Echocardiography-Based Pulmonary Artery Pulsatility Index Correlates with Outcomes in Patients with Acute Pulmonary Embolism
Abstract
1. Introduction
2. Methods
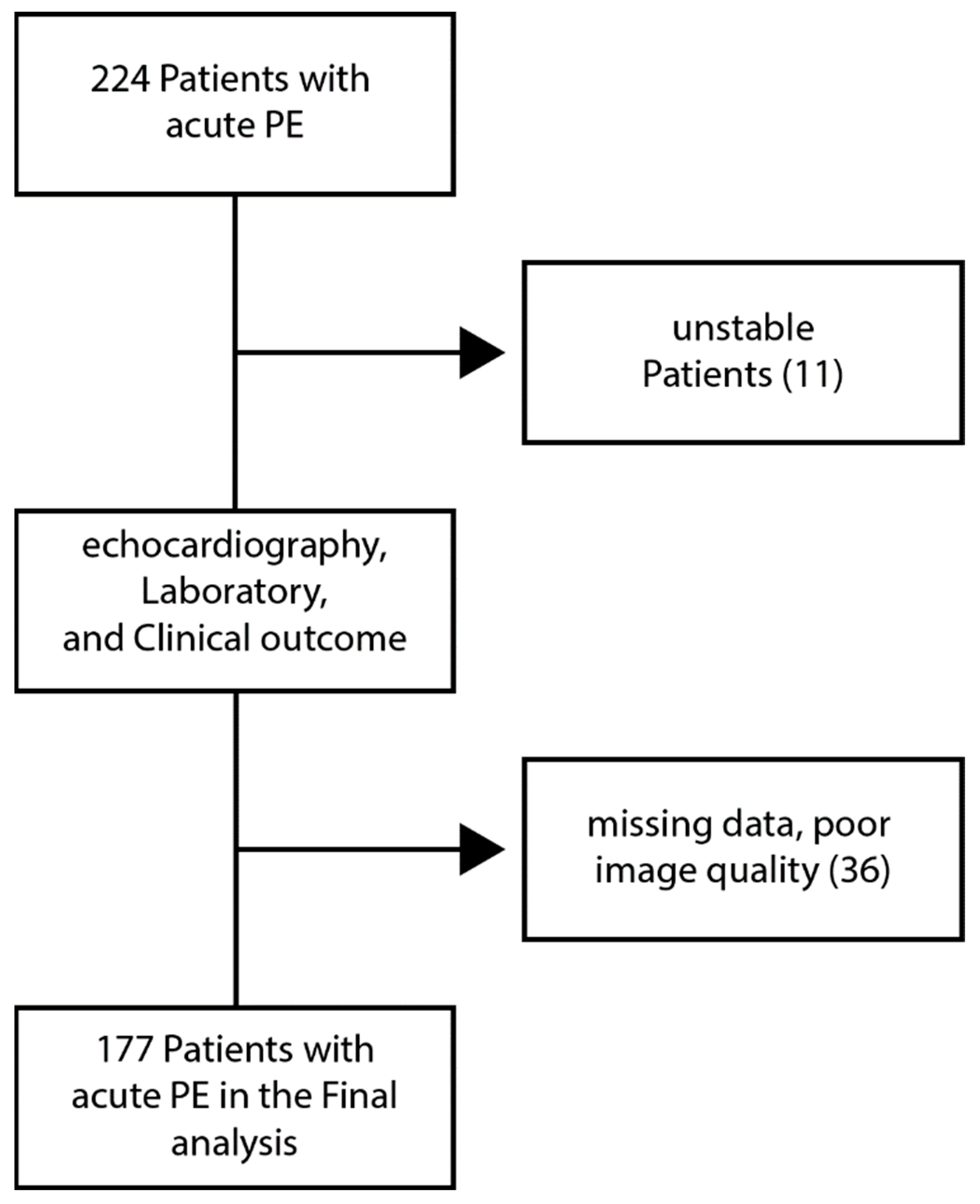
2.1. Study Population
2.2. Echocardiographic Measurements
2.3. Laboratory Data
2.4. Outcomes
2.5. Statistical Analysis
3. Results
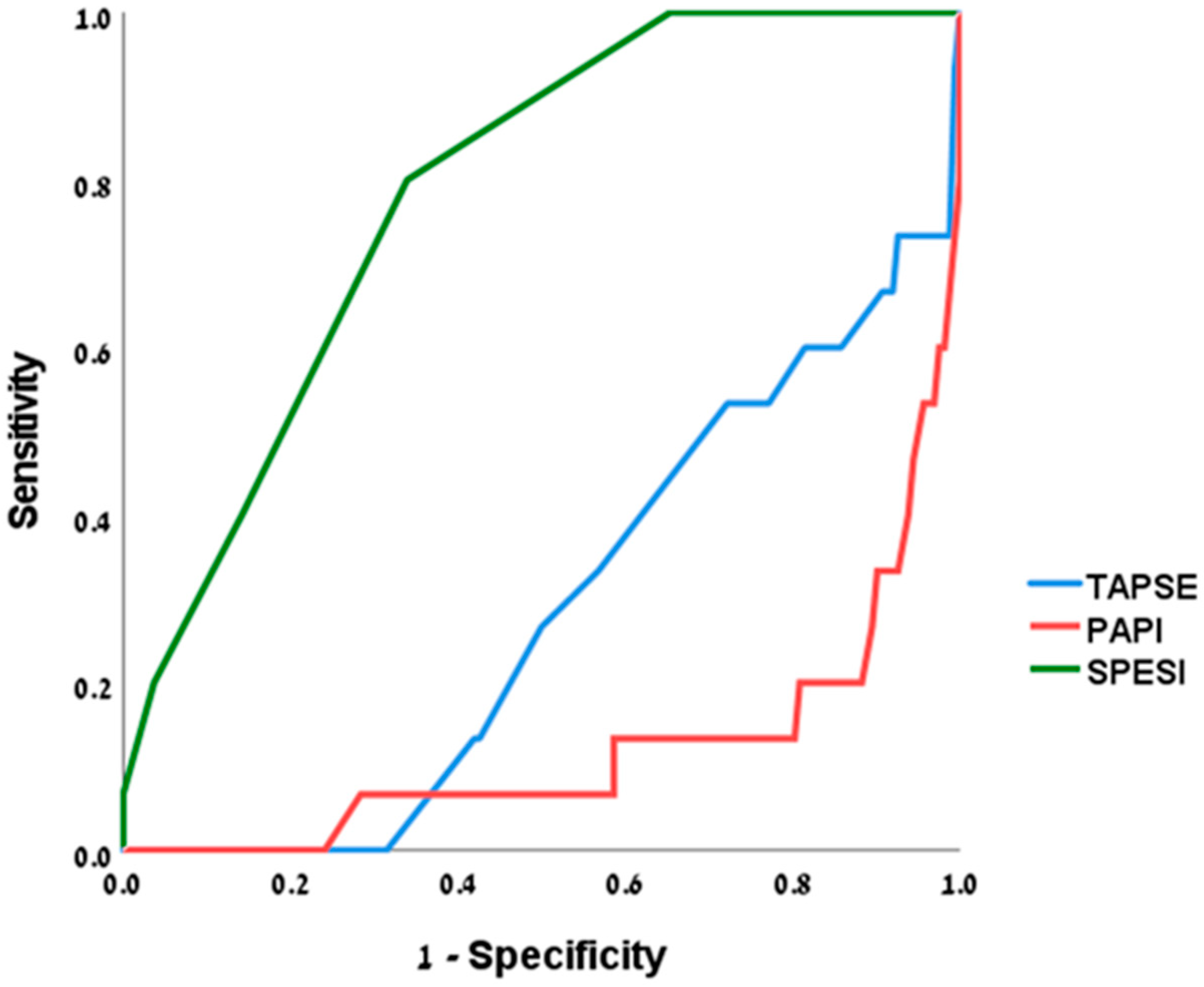
3.1. Outcomes
3.2. Discussion
3.3. Limitations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lim, H.S.; Gustafsson, F. Pulmonary artery pulsatility index: Physiological basis and clinical application. Eur. J. Heart Fail. 2020, 22, 32–38. [Google Scholar] [CrossRef]
- Mazimba, S.; Welch, T.S.; Mwansa, H.; Breathett, K.K.; Kennedy, J.L.W.; Mihalek, A.D.; Harding, W.C.; Mysore, M.M.; Zhuo, D.X.; Bilchick, K.C. Haemodynamically derived pulmonary artery pulsatility index predicts mortality in pulmonary arterial hypertension. Heart Lung Circ. 2019, 28, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Kochav, S.M.; Flores, R.J.; Truby, L.K.; Topkara, V.K. Prognostic impact of pulmonary artery pulsatility index (PAPi) in patients with advanced heart failure: Insights from the ESCAPE trial. J. Card. Fail. 2018, 24, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Parasuraman, S.; Walker, S.; Loudon, B.L.; Gollop, N.D.; Wilson, A.M.; Lowery, C.; Frenneaux, M.P. Assessment of pulmonary artery pressure by echocardiography-A comprehensive review. Int. J. Cardiol. Heart Vasc. 2016, 12, 45–51. [Google Scholar] [CrossRef]
- Kane, C.J.; Salama, A.A.; Pislaru, C.; Kane, G.C.; Pislaru, S.V.; Lin, G. Low Pulmonary Artery Pulsatility Index by Echocardiography Is Associated With Increased Mortality in Pulmonary Hypertension. J. Am. Soc. Echocardiogr. 2023, 36, 189–195. [Google Scholar] [CrossRef]
- Boretto, P.; Gravinese, C.; Frea, S.; Pidello, S.; De Ferrari, G.M. Echocardiographic-derived Pulmonary Artery Pulsatility Index: Towards non-invasive evaluation of right ventricular function. Eur. Heart J.-Cardiovasc. Imaging 2022, 23, jeab289.385. [Google Scholar] [CrossRef]
- SMirza, A.; Khalif, S.; Khodjaev, C.; Alpert, A.; Raina, M.; Kanwar, S.; Murali, R.; Benza, A. Hadi. Echocardiographic Estimation of Pulmonary Artery Pulsatility Index in Pulmonary Hypertension. J. Heart Lung Transplant. 2019, 38, S492. [Google Scholar]
- Kang, G.; Ha, R.; Banerjee, D. Pulmonary artery pulsatility index predicts right ventricular failure after left ventricular assist device implantation. J. Heart Lung Transplant. 2016, 35, 67–73. [Google Scholar] [CrossRef]
- Bayram, Z.; Dogan, C.; Efe, S.C.; Karagoz, A.; Guvendi, B.; Uysal, S.; Akbas, R.B.; Acar, R.D.; Akbal, O.Y.; Yilmaz, F.; et al. Prognostic Importance of Pulmonary Artery Pulsatility Index and Right Ventricular Stroke Work Index in End-Stage Heart Failure Patients. Cardiology 2022, 147, 143–153. [Google Scholar] [CrossRef]
- Morine, K.J.; Kiernan, M.S.; Pham, D.T.; Paruchuri, V.; Denofrio, D.; Kapur, N.K. Pulmonary Artery Pulsatility Index Is Associated With Right Ventricular Failure After Left Ventricular Assist Device Surgery. J. Card. Fail. 2016, 22, 110–116. [Google Scholar] [CrossRef]
- Raymer, D.S.; Moreno, J.D.; Sintek, M.A.; Nassif, M.E.; Sparrow, C.T.; Adamo, L.; Novak, E.L.; LaRue, S.J.; Vader, J.M. The Combination of Tricuspid Annular Plane Systolic Excursion and HeartMate Risk Score Predicts Right Ventricular Failure After Left Ventricular Assist Device Implantation. ASAIO J. 2019, 65, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, B.N.; Truesdell, A.G.; Sherwood, M.W.; Desai, S.; Tran, H.A.; Epps, K.C.; Singh, R.; Psotka, M.; Shah, P.; Cooper, L.B.; et al. Standardized Team-Based Care for Cardiogenic Shock. J. Am. Coll. Cardiol. 2019, 73, 1659–1669. [Google Scholar] [CrossRef] [PubMed]
- Korabathina, R.; Heffernan, K.S.; Paruchuri, V.; Patel, A.R.; Mudd, J.O.; Prutkin, J.M.; Orr, N.M.; Weintraub, A.; Kimmelstiel, C.D.; Kapur, N.K. The pulmonary artery pulsatility index identifies severe right ventricular dysfunction in acute inferior myocardial infarction. Catheter. Cardiovasc. Interv. 2012, 80, 593–600. [Google Scholar] [CrossRef]
- Zern, E.K.; Wang, D.; Rambarat, P.; Bernard, S.; Paniagua, S.M.; Liu, E.E.; McNeill, J.; Wang, J.K.; Andrews, C.T.; Pomerantsev, E.V.; et al. Association of Pulmonary Artery Pulsatility Index With Adverse Cardiovascular Events Across a Hospital-Based Sample. Circ. Heart Fail. 2022, 15, e009085. [Google Scholar] [CrossRef]
- Wendelboe, A.M.; Raskob, G.E. Global Burden of Thrombosis: Epidemiologic Aspects. Circ. Res. 2016, 118, 1340–1347. [Google Scholar] [CrossRef]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur. Respir. J. 2019, 54, 1901647. [Google Scholar] [PubMed]
- Elias, A.; Mallett, S.; Daoud-Elias, M.; Poggi, J.N.; Clarke, M. Prognostic models in acute pulmonary embolism: A systematic review and meta-analysis. BMJ Open 2016, 6, e010324. [Google Scholar] [CrossRef]
- Lankhaar, J.W.; Westerhof, N.; Faes, T.J.; Gan, C.T.; Marques, K.M.; Boonstra, A.; van den Berg, F.G.; Postmus, P.E.; Vonk-Noordegraaf, A. Pulmonary vascular resistance and compliance stay inversely related during treatment of pulmonary hypertension. Eur. Heart J. 2008, 29, 1688–1695. [Google Scholar] [CrossRef]
- Saouti, N.; Westerhof, N.; Postmus, P.E.; Vonk-Noordegraaf, A. The arterial load in pulmonary hypertension. Eur. Respir. Rev. 2010, 117, 197–203. [Google Scholar] [CrossRef]
- Tedford, R.J.; Hassoun, P.M.; Mathai, S.C.; Girgis, R.E.; Russell, S.D.; Thiemann, D.R.; Cingolani, O.H.; Mudd, J.O.; Borlaug, B.A.; Redfield, M.M.; et al. Pulmonary capillary wedge pressure augments right ventricular pulsatile loading. Circulation 2012, 2, 289–297. [Google Scholar] [CrossRef]
- Barco, S.; Mahmoudpour, S.H.; Valerio, L.; Klok, F.A.; Münzel, T.; Middeldorp, S.; Ageno, W.; Cohen, A.T.; Hunt, B.J.; Konstantinides, S.V. Trends in mortality related to pulmonary embolism in the European Region, 2000–2015: Analysis of vital registration data from the WHO Mortality Database. Lancet Respir. Med. 2020, 8, 277–287. [Google Scholar] [CrossRef]
- Cimini, L.A.; Candeloro, M.; Pływaczewska, M.; Maraziti, G.; Di Nisio, M.; Pruszczyk, P.; Agnelli, G.; Becattini, C. Prognostic role of different findings at echocardiography in acute pulmonary embolism: A critical review and meta-analysis. ERJ Open Res. 2023, 9, 00641-2022. [Google Scholar] [CrossRef]
- Pruszczyk, P.; Goliszek, S.; Lichodziejewska, B.; Kostrubiec, M.; Ciurzyński, M.; Kurnicka, K.; Dzikowska-Diduch, O.; Palczewski, P.; Wyzgal, A. Prognostic value of echocardiography in normotensive patients with acute pulmonary embolism. JACC Cardiovasc. Imaging 2014, 7, 553–560. [Google Scholar] [CrossRef]
- Lyhne, M.D.; Kabrhel, C.; Giordano, N.; Andersen, A.; Nielsen-Kudsk, J.E.; Zheng, H.; Dudzinski, D.M. The echocardiographic ratio tricuspid annular plane systolic excursion/pulmonary arterial systolic pressure predicts short-term adverse outcomes in acute pulmonary embolism. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Tello, K.; Axmann, J.; Ghofrani, H.A.; Naeije, R.; Narcin, N.; Rieth, A.; Seeger, W.; Gall, H.; Richter, M.J. Relevance of the TAPSE/PASP ratio in pulmonary arterial hypertension. Int. J. Cardiol. 2018, 266, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Bosch, L.; Lam, C.S.P.; Gong, L.; Chan, S.P.; Sim, D.; Yeo, D.; Jaufeerally, F.; Leong, K.T.G.; Ong, H.Y.; Ng, T.P.; et al. Right ventricular dysfunction in left-sided heart failure with preserved versus reduced ejection fraction. Eur. J. Heart Fail. 2017, 19, 1664–1671. [Google Scholar] [CrossRef]
- Guazzi, M.; Bandera, F.; Pelissero, G.; Castelvecchio, S.; Menicanti, L.; Ghio, S.; Temporelli, P.L.; Arena, R. Tricuspid annular plane systolic excursion and pulmonary arterial systolic pressure relationship in heart failure: An index of right ventricular contractile function and prognosis. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H1373–H1381. [Google Scholar] [CrossRef] [PubMed]
- Weekes, A.J.; Raper, J.D.; Esener, D.; Davison, J.; Boyd, J.S.; Kelly, C.; Nomura, J.T.; Thomas, A.M.; Lupez, K.; Cox, C.A.; et al. Comparing predictive performance of pulmonary embolism risk stratification tools for acute clinical deterioration. J. Am. Coll. Emerg. Physicians Open 2023, 4, e12983. [Google Scholar] [CrossRef]
- Santolicandro, A.; Prediletto, R.; Fornai, E.; Formichi, B.; Begliomini, E.; Giannella-Neto, A.; Giuntini, C. Mechanisms of hypoxemia and hypocapnia in pulmonary embolism. Am. J. Respir. Crit. Care Med. 1995, 152, 336–347. [Google Scholar] [CrossRef]
- Goldhaber, S.Z.; Elliott, C.G. Acute pulmonary embolism: Part I: Epidemiology, pathophysiology, and diagnosis. Circulation 2003, 108, 2726–2729. [Google Scholar] [CrossRef]
- Donadini, M.P.; Dentali, F.; Castellaneta, M.; Gnerre, P.; La Regina, M.; Masotti, L.; Pieralli, F.; Pomero, F.; Re, R.; Guasti, L.; et al. LORPELHS study group. Pulmonary embolism prognostic factors and length of hospital stay: A cohort study. Thromb. Res. 2017, 156, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Sławek-Szmyt, S.; Araszkiewicz, A.; Jankiewicz, S.; Grygier, M.; Mularek-Kubzdela, T.; Lesiak, M. Prognostic Value of Pulmonary Artery Pulsatility Index in Right Ventricle Failure-Related Mortality in Inoperable Chronic Thromboembolic Pulmonary Hypertension. J. Clin. Med. 2022, 11, 2735. [Google Scholar] [CrossRef] [PubMed]


| n | 177 |
| Age (mean ± SD), years | 67 ± 15 |
| Male (%) | 96 (54.2) |
| BMI (mean ± SD), Kg/m2 | 23.2 ± 3.1 |
| Hypertension (%) | 122 (68.9) |
| Hyperlipidemia (%) | 103 (58.2) |
| Diabetes mellitus (%) | 62 (35) |
| Chronic kidney disease (%) | 38 (21.5) |
| Tobacco use (%) | 109 (61.9) |
| Ischemic heart disease (%) | 56 (31.6) |
| History of stroke (%) | 14 (7.9) |
| Heart failure | 26 (14.7) |
| Pacemaker/ICD (%) | 22 (12.4) |
| Medications | |
| ACE inhibitor/ARB (%) | 85 (48) |
| Beta blockers (%) | 105 (59.3) |
| Aspirin (%) | 92 (52) |
| Statins (%) | 122 (68.9) |
| Spironolactone (%) | 31 (17.5) |
| SGLT2 inhibitors (%) | 70 (39.5) |
| Anticoagulants (%) | 19 (10.7) |
| Hemoglobin (mean ± SD), gr/dL | 12.7 ± 2.3 |
| WBC (mean ± SD), × 109/L | 10.4 ± 3.9 |
| Creatinine (mean ± SD), mg/dL | 1.1 ± 0.6 |
| CRP (median, IQR), mg/L | 56 [23, 154] |
| Troponin (median, IQR), ng/L | 21 [6, 138] |
| n | 177 |
| SBP (mean ± SD), mmHg | 131 ± 22 |
| Hear rate (mean ± SD), bpm | 87 ± 17 |
| LVEF (mean ± SD), (%) | 56 ± 11 |
| Estimated RAP (median, IQR), mmHg | 6 [5, 10] |
| Estimated PASP (median, IQR), mmHg | 40 [30, 55] |
| Estimated PADP (median, IQR), mmHg | 10 [9, 15] |
| Estimated PAPI (median, IQR) | 4.3 [2.9, 6] |
| TAPSE (mean ± SD), cm | 1.9 ± 0.5 |
| <1.7 (%) | 44 (25) |
| >1.7 (%) | 133 (75) |
| SPESI score (median, IQR) | 1 [0, 2] |
| RV involvement (%) | 33 (18.6) |
| Need for oxygen (%) | 47 (27) |
| Mechanical ventilation (%) | 13 (7.3) |
| Inotropes (%) | 10 (5.6) |
| Thrombolysis (%) | 8 (4.5) |
| CPR (%) | 15 (8.5) |
| Length of stay (median, IQR), days | 6 [4, 10] |
| 30-day mortality (%) | 15 (8.5) |
| Low Risk | Intermediate-High Risk | p Value | |
|---|---|---|---|
| n | 111 | 66 | |
| Age (mean ± SD), years | 65 ± 18 | 70 ± 10 | <0.05 |
| Male (%) | 58 (52%) | 38 (57%) | 0.49 |
| BMI (mean ± SD), Kg/m2 | 22.1 ± 2.5 | 25 ± 3.1 | <0.05 |
| Diabetes mellitus (%) | 37 (33) | 25 (38) | 0.54 |
| Hypertension (%) | 76 (69) | 46 (70) | 0.86 |
| Hyperlipidemia (%) | 67 (60) | 36 (55) | 0.45 |
| Tobacco use (%) | 70 (63) | 39 (59) | 0.59 |
| Chronic kidney disease (%) | 23 (21) | 15 (23) | 0.75 |
| Ischemic heart disease (%) | 38 (34) | 18 (27) | 0.34 |
| History of stroke (%) | 7 (6) | 7 (11) | 0.31 |
| Pacemaker/ICD (%) | 16 (14) | 6 (9) | 0.29 |
| Hemoglobin | 12.6 ± 2.1 | 12.9 ±2.5 | 0.39 |
| LVEF (%) | 56 ± 12 | 55 ± 8 | 0.54 |
| OR | 95% CI | p Value | |
|---|---|---|---|
| Age | 1.03 | 0.96–1.09 | 0.48 |
| Male | 1.5 | 0.28–8.3 | 0.63 |
| Diabetes mellitus | 5.3 | 0.76–37.0 | 0.09 |
| Chronic kidney disease | 3.1 | 0.87–11.2 | 0.08 |
| TAPSE | 0.09 | 0.0001–0.186 | <0.05 |
| SPESI | 3.2 | 1.5–6.6 | <0.05 |
| Low PAPI | 0.14 | 0.045–0.43 | <0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moady, G.; Mobarki, L.; Or, T.; Shturman, A.; Atar, S. Echocardiography-Based Pulmonary Artery Pulsatility Index Correlates with Outcomes in Patients with Acute Pulmonary Embolism. J. Clin. Med. 2025, 14, 2685. https://doi.org/10.3390/jcm14082685
Moady G, Mobarki L, Or T, Shturman A, Atar S. Echocardiography-Based Pulmonary Artery Pulsatility Index Correlates with Outcomes in Patients with Acute Pulmonary Embolism. Journal of Clinical Medicine. 2025; 14(8):2685. https://doi.org/10.3390/jcm14082685
Chicago/Turabian StyleMoady, Gassan, Loai Mobarki, Tsafrir Or, Alexander Shturman, and Shaul Atar. 2025. "Echocardiography-Based Pulmonary Artery Pulsatility Index Correlates with Outcomes in Patients with Acute Pulmonary Embolism" Journal of Clinical Medicine 14, no. 8: 2685. https://doi.org/10.3390/jcm14082685
APA StyleMoady, G., Mobarki, L., Or, T., Shturman, A., & Atar, S. (2025). Echocardiography-Based Pulmonary Artery Pulsatility Index Correlates with Outcomes in Patients with Acute Pulmonary Embolism. Journal of Clinical Medicine, 14(8), 2685. https://doi.org/10.3390/jcm14082685







