Repurposing Antiepileptic Drugs for Cancer: A Promising Therapeutic Strategy
Abstract
:1. Introduction
1.1. Background on Cancer
1.2. Objectives of the Review
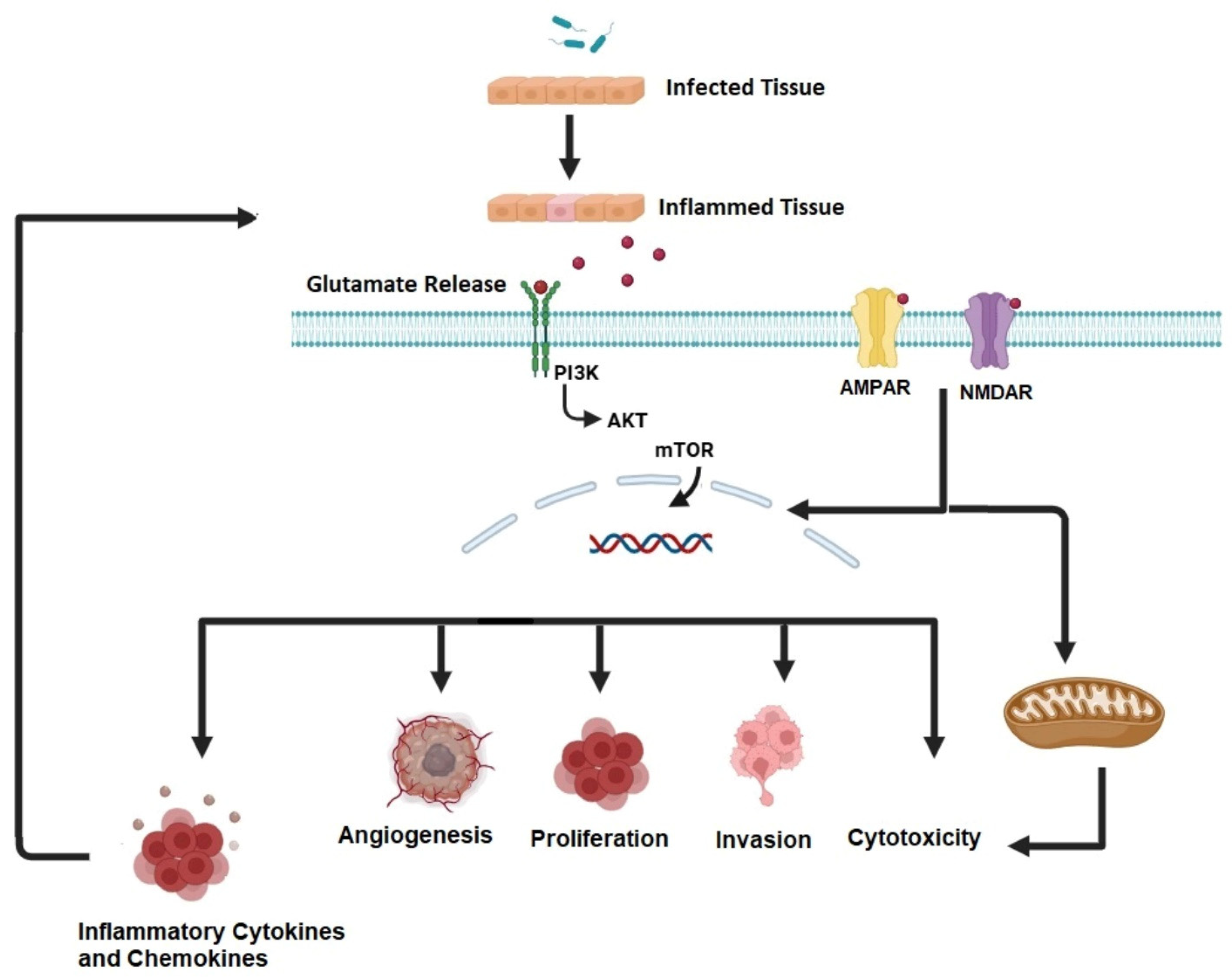
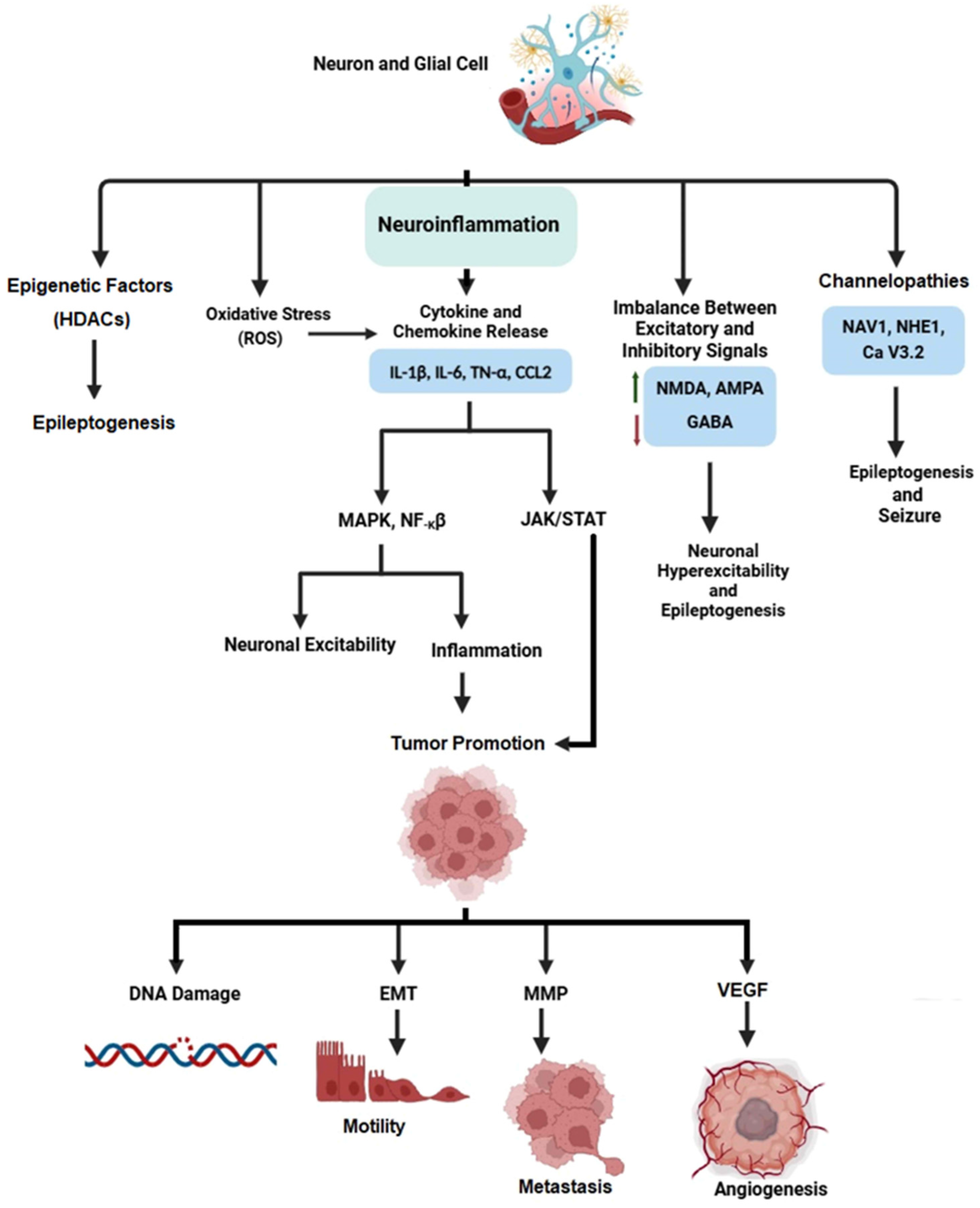
1.3. Cancer and Epilepsy
1.4. The Need for Drug Repurposing (DR)
1.5. A Practical Approach to DR
1.6. Why Are Antiepileptic Drugs Repurposed for Cancer Treatment?
2. Repurposed Antiepileptic Drugs with Anticancer Potential
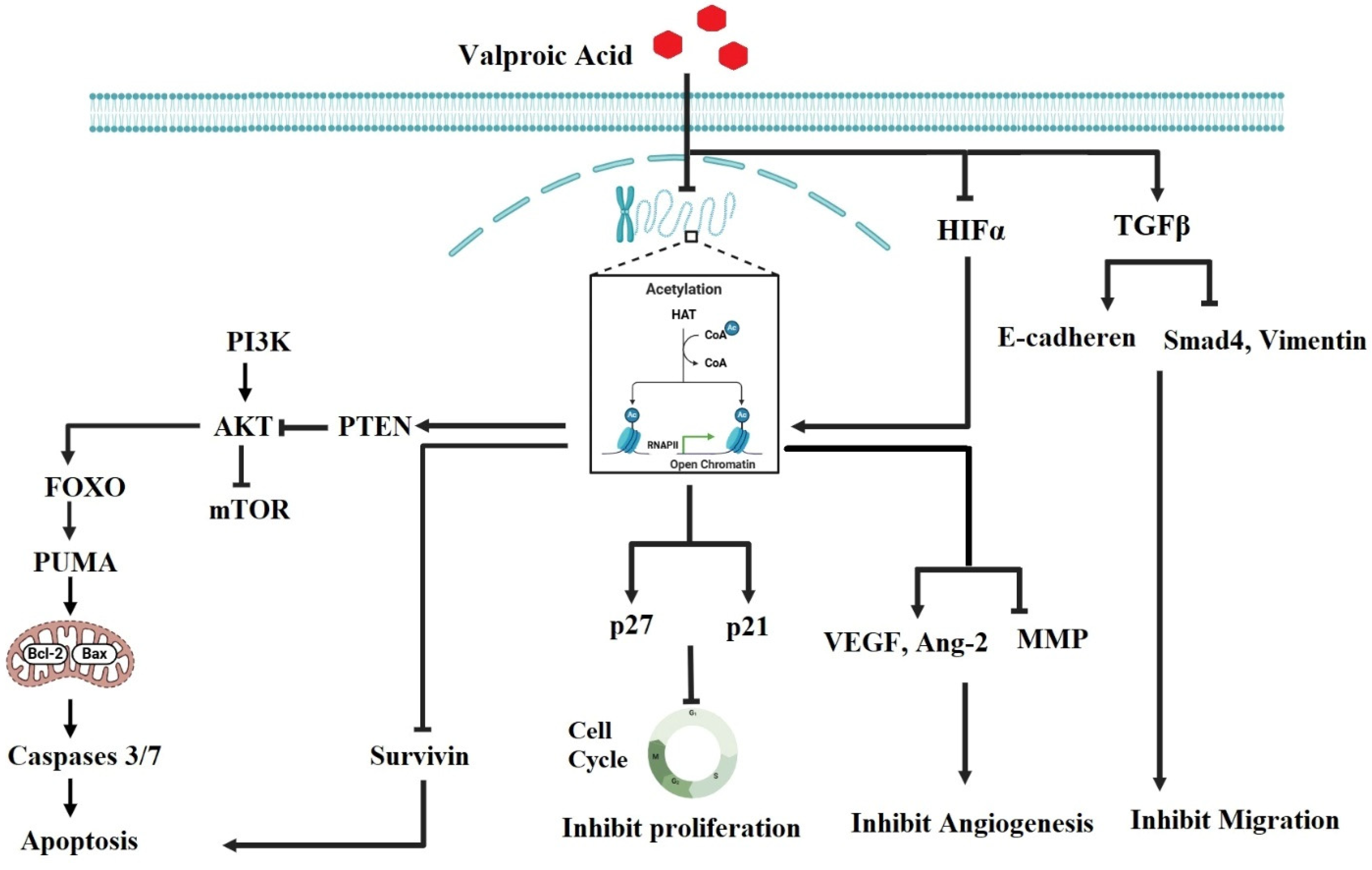
2.1. Valproic Acid (VPA)
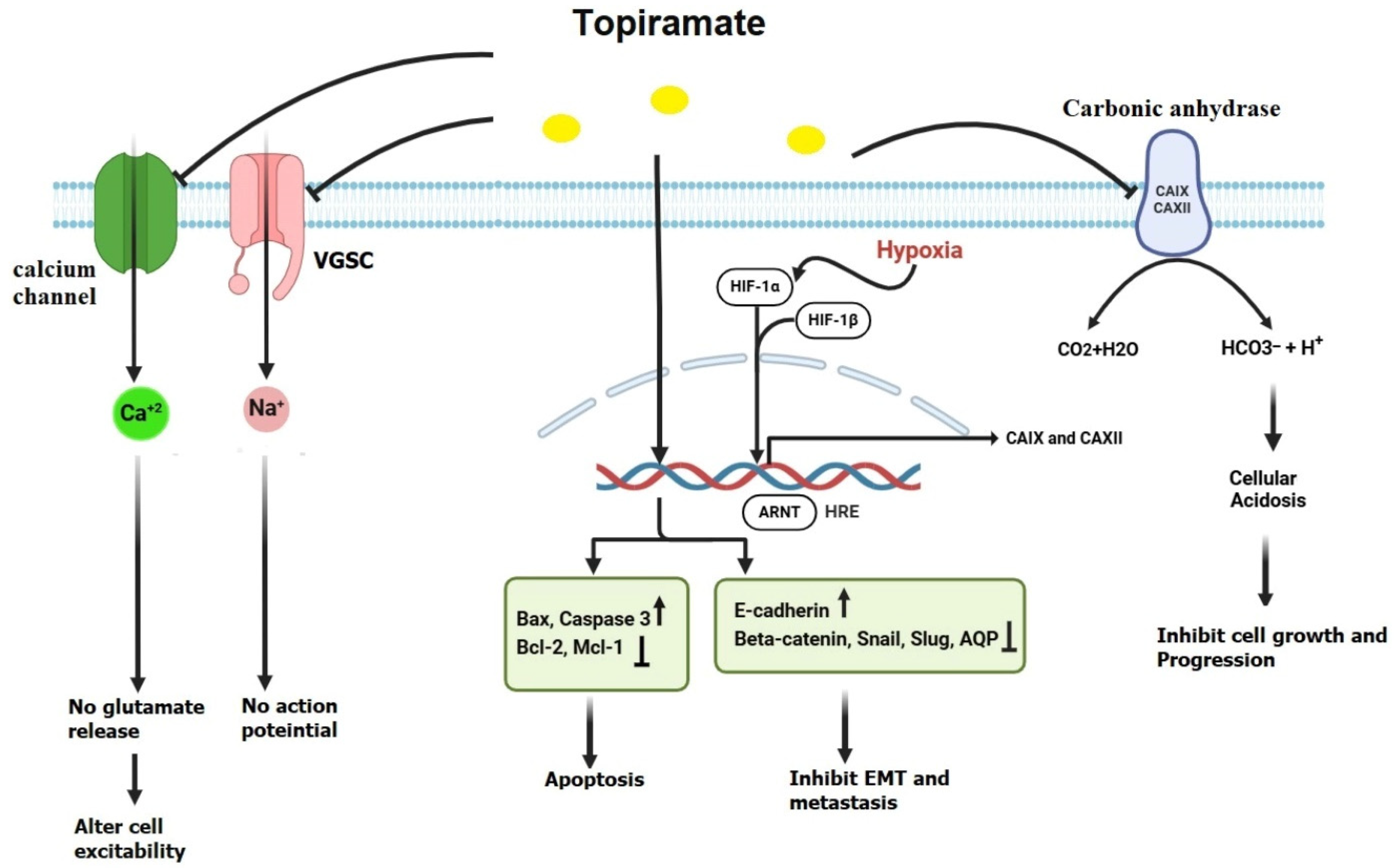
2.2. Topiramate (TPM)
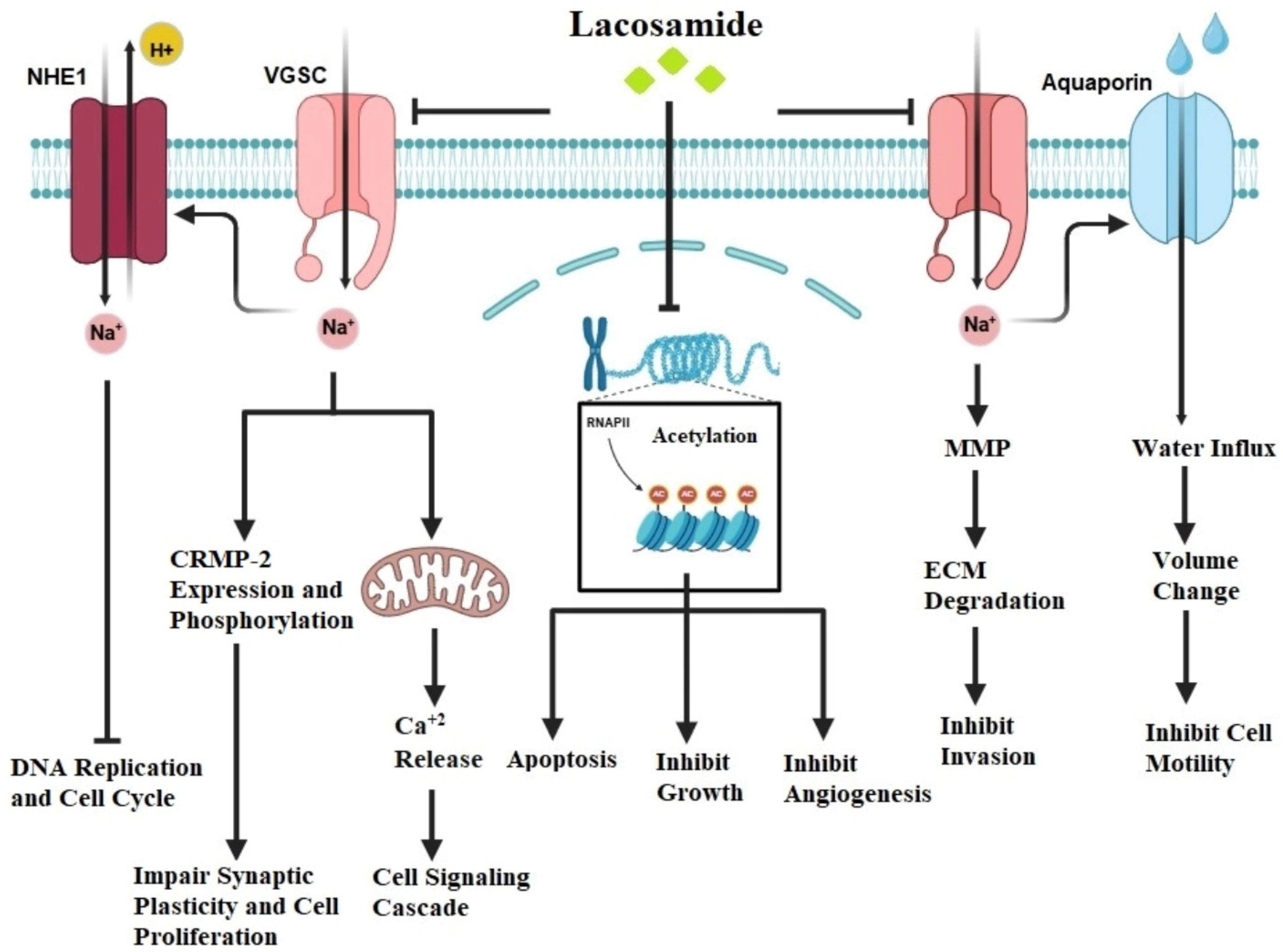
2.3. Lacosamide (LCM)
3. Challenges and Considerations
3.1. Dosage and Pharmacokinetics
3.2. Drug Interactions
4. Clinical Trials and Future Directions
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yahya, E.B.; Alqadhi, A.M. Recent Trends in Cancer Therapy: A Review on the Current State of Gene Delivery. Life Sci. 2021, 269, 119087. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yang, J.; Xu, B.; Zhang, X. Tumor Metastasis: Mechanistic Insights and Therapeutic Interventions. MedComm 2020, 2, 587–617. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Sundaram, C.; Harikumar, K.B.; Tharakan, S.T.; Lai, O.S.; Sung, B.; Aggarwal, B.B. Cancer Is a Preventable Disease That Requires Major Lifestyle Changes. Pharm. Res. 2008, 25, 2097–2116. [Google Scholar] [CrossRef] [PubMed]
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New Approaches and Procedures for Cancer Treatment: Current Perspectives. SAGE Open Med. 2021, 9, 20503121211034366. [Google Scholar] [CrossRef]
- Anwar, S.; Malik, J.A.; Ahmed, S.; Kameshwar, V.A.; Alanazi, J.; Alamri, A.; Ahemad, N. Can Natural Products Targeting EMT Serve as the Future Anticancer Therapeutics? Molecules 2022, 27, 7668. [Google Scholar] [CrossRef]
- Juárez-López, D.; Schcolnik-Cabrera, A. Drug Repurposing: Considerations to Surpass while Re-directing Old Compounds for New Treatments. Arch. Med. Res. 2021, 52, 243–251. [Google Scholar] [CrossRef]
- Aronica, E.; Ciusani, E.; Coppola, A.; Costa, C.; Russo, E.; Salmaggi, A.; Perversi, F.; Maschio, M. Epilepsy and Brain Tumors: Two Sides of the Same Coin. J. Neurol. Sci. 2023, 446, 120584. [Google Scholar] [CrossRef]
- Krajewski, S.; Wójcik, M.; Harat, M.; Furtak, J. Influence of Epilepsy on the Quality of Life of Patients with Brain Tumors. Int. J. Environ. Res. Public Health 2021, 18, 6390. [Google Scholar] [CrossRef]
- Zoccarato, M.; Nardetto, L.; Basile, A.M.; Giometto, B.; Zagonel, V.; Lombardi, G. Seizures, Edema, Thrombosis, and Hemorrhages: An Update Review on the Medical Management of Gliomas. Front. Oncol. 2021, 11, 617966. [Google Scholar] [CrossRef]
- Hoxhaj, P.; Habiya, S.K.; Sayabugari, R.; Balaji, R.; Xavier, R.; Ahmad, A.; Khanam, M.; Kachhadia, M.P.; Patel, T.; Abdin, Z.U.; et al. Investigating the Impact of Epilepsy on Cognitive Function: A Narrative Review. Cureus 2023, 15, e41223. [Google Scholar] [CrossRef]
- Julie, D.A.R.; Ahmed, Z.; Karceski, S.C.; Pannullo, S.C.; Schwartz, T.H.; Parashar, B.; Wernicke, A.G. An Overview of Anti-Epileptic Therapy Management of Patients with Malignant Tumors of the Brain Undergoing Radiation Therapy. Seizure 2019, 70, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; van den Bent, M.; Preusser, M.; Le Rhun, E.; Tonn, J.C.; Minniti, G.; Bendszus, M.; Balana, C.; Chinot, O.; Dirven, L.; et al. EANO Guidelines on the Diagnosis and Treatment of Diffuse Gliomas of Adulthood. Nat. Rev. Clin. Oncol. 2021, 18, 170–186. [Google Scholar] [CrossRef] [PubMed]
- van der Meer, P.B.; Taphoorn, M.J.B.; Koekkoek, J.A.F. Management of Epilepsy in Brain Tumor Patients. Curr. Opin. Oncol. 2022, 34, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Graber, J.J. Overview of Prognostic Factors in Adult Gliomas. Ann. Palliat. Med. 2021, 10, 863–874. [Google Scholar] [CrossRef]
- Monsour, M.A.; Kelly, P.D.; Chambless, L.B. Antiepileptic Drugs in the Management of Cerebral Metastases. Neurosurg. Clin. N. Am. 2020, 31, 589–601. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Salminen, J.K.; Mehtola, A.; Talala, K.; Taari, K.; Mäkinen, J.; Peltola, J.; Tammela, T.L.J.; Auvinen, A.; Murtola, T.J. Anti-epileptic drugs and prostate cancer-specific mortality compared to non-users of anti-epileptic drugs in the Finnish Randomized Study of Screening for Prostate Cancer. Br. J. Cancer 2022, 127, 704–711. [Google Scholar] [CrossRef]
- Cao, D.-F.; Zhou, X.-Y.; Guo, Q.; Xiang, M.-Y.; Bao, M.-H.; He, B.-S.; Mao, X.-Y. Unveiling the role of histone deacetylases in neurological diseases: Focus on epilepsy. Biomark. Res. 2024, 12, 142. [Google Scholar] [CrossRef]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and Cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef]
- Abusara, O.; Hammad, A.M.; Debas, R.; Al-Shalabi, E.; Waleed, M.; Hall, F.S. The Inflammation and Oxidative Status of Rat Lung Tissue Following Smoke/Vapor Exposure via E-Cigarette, Cigarette, and Waterpipe. Gene 2025, 935, 149066. [Google Scholar] [CrossRef]
- Aroosa, M.; Malik, J.A.; Ahmed, S.; Bender, O.; Ahemad, N.; Anwar, S. The Evidence for Repurposing Anti-Epileptic Drugs to Target Cancer. Mol. Biol. Rep. 2023, 50, 7667–7680. [Google Scholar] [CrossRef]
- Abdul-Rahman, T.; Ghosh, S.; Badar, S.M.; Nazir, A.; Bamigbade, G.B.; Aji, N.; Roy, P.; Kachani, H.; Garg, N.; Lawal, L.; et al. The Paradoxical Role of Cytokines and Chemokines at the Tumor Microenvironment: A Comprehensive Review. Eur. J. Med. Res. 2024, 29, 124. [Google Scholar] [CrossRef] [PubMed]
- Sarlo, G.L.; Holton, K.F. Brain Concentrations of Glutamate and GABA in Human Epilepsy: A Review. Seizure 2021, 91, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.S.; Huang, T.H.; Lai, M.C.; Huang, C.W. The Role of Glutamate Receptors in Epilepsy. Biomedicines 2023, 11, 783. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, N.; Karimpour, M.; Bahrami, H.; Zali, M.R.; Chaleshi, V.; Riccio, A.; Nazemalhosseini-Mojarad, E.; Totonchi, M. Current Trends and Future Prospects of Drug Repositioning in Gastrointestinal Oncology. Front. Pharmacol. 2024, 14, 1329244. [Google Scholar] [CrossRef]
- Cruz-Burgos, M.; Losada-Garcia, A.; Cruz-Hernández, C.D.; Cortés-Ramírez, S.A.; Camacho-Arroyo, I.; Gonzalez-Covarrubias, V.; Morales-Pacheco, M.; Trujillo-Bornios, S.I.; Rodríguez-Dorantes, M. New Approaches in Oncology for Repositioning Drugs: The Case of PDE5 Inhibitor Sildenafil. Front. Oncol. 2021, 11, 627229. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Desborough, M.J.R.; Keeling, D.M. The Aspirin Story—From Willow to Wonder Drug. Br. J. Haematol. 2017, 177, 674–683. [Google Scholar] [CrossRef] [PubMed]
- De Lellis, L.; Veschi, S.; Tinari, N.; Mokini, Z.; Carradori, S.; Brocco, D.; Florio, R.; Grassadonia, A.; Cama, A. Drug Repurposing, an Attractive Strategy in Pancreatic Cancer Treatment: Preclinical and Clinical Updates. Cancers 2021, 13, 3946. [Google Scholar] [CrossRef]
- Aggarwal, S.; Verma, S.S.; Aggarwal, S.; Gupta, S.C. Drug Repurposing for Breast Cancer Therapy: Old Weapon for New Battle. Semin. Cancer Biol. 2021, 68, 8–20. [Google Scholar] [CrossRef]
- Doumat, G.; Daher, D.; Zerdan, M.B.; Nasra, N.; Bahmad, H.F.; Recine, M.; Poppiti, R. Drug Repurposing in Non-Small Cell Lung Carcinoma: Old Solutions for New Problems. Curr. Oncol. 2023, 30, 704–719. [Google Scholar] [CrossRef]
- Sidorova, M.; Petrikaitė, V. The Effect of Beta Adrenoreceptor Blockers on Viability and Cell Colony Formation of Non-Small Cell Lung Cancer Cell Lines A549 and H1299. Molecules 2022, 27, 1938. [Google Scholar] [CrossRef]
- Gales, L.; Forsea, L.; Mitrea, D.; Stefanica, I.; Stanculescu, I.; Mitrica, R.; Georgescu, M.; Trifanescu, O.; Anghel, R.; Serbanescu, L. Antidiabetics, Anthelmintics, Statins, and Beta-Blockers as Co-Adjuvant Drugs in Cancer Therapy. Medicina 2022, 58, 1239. [Google Scholar] [CrossRef] [PubMed]
- Montoya, A.; Varela-Ramirez, A.; Dickerson, E.; Pasquier, E.; Torabi, A.; Aguilera, R.; Nahleh, Z.; Bryan, B. The Beta Adrenergic Receptor Antagonist Propranolol Alters Mitogenic and Apoptotic Signaling in Late Stage Breast Cancer. Biomed. J. 2019, 42, 155–165. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, D.; Ma, Q.Y.; Hu, H.-T.; Zhang, M. β2-adrenergic antagonists suppress pancreatic cancer cell invasion by inhibiting CREB, NF-κB and AP-1. Cancer Biol. Ther. 2010, 10, 19–29. [Google Scholar] [CrossRef]
- Ma, T.; Tian, X.; Zhang, B.; Li, M.; Wang, Y.; Yang, C.; Wu, J.; Wei, X.; Qu, Q.; Yu, Y.; et al. Low-Dose Metformin Targets the Lysosomal AMPK Pathway through PEN2. Nature 2022, 603, 159–165. [Google Scholar] [CrossRef]
- Galal, M.A.; Al-Rimawi, M.; Hajeer, A.; Dahman, H.; Alouch, S.; Aljada, A. Metformin: A Dual-Role Player in Cancer Treatment and Prevention. Int. J. Mol. Sci. 2024, 25, 4083. [Google Scholar] [CrossRef]
- Shao, S.; Zhao, L.; An, G.; Zhang, L.; Jing, X.; Luo, M.; Li, W.; Meng, D.; Ning, Q.; Zhao, X.; et al. Metformin Suppresses HIF-1α Expression in Cancer-Associated Fibroblasts to Prevent Tumor-Stromal Cross Talk in Breast Cancer. FASEB J. 2020, 34, 10860–10870. [Google Scholar] [CrossRef] [PubMed]
- Nassif, R.M.; Chalhoub, E.; Chedid, P.; Hurtado-Nedelec, M.; Raya, E.; Dang, P.M.; Marie, J.C.; El-Benna, J. Metformin Inhibits ROS Production by Human M2 Macrophages via the Activation of AMPK. Biomedicines 2022, 10, 319. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huynh, T.Y.L.; Oscilowska, I.; Sáiz, J.; Nizioł, M.; Baszanowska, W.; Barbas, C.; Palka, J. Metformin Treatment or PRODH/POX-Knock out Similarly Induces Apoptosis by Reprograming of Amino Acid Metabolism, TCA, Urea Cycle and Pentose Phosphate Pathway in MCF-7 Breast Cancer Cells. Biomolecules 2021, 11, 1888. [Google Scholar] [CrossRef]
- Bhaw-Luximon, A.; Jhurry, D. Metformin in Pancreatic Cancer Treatment: From Clinical Trials through Basic Research to Biomarker Quantification. J. Cancer Res. Clin. Oncol. 2016, 142, 2159–2171. [Google Scholar] [CrossRef]
- Zamanian, M.Y.; Golmohammadi, M.; Yumashev, A.; Hjazi, A.; Toama, M.A.; AbdRabou, M.A.; Gehlot, A.; Alwaily, E.R.; Shirsalimi, N.; Yadav, P.K.; et al. Effects of Metformin on Cancers in Experimental and Clinical Studies: Focusing on Autophagy and AMPK/mTOR Signaling Pathways. Cell Biochem. Funct. 2024, 42, e4071. [Google Scholar] [CrossRef] [PubMed]
- Sekar, A.P.; Nurmala, S.; Matsuura, E.; Tan, X.W.; Rahmasari, R.; Sauriasari, R. Estrogen Receptor Is Required for Metformin-Induced Apoptosis in Breast Cancer Cells Under Hyperglycemic Conditions. Breast Cancer Basic Clin. Res. 2024, 18, 11782234241240173. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shao, X.; Li, C.; Liang, J.; Changzhong, L. Metformin Enhances Epithelial Cell Growth Inhibition via the Protein Kinase-Insulin-Like Growth Factor Binding Protein-1 Pathway. J. Obstet. Gynaecol. 2024, 44, 2321651. [Google Scholar] [CrossRef] [PubMed]
- Turanli, B.; Grøtli, M.; Boren, J.; Nielsen, J.; Uhlen, M.; Arga, K.Y.; Mardinoglu, A. Drug Repositioning for Effective Prostate Cancer Treatment. Front. Physiol. 2018, 9, 500. [Google Scholar] [CrossRef]
- Ferreira, L.G.; Dos Santos, R.N.; Oliva, G.; Andricopulo, A.D. Molecular Docking and Structure-Based Drug Design Strategies. Molecules 2015, 20, 13384–13421. [Google Scholar] [CrossRef]
- Vitiello, L.; Tibaudo, L.; Pegoraro, E.; Bello, L.; Canton, M. Teaching an Old Molecule New Tricks: Drug Repositioning for Duchenne Muscular Dystrophy. Int. J. Mol. Sci. 2019, 20, 6053. [Google Scholar] [CrossRef]
- Pushpakom, S.; Ianev, V.; Santos, R. Drug repurposing: Exploring the unexplored. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef]
- Ekeomodi, C.C.; Obetta, K.I.; Okolocha, M.L.; Nnacho, S.; Isijola, M.O.; Ejiofor, I.I.C. Computational Approaches in Drug Repurposing. In Drug Repurposing—Advances, Scopes and Opportunities in Drug Discovery: Computational Approaches in Drug Repurposing; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Sidhu, H.S.; Sadhotra, A. Current Status of the New Antiepileptic Drugs in Chronic Pain. Front. Pharmacol. 2016, 7, 276. [Google Scholar] [CrossRef]
- Zhang, Y.; Jing, Y.; Pan, R.; Ding, K.; Chen, R.; Meng, Q. Mechanisms of Cancer Inhibition by Local Anesthetics. Front. Pharmacol. 2021, 12, 770694. [Google Scholar] [CrossRef]
- Garmon, E.H.; Huecker, M.R. Topical, Local, and Regional Anesthesia and Anesthetics. [Updated 2023 Aug 28]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK430894/ (accessed on 6 February 2025).
- Agbo, J.; Ibrahim, Z.G.; Magaji, S.Y.; Mutalub, Y.B.; Mshelia, P.P.; Mhyha, D.H. Therapeutic Efficacy of Voltage-Gated Sodium Channel Inhibitors in Epilepsy. Acta Epileptol. 2023, 5, 16. [Google Scholar] [CrossRef]
- Rusciano, D. Molecular Mechanisms and Therapeutic Potential of Gabapentin with a Focus on Topical Formulations to Treat Ocular Surface Diseases. Pharmaceuticals 2024, 17, 623. [Google Scholar] [CrossRef]
- Chang, Y.C.; Rapoport, S.I.; Rao, J.S. Chronic Administration of Mood Stabilizers Upregulates BDNF and Bcl-2 Expression Levels in Rat Frontal Cortex. Neurochem. Res. 2009, 34, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Safdar, A.; Ismail, F. A Comprehensive Review on Pharmacological Applications and Drug-Induced Toxicity of Valproic Acid. Saudi Pharm. J. 2023, 31, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Romoli, M.; Mazzocchetti, P.; D’Alonzo, R.; Siliquini, S.; Rinaldi, V.E.; Verrotti, A.; Calabresi, P.; Costa, C. Valproic Acid and Epilepsy: From Molecular Mechanisms to Clinical Evidences. Curr. Neuropharmacol. 2019, 17, 926–946. [Google Scholar] [CrossRef] [PubMed]
- Perković Vukčević, N.; Mijatović Jovin, V.; Vuković Ercegović, G.; Antunović, M.; Kelečević, I.; Živanović, D.; Vučinić, S. Carbapenems as Antidotes for the Management of Acute Valproic Acid Poisoning. Pharmaceuticals 2024, 17, 257. [Google Scholar] [CrossRef]
- Sanaei, M.; Kavoosi, F.; Atashpour, S. Effect of Valproic Acid on Proliferation and Apoptosis of Colon Cancer HT29 Cell Line. Glob. J. Med. Res. Stud. 2016, 3, 21–26. [Google Scholar]
- Sanaei, M.; Kavoosi, F.; Roustazadeh, A.; Shahsavani, H. In vitro Effect of the Histone Deacetylase Inhibitor Valproic Acid on Viability and Apoptosis of the PLC/PRF5 Human Hepatocellular Carcinoma Cell Line. Asian Pac. J. Cancer Prev. 2018, 19, 2507–2510. [Google Scholar] [CrossRef]
- Wawruszak, A.; Halasa, M.; Okon, E.; Kukula-Koch, W.; Stepulak, A. Valproic Acid and Breast Cancer: State of the Art in 2021. Cancers 2021, 13, 3409. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, G.; Wang, L.; Song, C.; Leng, Y.; Wang, X.; Kang, J. VPA Inhibits Breast Cancer Cell Migration by Specifically Targeting HDAC2 and Down-Regulating Survivin. Mol. Cell. Biochem. 2012, 361, 39–45. [Google Scholar] [CrossRef]
- Sheng, L.; Wan, B.; Feng, P.; Sun, J.; Rigo, F.; Bennett, C.F.; Akerman, M.; Krainer, A.R.; Hua, Y. Downregulation of Survivin Contributes to Cell-Cycle Arrest During Postnatal Cardiac Development in a Severe Spinal Muscular Atrophy Mouse Model. Hum. Mol. Genet. 2018, 27, 486–498. [Google Scholar] [CrossRef]
- Tarawneh, N.; Hamadneh, L.; Abu-Irmaileh, B.; Shraideh, Z.; Bustanji, Y.; Abdalla, S. Berberine Inhibited Growth and Migration of Human Colon Cancer Cell Lines by Increasing Phosphatase and Tensin and Inhibiting Aquaporins 1, 3 and 5 Expressions. Molecules 2023, 28, 3823. [Google Scholar] [CrossRef]
- Tarawneh, N.; Hamadneh, L.; Alshaer, W.; Al Bawab, A.Q.; Bustanji, Y.; Abdalla, S. Downregulation of Aquaporins and PI3K/AKT and Upregulation of PTEN Expression Induced by the Flavone Scutellarein in Human Colon Cancer Cell Lines. Heliyon 2024, 10, e39402. [Google Scholar] [CrossRef] [PubMed]
- Warrier, N.M.; Agarwal, P.; Kumar, P. Emerging Importance of Survivin in Stem Cells and Cancer: The Development of New Cancer Therapeutics. Stem Cell Rev. Rep. 2020, 16, 828–852. [Google Scholar] [CrossRef] [PubMed]
- Terada, Y. Role of Chromosomal Passenger Complex in Chromosome Segregation and Cytokinesis. Cell Struct. Funct. 2001, 26, 653–657. [Google Scholar] [CrossRef]
- Jan, R.; Chaudhry, G.E. Understanding Apoptosis and Apoptotic Pathways Targeted Cancer Therapeutics. Adv. Pharm. Bull. 2019, 9, 205–218. [Google Scholar] [CrossRef]
- Huo, Z.; Lomora, M.; Kym, U.; Palivan, C.; Holland-Cunz, S.G.; Gros, S.J. AQP1 Is Up-Regulated by Hypoxia and Leads to Increased Cell Water Permeability, Motility, and Migration in Neuroblastoma. Front. Cell Dev. Biol. 2021, 9, 605272. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Chang, M.; Mu, R.; Zhu, J. Effect of RNAi-Mediated Survivin and Hypoxia-Inducible Factor 1α Gene Silencing on Proliferation, Invasion, Migration and Apoptosis of Gastric Cancer BGC-823 Cells. Mol. Biotechnol. 2024, 66, 1872–1882. [Google Scholar] [CrossRef]
- Chen, X.; Duan, N.; Zhang, C.; Zhang, W. Survivin and Tumorigenesis: Molecular Mechanisms and Therapeutic Strategies. J. Cancer 2016, 7, 314–323. [Google Scholar] [CrossRef]
- Mao, S.; Lu, G.; Lan, X.; Yuan, C.; Jiang, W.; Chen, Y.; Jin, X.; Xia, Q. Valproic Acid Inhibits Epithelial Mesenchymal Transition in Renal Cell Carcinoma by Decreasing SMAD4 Expression. Mol. Med. Rep. 2017, 16, 6190–6199. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Wang, X.H. Valproic Acid Inhibits Glioma and Its Mechanisms. J. Healthc. Eng. 2022, 2022, 4985781. [Google Scholar] [CrossRef]
- Jahani, M.; Khanahmad, H.; Nikpour, P. Evaluation of the Effects of Valproic Acid Treatment on Cell Survival and Epithelial-Mesenchymal Transition-Related Features of Human Gastric Cancer Cells. J. Gastrointest. Cancer 2021, 52, 676–681. [Google Scholar] [CrossRef]
- Niknami, Z.; Muhammadnejad, A.; Ebrahimi, A.; Harsani, Z.; Shirkoohi, R. Significance of E-cadherin and Vimentin as Epithelial-Mesenchymal Transition Markers in Colorectal Carcinoma Prognosis. EXCLI J. 2020, 19, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Al-Khafaji, A.S.K.; Wang, L.M.; Alabdei, H.H.; Liloglou, T. Effect of Valproic Acid on Histone Deacetylase Expression in Oral Cancer (Review). Oncol. Lett. 2024, 27, 197. [Google Scholar] [CrossRef] [PubMed]
- Zeinali, E.; Bagheri, V.; Rostami, E.; Ramezani, M.; Anani Sarab, G. Inhibition of CIP2A/C-MYC/PI3K/Akt/mTOR Signaling Molecules and PD-L1 by Valproic Acid in Breast Cancer Cells. Res. Sq. 2023; preprint. [Google Scholar] [CrossRef]
- Riva, G.; Cilibrasi, C.; Bazzoni, R.; Cadamuro, M.; Negroni, C.; Butta, V.; Strazzabosco, M.; Dalprà, L.; Lavitrano, M.; Bentivegna, A. Valproic Acid Inhibits Proliferation and Reduces Invasiveness in Glioma Stem Cells Through Wnt/β Catenin Signalling Activation. Genes 2018, 9, 522. [Google Scholar] [CrossRef]
- Ozman, Z.; Ozbek Iptec, B.; Sahin, E.; Eskiler, G.G.; Ozkan, A.D.; Kaleli, S. Regulation of Valproic Acid Induced EMT by AKT/GSK3β/β-Catenin Signaling Pathway in Triple Negative Breast Cancer. Mol. Biol. Rep. 2021, 48, 1335–1343. [Google Scholar] [CrossRef]
- Tsai, H.C.; Wei, K.C.; Chen, P.Y.; Huang, C.Y.; Chen, K.T.; Lin, Y.J.; Cheng, H.W.; Chen, Y.R.; Wang, H.T. Valproic Acid Enhanced Temozolomide-Induced Anticancer Activity in Human Glioma Through the p53-PUMA Apoptosis Pathway. Front. Oncol. 2021, 11, 722754. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Z.; Gao, C.; Wu, S.; Duan, Q.; Wu, H.; Wang, C.; Shen, Q.; Yin, T. Combination Chemotherapy of Valproic Acid (VPA) and Gemcitabine Regulates STAT3/Bmi1 Pathway to Differentially Potentiate the Motility of Pancreatic Cancer Cells. Cell Biosci. 2019, 9, 50. [Google Scholar] [CrossRef]
- Malla, R.; Viswanathan, S.; Makena, S.; Kapoor, S.; Verma, D.; Raju, A.A.; Dunna, M.; Muniraj, N. Revitalizing Cancer Treatment: Exploring the Role of Drug Repurposing. Cancers 2024, 16, 1463. [Google Scholar] [CrossRef]
- Granit Mizrahi, A.; Gugenheim, A.; Hamad, H.; Hamed, R.; Tetro, N.; Maimon, O.; Khutsurauli, S.; Nechushtan, H.; Nisman, B.; Duran, D.; et al. Valproic Acid Reprograms the Metabolic Aberration of Cisplatin Treatment via ALDH Modulation in Triple-Negative Breast Cancer Cells. Front. Cell Dev. Biol. 2023, 11, 1217149. [Google Scholar] [CrossRef]
- Iannelli, F.; Lombardi, R.; Costantini, S.; Roca, M.S.; Addi, L.; Bruzzese, F.; Di Gennaro, E.; Budillon, A.; Pucci, B. Integrated Proteomics and Metabolomics Analysis Reveals New Insight into the Synergistic Antitumor Effect of Valproic Acid Plus Simvastatin in Prostate Cancer Xenograft Model Associated with Downmodulation of YAP/TAZ Signaling. Res. Sq. 2023; preprint. [Google Scholar] [CrossRef]
- Diniz, M.S., Jr.; Freire, P.T.C.; Filho, J.M.; Melo, F.E.A.; Pontes, F.M.; Longo, E.; Ferreira, O.P.; Alves, O.L. Vibrational and Thermal Properties of Crystalline Topiramate. J. Braz. Chem. Soc. 2008, 19, 1607–1613. [Google Scholar] [CrossRef]
- Tippayachai, P.; Leelakanok, N.; Methaneethorn, J. Significant Predictors for Topiramate Pharmacokinetics: A Systematic Review of Population Pharmacokinetic Studies. J. Pharm. Pract. Res. 2022, 52, 94–107. [Google Scholar] [CrossRef]
- Lee, S.; Kim, H.C.; Jang, Y.; Lee, H.S.; Ahn, S.J.; Lee, S.T.; Jung, K.H.; Park, K.I.; Jung, K.Y.; Oh, J.; et al. Topiramate Dosage Optimization for Effective Antiseizure Management via Population Pharmacokinetic Modeling. Ann. Clin. Transl. Neurol. 2024, 11, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.F.; Zeng, C.; Jia, M.; Xiao, B. Molecular Mechanisms of Topiramate and Its Clinical Value in Epilepsy. Seizure 2022, 98, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Fang, Z.; Clark, L.H.; Sun, W.; Yin, Y.; Zhang, R.; Sullivan, S.A.; Tran, A.Q.; Kong, W.; Wang, J.; et al. Topiramate Exhibits Anti-Tumorigenic and Metastatic Effects in Ovarian Cancer Cells. Am. J. Transl. Res. 2018, 10, 1663–1676. [Google Scholar] [PubMed] [PubMed Central]
- Pastorekova, S.; Gillies, R.J. The Role of Carbonic Anhydrase IX in Cancer Development: Links to Hypoxia, Acidosis, and Beyond. Cancer Metastasis Rev. 2019, 38, 65–77. [Google Scholar] [CrossRef]
- Obeidat, N.M.; Zihlif, M.A.; Alqudah, D.A.; Alshaer, W.; Alqaraleh, M.; Sharab, A.; Abdalla, S.S. Effects of Cyclic Acute and Chronic Hypoxia on the Expression Levels of Metabolism-Related Genes in a Pancreatic Cancer Cell Line. Biomed. Rep. 2022, 17, 81. [Google Scholar] [CrossRef]
- Lee, S.C.S.; Pyo, A.H.A.; Koritzinsky, M. Longitudinal Dynamics of the Tumor Hypoxia Response: From Enzyme Activity to Biological Phenotype. Sci. Adv. 2023, 9, eadj6409. [Google Scholar] [CrossRef]
- Daunys, S.; Petrikaitė, V. The Roles of Carbonic Anhydrases IX and XII in Cancer Cell Adhesion, Migration, Invasion and Metastasis. Biol. Cell 2020, 112, 383–397. [Google Scholar] [CrossRef]
- Daverio, Z.; Balcerczyk, A.; Rautureau, G.J.P.; Panthu, B. How Warburg-Associated Lactic Acidosis Rewires Cancer Cell Energy Metabolism to Resist Glucose Deprivation. Cancers 2023, 15, 1417. [Google Scholar] [CrossRef]
- Ward, C.; Meehan, J.; Gray, M.E.; Murray, A.F.; Argyle, D.J.; Kunkler, I.H.; Langdon, S.P. The Impact of Tumour pH on Cancer Progression: Strategies for Clinical Intervention. Explor. Target. Anti-Tumor Ther. 2020, 1, 71–100. [Google Scholar] [CrossRef] [PubMed]
- Dodgson, S.J.; Shank, R.P.; Maryanoff, B.E. Topiramate as an Inhibitor of Carbonic Anhydrase Isoenzymes. Epilepsia 2000, 41, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Xiang, Y.; Li, T.; Yu, H.M.; Li, X.J. Inhibitory Effect of Topiramate on Lewis Lung Carcinoma Metastasis and Its Relation with AQP1 Water Channel. Acta Pharmacol. Sin. 2004, 25, 54–60. [Google Scholar] [PubMed]
- Marathe, K.; McVicar, N.; Li, A.; Bellyou, M.; Meakin, S.; Bartha, R. Topiramate Induces Acute Intracellular Acidification in Glioblastoma. J. Neurooncol. 2016, 130, 465–472. [Google Scholar] [CrossRef]
- Chao, L.; Zhang, S.; Zhang, J.; Cai, L.; Wang, X.; Meng, F.; Cai, W. Topiramate Inhibits the Proliferation of Bladder Cancer Cells via the PI3K/AKT/mTOR Signaling Pathway. Trop. J. Pharm. Res. 2022, 21, 1–10. [Google Scholar] [CrossRef]
- Contreras-Zárate, M.J.; Alvarez-Eraso, K.L.; Jaramillo-Gómez, J.A.; Littrell, Z.; Tsuji, N.; Ormond, D.R.; Karam, S.D.; Kabos, P.; Cittelly, D.M. Short-Term Topiramate Treatment Prevents Radiation-Induced Cytotoxic Edema in Preclinical Models of Breast-Cancer Brain Metastasis. bioRxiv, 2023; preprint. [Google Scholar] [CrossRef]
- Bauer, S.; Willems, L.M.; Paule, E.; Petschow, C.; Zöllner, J.P.; Rosenow, F.; Strzelczyk, A. The Efficacy of Lacosamide as Monotherapy and Adjunctive Therapy in Focal Epilepsy and Its Use in Status Epilepticus: Clinical Trial Evidence and Experience. Ther. Adv. Neurol. Disord. 2017, 10, 103–126. [Google Scholar] [CrossRef]
- Yang, C.; Peng, Y.; Zhang, L.; Zhao, L. Safety and Tolerability of Lacosamide in Patients with Epilepsy: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2021, 12, 694381. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Newton, H.B.; Wojkowski, J. Antiepileptic Strategies for Patients with Primary and Metastatic Brain Tumors. Curr. Treat. Options Oncol. 2024, 25, 389–403. [Google Scholar] [CrossRef]
- Niespodziany, I.; Leclère, N.; Vandenplas, C.; Foerch, P.; Wolff, C. Comparative Study of Lacosamide and Classical Sodium Channel Blocking Antiepileptic Drugs on Sodium Channel Slow Inactivation. J. Neurosci. Res. 2013, 91, 436–443. [Google Scholar] [CrossRef]
- Liu, A.; Gu, Q.; Wang, M. Effects of Levetiracetam and Lacosamide on Therapeutic Efficacy and Neural Function in Patients with Epilepsy. Exp. Ther. Med. 2020, 20, 3687–3694. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.M.; Khanna, R. Specific Binding of Lacosamide to Collapsin Response Mediator Protein 2 (CRMP2) and Direct Impairment of Its Canonical Function: Implications for the Therapeutic Potential of Lacosamide. Mol. Neurobiol. 2015, 51, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Moutal, A.; Dustrude, E.T.; Largent-Milnes, T.M.; Vanderah, T.W.; Khanna, M.; Khanna, R. Blocking CRMP2 SUMOylation reverses neuropathic pain. Mol. Psychiatry 2018, 23, 2119–2121. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Toledo, M.; Molins, A.; Quintana, M.; Santamarina, E.; Martinez-Ricarte, F.; Martínez-Saez, E.; Salas-Puig, J. Outcome of Cancer-Related Seizures in Patients Treated with Lacosamide. Acta Neurol. Scand. 2018, 137, 67–75. [Google Scholar] [CrossRef]
- Angus, M.; Ruben, P. Voltage gated sodium channels in cancer and their potential mechanisms of action. Channels 2019, 13, 400–409. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roger, S.; Gillet, L.; Le Guennec, J.Y.; Besson, P. Voltage-Gated Sodium Channels and Cancer: Is Excitability Their Primary Role? Front. Pharmacol. 2015, 6, 152. [Google Scholar] [CrossRef]
- Malcolm, J.R.; Sajjaboontawee, N.; Yerlikaya, S.; Plunkett-Jones, C.; Boxall, P.J.; Brackenbury, W.J. Voltage-Gated Sodium Channels, Sodium Transport and Progression of Solid Tumours. Curr. Top. Membr. 2023, 92, 71–98. [Google Scholar] [CrossRef]
- Liu, H.; Weng, J.; Huang, C.L.; Jackson, A.P. Voltage-Gated Sodium Channels in Cancers. Biomark. Res. 2024, 12, 70. [Google Scholar] [CrossRef]
- Giammello, F.; Biella, C.; Priori, E.C.; Filippo, M.A.D.S.; Leone, R.; D’Ambrosio, F.; Paterno’, M.; Cassioli, G.; Minetti, A.; Macchi, F.; et al. Modulating Voltage-Gated Sodium Channels to Enhance Differentiation and Sensitize Glioblastoma Cells to Chemotherapy. Cell Commun. Signal. 2024, 22, 434. [Google Scholar] [CrossRef]
- Morishita, K.; Watanabe, K.; Ichijo, H. Cell Volume Regulation in Cancer Cell Migration Driven by Osmotic Water Flow. Cancer Sci. 2019, 110, 2337–2347. [Google Scholar] [CrossRef]
- Sanchez-Sandoval, A.L.; Hernández-Plata, E.; Gomora, J.C. Voltage-Gated Sodium Channels: From Roles and Mechanisms in the Metastatic Cell Behavior to Clinical Potential as Therapeutic Targets. Front. Pharmacol. 2023, 14, 1206136. [Google Scholar] [CrossRef] [PubMed]
- Granit, A.; Tetro, N.; Shmuel, M.; Peretz, T.; Eyal, S. Lacosamide at Therapeutic Concentrations Induces Histone Hyperacetylation In vitro. Epilepsia Open 2018, 3, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Karisetty, B.C.; Duscharla, D.; Vijay, V.; Patel, S.; Soren, K.; Kumar, A.; Ummanni, R.; Chakravarty, S. Proteome Profile of Nucleus Accumbens (NAc) Uncovers the Differential and Sex-Specific Role of CRMP2 in CVMS-Induced Mouse Model of Depression. J. Proteins Proteom. 2024, 15, 561–576. [Google Scholar] [CrossRef]
- Lin, B.; Li, Y.; Wang, T.; Qiu, Y.; Chen, Z.; Zhao, K.; Lu, N. CRMP2 is a Therapeutic Target That Suppresses the Aggressiveness of Breast Cancer Cells by Stabilizing RECK. Oncogene 2020, 39, 6024–6040. [Google Scholar] [CrossRef] [PubMed]
- Morales, X.; Peláez, R.; Garasa, S.; Ortiz de Solórzano, C.; Rouzaut, A. CRMP2 as a Candidate Target to Interfere with Lung Cancer Cell Migration. Biomolecules 2021, 11, 1533. [Google Scholar] [CrossRef]
- Jin, Y.; Bian, S.; Wang, H.; Mo, J.; Fei, H.; Li, L.; Chen, T.; Jiang, H. CRMP2 Derived from Cancer Associated Fibroblasts Facilitates Progression of Ovarian Cancer via HIF-1α-Glycolysis Signaling Pathway. Cell Death Dis. 2022, 13, 675. [Google Scholar] [CrossRef]
- Rizzo, A.; Donzelli, S.; Girgenti, V.; Sacconi, A.; Vasco, C.; Salmaggi, A.; Blandino, G.; Maschio, M.; Ciusani, E. In Vitro Antineoplastic Effects of Brivaracetam and Lacosamide on Human Glioma Cells. J. Exp. Clin. Cancer Res. 2017, 36, 76. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yagi, C.; Tatsuoka, J.; Sano, E.; Hanashima, Y.; Ozawa, Y.; Yoshimura, S.; Yamamuro, S.; Sumi, K.; Hara, H.; Katayama, Y.; et al. Anti-Tumor Effects of Anti-Epileptic Drugs in Malignant Glioma Cells. Oncol. Rep. 2022, 48, 216. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Patsalos, P.N.; Spencer, E.P.; Berry, D.J. Therapeutic Drug Monitoring of Antiepileptic Drugs in Epilepsy: A 2018 Update. Ther. Drug Monit. 2018, 40, 526–548. [Google Scholar] [CrossRef]
- Methaneethorn, J. A systematic review of population pharmacokinetics of valproic acid. Br. J. Clin. Pharmacol. 2018, 84, 816–834. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fishman, J.; Kalilani, L.; Song, Y.; Swallow, E.; Wild, I. Antiepileptic Drug Titration and Related Health Care Resource Use and Costs. J. Manag. Care Spec. Pharm. 2018, 24, 929–938. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Charlier, B.; Coglianese, A.; De Rosa, F.; de Grazia, U.; Operto, F.F.; Coppola, G.; Filippelli, A.; Dal Piaz, F.; Izzo, V. The Effect of Plasma Protein Binding on the Therapeutic Monitoring of Antiseizure Medications. Pharmaceutics 2021, 13, 1208. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nazeri, A.; Jalai, M.; Aliasgharpour, M.; Khosravie, F. Comparison of Serum Valproic Acid Determination Through Gas and High Performance Liquid Chromatography Methods. Health Scope 2014, 3, e12085. [Google Scholar] [CrossRef]
- Shank, R.P.; Maryanoff, B.E. Molecular pharmacodynamics, clinical therapeutics, and pharmacokinetics of topiramate. CNS Neurosci. Ther. 2008, 14, 120–142. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beydoun, A.; D’Souza, J.; Hebert, D.; Doty, P. Lacosamide: Pharmacology, mechanisms of action and pooled efficacy and safety data in partial-onset seizures. Expert Rev. Neurother. 2009, 9, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Moseley, B.D.; Chanteux, H.; Nicolas, J.M.; Laloyaux, C.; Gidal, B.; Stockis, A. A review of the drug-drug interactions of the antiepileptic drug brivaracetam. Epilepsy Res. 2020, 163, 106327. [Google Scholar] [CrossRef] [PubMed]
- Johannessen Landmark, C. Antiepileptic Drugs in Non-Epilepsy Disorders: Relations between Mechanisms of Action and Clinical Efficacy. CNS Drugs 2008, 24, 37–47. [Google Scholar] [CrossRef]
- Bénit, C.P.; Vecht, C.J. Seizures and Cancer: Drug Interactions of Anticonvulsants with Chemotherapeutic Agents, Tyrosine Kinase Inhibitors and Glucocorticoids. Neurooncol. Pract. 2016, 3, 245–260. [Google Scholar] [CrossRef]
- Saha, S.K.; Yin, Y.; Kim, K.; Yang, G.M.; Dayem, A.A.; Choi, H.Y.; Cho, S.G. Valproic Acid Induces Endocytosis-Mediated Doxorubicin Internalization and Shows Synergistic Cytotoxic Effects in Hepatocellular Carcinoma Cells. Int. J. Mol. Sci. 2017, 18, 1048. [Google Scholar] [CrossRef]
- Iannelli, F.; Zotti, A.I.; Roca, M.S.; Grumetti, L.; Lombardi, R.; Moccia, T.; Vitagliano, C.; Milone, M.R.; Ciardiello, C.; Bruzzese, F.; et al. Valproic Acid Synergizes with Cisplatin and Cetuximab in vitro and in vivo in Head and Neck Cancer by Targeting the Mechanisms of Resistance. Front. Cell Dev. Biol. 2020, 8, 732. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, X.; Wang, Y.; Chen, Y.; Lu, H.; Meng, X.; Ye, X.; Chen, W. Activation/Inactivation of Anticancer Drugs by CYP3A4: Influencing Factors for Personalized Cancer Therapy. Drug Metab. Dispos. 2023, 51, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, M.; Wang, X. The Adverse-Effect Profile of Lacosamide. Expert Opin. Drug Saf. 2020, 19, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Was, H.; Borkowska, A.; Bagues, A.; Tu, L.; Liu, J.Y.H.; Lu, Z.; Rudd, J.A.; Nurgali, K.; Abalo, R. Mechanisms of Chemotherapy-Induced Neurotoxicity. Front. Pharmacol. 2022, 13, 750507. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zamiri, R.E.; Charsouei, S. Investigating the Relationship Between Seizures, Cancer, and Chemotherapy Effects: Risk Factors, Underlying Mechanisms, and Therapeutic Approaches. Eurasian J. Chem. Med. Pet. Res. 2024, 3, 1299–1312. [Google Scholar]
- Michaelis, M.; Michaelis, U.R.; Fleming, I.; Suhan, T.; Cinatl, J.; Blaheta, R.A.; Hoffmann, K.; Kotchetkov, R.; Busse, R.; Nau, H.; et al. Valproic Acid Inhibits Angiogenesis In vitro and In vivo. Mol. Pharmacol. 2004, 65, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Simões, R.B.; Simões, M.L.P.B.; Ioshii, S.O.; Robes, R.R.; Dall’antonia, M.O.; Goehr, M.P.; Neves, P.J.F. Effects of Valproic Acid on Wound Healing of the Abdominal Wall Musculoaponeurotic Layer: An Experimental Study in Rats. Rev. Col. Bras. Cir. 2024, 51, e20243676. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarawneh, N.; Hussein, S.A.; Abdalla, S. Repurposing Antiepileptic Drugs for Cancer: A Promising Therapeutic Strategy. J. Clin. Med. 2025, 14, 2673. https://doi.org/10.3390/jcm14082673
Tarawneh N, Hussein SA, Abdalla S. Repurposing Antiepileptic Drugs for Cancer: A Promising Therapeutic Strategy. Journal of Clinical Medicine. 2025; 14(8):2673. https://doi.org/10.3390/jcm14082673
Chicago/Turabian StyleTarawneh, Noor, Shaymaa A. Hussein, and Shtaywy Abdalla. 2025. "Repurposing Antiepileptic Drugs for Cancer: A Promising Therapeutic Strategy" Journal of Clinical Medicine 14, no. 8: 2673. https://doi.org/10.3390/jcm14082673
APA StyleTarawneh, N., Hussein, S. A., & Abdalla, S. (2025). Repurposing Antiepileptic Drugs for Cancer: A Promising Therapeutic Strategy. Journal of Clinical Medicine, 14(8), 2673. https://doi.org/10.3390/jcm14082673






