical reorganization [26], ↑ movement quality [14] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forelli, F.; Moiroux-Sahraoui, A.; Mazeas, J.; Dugernier, J.; Cerrito, A. Rethinking the Assessment of Arthrogenic Muscle Inhibition After ACL Reconstruction: Implications for Return-to-Sport Decision-Making—A Narrative Review. J. Clin. Med. 2025, 14, 2633. https://doi.org/10.3390/jcm14082633
Forelli F, Moiroux-Sahraoui A, Mazeas J, Dugernier J, Cerrito A. Rethinking the Assessment of Arthrogenic Muscle Inhibition After ACL Reconstruction: Implications for Return-to-Sport Decision-Making—A Narrative Review. Journal of Clinical Medicine. 2025; 14(8):2633. https://doi.org/10.3390/jcm14082633
Chicago/Turabian StyleForelli, Florian, Ayrton Moiroux-Sahraoui, Jean Mazeas, Jonathan Dugernier, and Adrien Cerrito. 2025. "Rethinking the Assessment of Arthrogenic Muscle Inhibition After ACL Reconstruction: Implications for Return-to-Sport Decision-Making—A Narrative Review" Journal of Clinical Medicine 14, no. 8: 2633. https://doi.org/10.3390/jcm14082633
APA StyleForelli, F., Moiroux-Sahraoui, A., Mazeas, J., Dugernier, J., & Cerrito, A. (2025). Rethinking the Assessment of Arthrogenic Muscle Inhibition After ACL Reconstruction: Implications for Return-to-Sport Decision-Making—A Narrative Review. Journal of Clinical Medicine, 14(8), 2633. https://doi.org/10.3390/jcm14082633








