Differential Association Between Ten Indices of Insulin Resistance and End-Organ Damage in a Community of African Ancestry in Africa
Abstract
1. Introduction
2. Methods
2.1. Study Group
2.2. Clinical, Demographic, Anthropometric, and Laboratory Information
2.3. Insulin and Indices of Insulin Resistance
- Homeostasis model assessment of insulin resistance (HOMA-IR) = (insulin [µU/mL] × glucose [mmol/L])/22.5.
- Quantitative insulin sensitivity check index (QUICKI) = 1/(log insulin[µU/mL] + log glucose [mg/dL]) [25].
- Metabolic score for insulin resistance (METS-IR) = (ln((2 × glucose [mg/dL]) + triglycerides [mg/dL])) × (BMI/(ln(HDL-cholesterol [mg/dL]))) [5].
- Triglyceride–glucose index (TyG) = ln(triglycerides [mg/dL] × glucose [mg/dL]/2) [6].
- Triglyceride–body mass index (TyG-BMI) = TyG × BMI [7].
- Triglyceride–waist circumference (TyG-WC) = TyG × waist circumference [7].
- Triglyceride–waist-to-height ratio (TyG-WHtR) = TyG × waist-to-height ratio [8].
- Triglyceride to high-density cholesterol concentration (TyG-HDL) = triglycerides [mg/dL]/HDL-cholesterol [mg/dL] [9].
- Lipid accumulation product (LAP) = (waist circumference − 65) × triglycerides [mmol/L] for men and LAP = (waist circumference − 58) × triglycerides [mmol/L] for women [10].
- Visceral adiposity index (VAI) = (waist circumference/(39.68 + (1.88 × BMI))) × (triglycerides [mmol/L]/1.03) × (1.31/HDL-cholesterol [mmol/L]) for men and VAI = (waist circumference/(36.58 + (1.89 × BMI))) × (triglycerides [mmol/L]/0.81) × (1.52/HDL-cholesterol [mmol/L]) for women [11].
2.4. End-Organ Measures
- Women with serum creatinine (SCr) ≤ 0.7 mg/dL: GFR = 142 × (SCr/0.7)−0.241 × (0.9938)Age × 1.012.
- Women with SCr > 0.7 mg/dL: GFR = 142 × (SCr/0.7)−1.200 × (0.9938)Age × 1.012.
- Men with SCr ≤ 0.9 mg/dL: GFR = 142 × (SCr/0.9)−0.302 × (0.9938)Age.
- Men with SCr > 0.9 mg/dL: GFR = 142 × (SCr/0.9)−1.200 × (0.9938)Age.
- Decreased GFR was identified as GFR less than 90 mL/min per 1.73 m2.
2.5. Data Analysis
3. Results
3.1. Participant Characteristics
3.2. Correlations Among Indices of Insulin Resistance
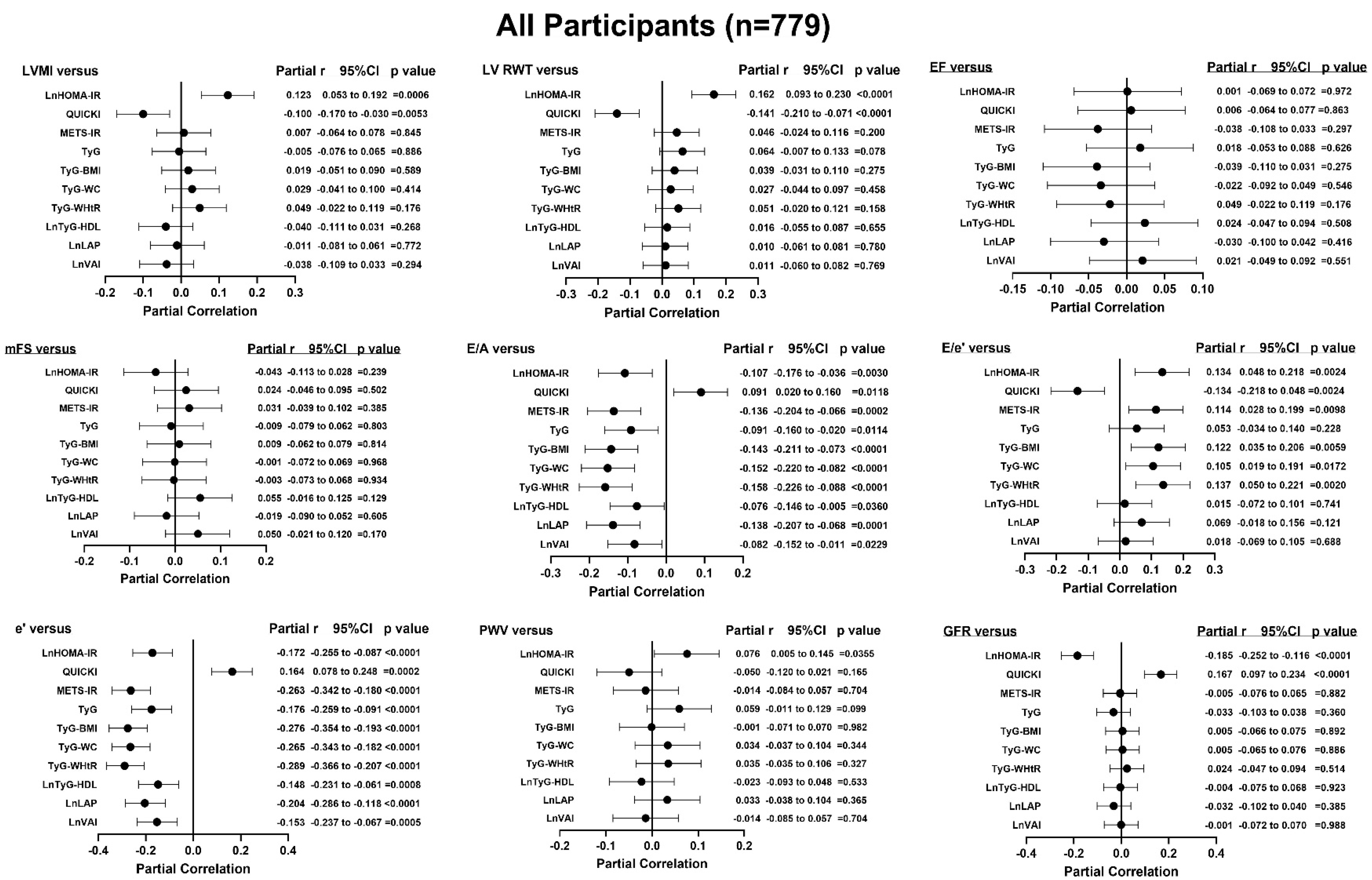
3.3. Unadjusted Associations Between Indices of Insulin Resistance and End-Organ Measures
3.4. Adjusted Associations Between Insulin and Blood Glucose and End-Organ Measures
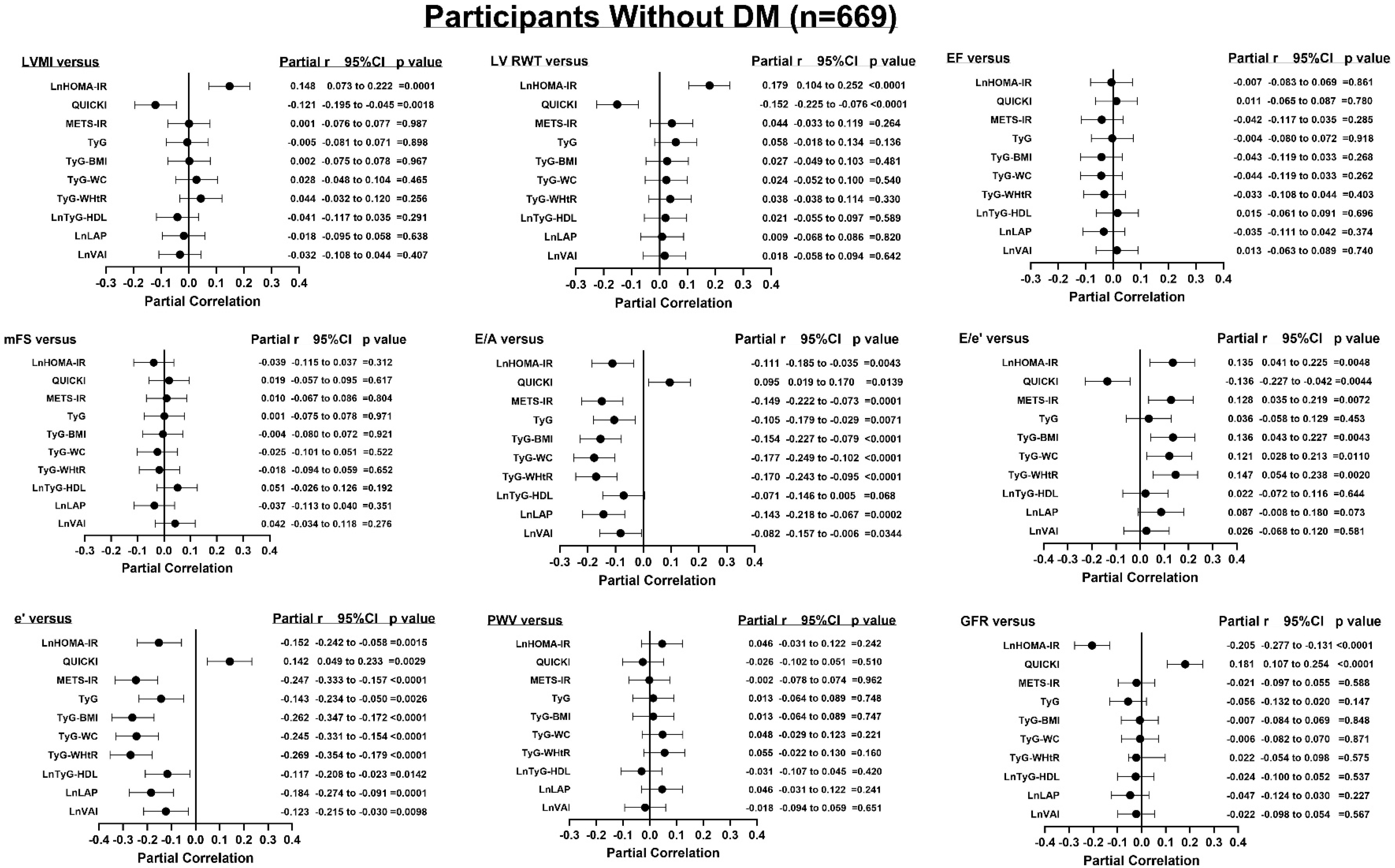
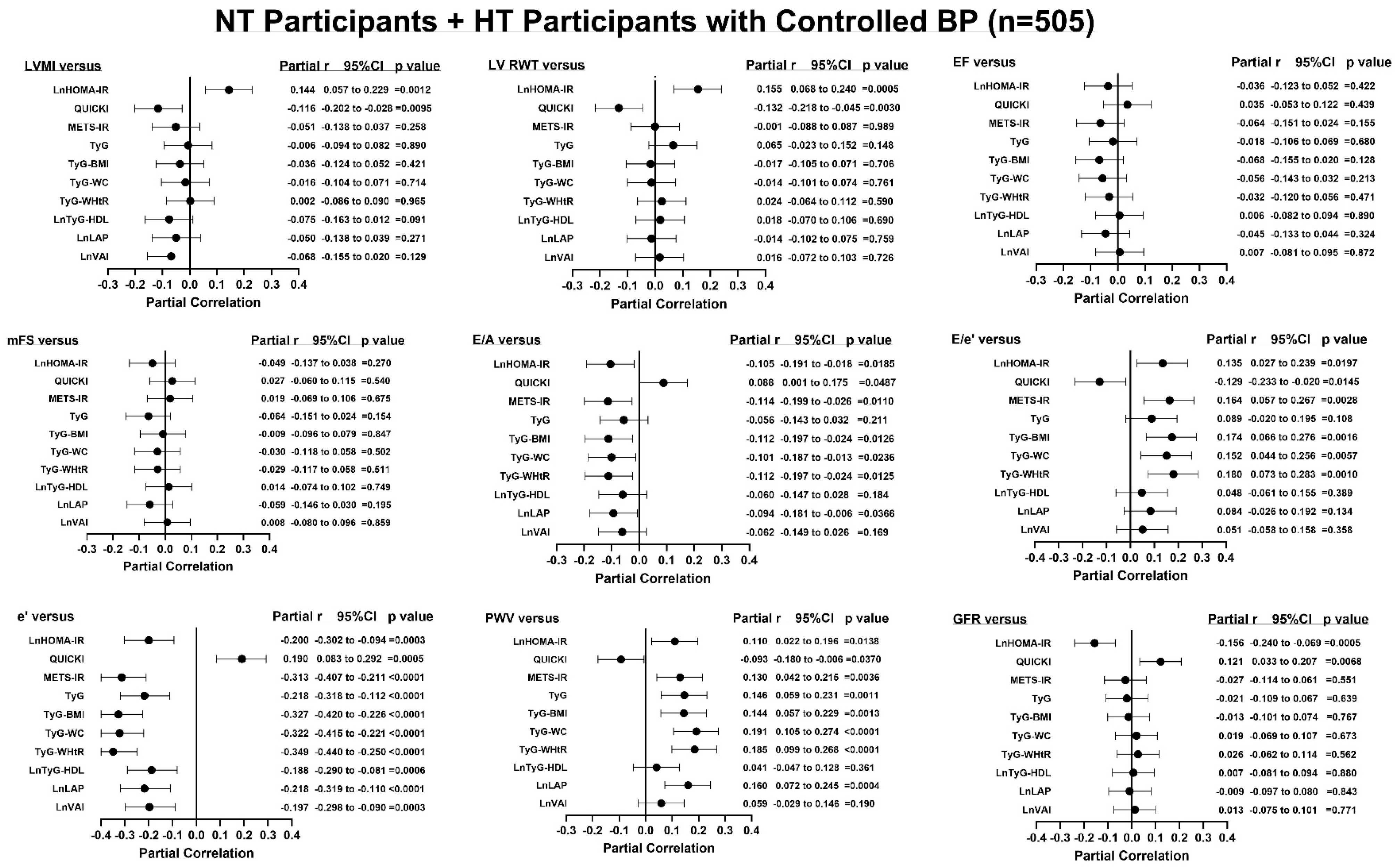
3.5. Adjusted Associations Between Indices of Insulin Resistance and End-Organ Measures
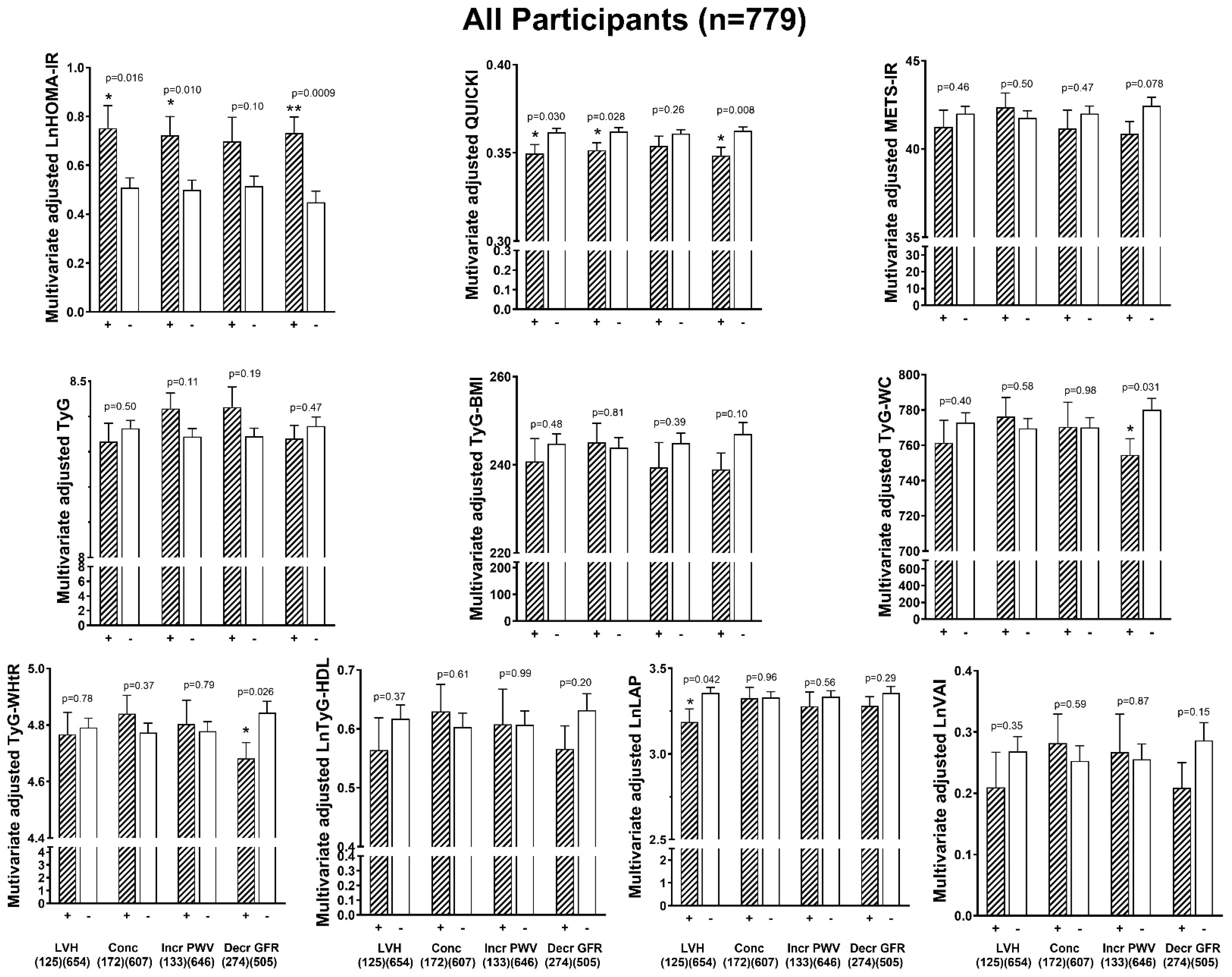
3.6. Associations Between Indices of Insulin Resistance and End-Organ Damage
3.7. HOMA-IR and Odds of End-Organ Damage Independent of CVS Risk Factors
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boutari, C.; Mantzoros, C.S. A 2022 Update on the Epidemiology of Obesity and a Call to Action: As its Twin COVID-19 Pandemic Appears to be Receding, the Obesity and Dysmetabolism Pandemic Continues to Rage on. Metabolism 2022, 133, 155217. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.A.; Yang, Y.; Zhang, L.; Sun, Z.; Jia, G.; Parrish, A.R.; Sowers, J.R. Insulin Resistance, Cardiovascular Stiffening and Cardiovascular Disease. Metabolism 2021, 119, 154766. [Google Scholar] [CrossRef] [PubMed]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuñiga, F.A. Association Between Insulin Resistance and the Development of Cardiovascular Disease. Cardiovasc. Diabetol. 2018, 17, 122. [Google Scholar] [CrossRef] [PubMed]
- Minh, H.V.; Tien, H.A.; Sinh, C.T.; Thang, D.C.; Chen, C.H.; Tay, J.C.; Siddique, S.; Wang, T.D.; Sogunuru, G.P.; Chia, Y.C.; et al. Assessment of Preferred Methods to Measure Insulin Resistance in Asian Patients with Hypertension. J. Clin. Hypertens. 2021, 23, 529–537. [Google Scholar] [CrossRef]
- Bello-Chavolla, O.Y.; Almeda-Valdes, P.; Gomez-Velasco, D.; Viveros-Ruiz, T.; Cruz-Bautista, I.; Romo-Romo, A.; Sánchez-Lázaro, D.; Meza-Oviedo, D.; Vargas-Vázquez, A.; Campos, O.A.; et al. METS-IR, a Novel Score to Evaluate Insulin Sensitivity, is Predictive of Visceral Adiposity and Incident Type 2 Diabetes. Eur. J. Endocrinol. 2018, 178, 533–544. [Google Scholar] [CrossRef]
- Simental-Mendia, L.E.; Rodriguez-Moran, M.; Guerrero-Romero, F. The Product of Fasting Glucose and Triglycerides as Surrogate for Identifying Insulin Resistance in Apparently Healthy Subjects. Metab. Syndr. Relat. Disord. 2008, 6, 299–304. [Google Scholar] [CrossRef]
- Er, L.K.; Wu, S.; Chou, H.H.; Hsu, L.A.; Teng, M.S.; Sun, Y.C.; Ko, Y.L. Triglyceride Glucose-Body Mass Index is a Simple and Clinically Useful Surrogate Marker for Insulin Resistance in Nondiabetic Individuals. PLoS ONE 2016, 11, e0149731. [Google Scholar] [CrossRef]
- Raimi, T.H.; Dele-Ojo, B.F.; Dada, S.A.; Fadare, J.O.; Ajayi, D.D.; Ajayi, E.A.; Ajayi, O.A. Triglyceride-Glucose Index and Related Parameters Predicted Metabolic Syndrome in Nigerians. Metab. Syndr. Relat. Disord. 2021, 19, 76–82. [Google Scholar] [CrossRef]
- Hanak, V.; Munoz, J.; Teague, J.; Stanley, A., Jr.; Bittner, V. Accuracy of the Triglyceride to High-Density Lipoprotein Cholesterol Ratio for Prediction of the Low-Density Lipoprotein Phenotype B. Am. J. Cardiol. 2004, 94, 219–222. [Google Scholar] [CrossRef]
- Kahn, H.S. The “Lipid Accumulation Product” Performs Better Than the Body Mass Index for Recognizing Cardiovascular Risk: A Population-Based Comparison. BMC Cardiovasc. Disord. 2005, 5, 26. [Google Scholar] [CrossRef]
- Amato, M.C.; Giordano, C.; Galia, M.; Criscimanna, A.; Vitabile, S.; Midiri, M.; Galluzzo, A. for the AlkaMeSy Study Group. Visceral Adiposity Index. A Reliable Indicator of Visceral Fat Function Associated with Cardiometabolic Risk. Diabetes Care 2010, 33, 920–922. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.; Zhao, X.; Li, S.; Miao, G.; Bai, L.; Zhang, Q.; Yang, W.; Zhao, X. Metabolic Score for Insulin Resistance (METS-IR) Predicts All-Cause and Cardiovascular Mortality in the General Population: Evidence from NHANES 2001–2018. Cardiovasc. Diabetol. 2024, 23, 243. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Larrad, M.T.; Lorenzo, C.; González-Villalpando, C.; Gabriel, R.; Haffner, S.M.; Serrano-Ríos, M. Associations Between Surrogate Measures of Insulin Resistance and Waist Circumference, Cardiovascular Risk and the Metabolic Syndrome Across Hispanic and non-Hispanic White Populations. Diabet. Med. 2012, 29, 1390–1394. [Google Scholar] [CrossRef] [PubMed]
- Howard, G.; O’Leary, D.H.; Zaccaro, D.; Haffner, S.; Rewers, M.; Hamman, R.; Selby, J.V.; Saad, M.F.; Savage, P.; Bergman, R. Insulin Sensitivity and Atherosclerosis. The Insulin Resistance Atherosclerosis Study (IRAS) investigators. Circulation 1996, 93, 1809–1817. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis Model Assessment: Insulin Resistance and Beta-Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Bertoni, A.G.; Wong, N.D.; Shea, S.; Ma, S.; Liu, K.; Preethi, S.; Jacobs, D.R., Jr.; Wu, C.; Saad, M.F.; Szklo, M. Insulin Resistance, Metabolic Syndrome, and Subclinical Atherosclerosis: The Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care 2007, 30, 2951–2956. [Google Scholar] [CrossRef]
- Osei, K. Metabolic Syndrome in Blacks: Are the Criteria Right? Curr. Diab. Rep. 2010, 10, 199–208. [Google Scholar] [CrossRef]
- McIntosh, M.S.; Kumar, V.; Kalynych, C.; Lott, M.; His, A.; Chang, J.L.; Lerman, R.H. Racial Differences in Blood Lipids Lead to Underestimation of Cardiovascular Risk in Black Women in a Nested Observational Study. Glob. Adv. Health. Med. 2013, 2, 76–79. [Google Scholar] [CrossRef]
- Shehu, M.N.; Adamu, U.G.; Ojji, D.B.; Ogah, O.S.; Sani, M.U. The Pandemic of Coronary Artery Disease in the Sub-Saharan Africa: What Clinicians Need to Know. Curr. Atheroscler. Rep. 2023, 25, 571–578. [Google Scholar] [CrossRef]
- Owobi, O.U.; Okonji, O.C.; Nzoputam, C.I.; Ekholuenetale, M. Country-Level Variations in Overweight and Obesity Among Reproductive-Aged Women in Sub-Saharan Countries. Women 2022, 2, 313–325. [Google Scholar] [CrossRef]
- Yoruk, A.; Boulos, P.K.; Bisognano, J.D. The State of Hypertension in Sub-Saharan Africa: Review and Commentary. Am. J. Hypertens. 2018, 31, 387–388. [Google Scholar] [CrossRef]
- Bibbins-Domingo, K.; Pletcher, M.J.; Lin, F.; Vittinghoff, E.; Gardin, J.M.; Arynchyn, A.; Lewis, C.E.; Williams, O.D.; Hulley, S.B. Racial Differences in Incident Heart Failure Among Young Adults. N. Engl. J. Med. 2009, 360, 1179–1190. [Google Scholar] [CrossRef]
- Woodiwiss, A.J.; Molebatsi, N.; Maseko, M.J.; Libhaber, E.; Libhaber, C.; Majane, O.H.; Paiker, J.; Dessein, P.; Brooksbank, R.; Sareli, P.; et al. Nurse-Recorded Auscultatory Blood Pressure at a Single Visit Predicts Target Organ Changes as well as Ambulatory Blood Pressure. J. Hypertens. 2009, 27, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Sibiya, M.J.; Norton, G.R.; Hodson, B.; Redelinghuys, M.; Maseko, M.J.; Majane, O.H.I.; Libhaber, E.; Woodiwiss, A.J. Gender-Specific Contribution of Aortic Augmentation Index to Variations in Left Ventricular Mass Index in a Community Sample of African Ancestry. Hypertens. Res. 2014, 37, 1021–1027. [Google Scholar] [CrossRef]
- Katz, A.; Nambi, S.S.; Mather, K.; Baron, A.D.; Follman, D.A.; Sullivan, G.; Quon, M.J. Quantitative Insulin Sensitivity Check Index: A Simple, Accurate Method for Assessing Insulin Sensitivity in Humans. J. Clin. Endocrinol. Metab. 2000, 85, 2402–2410. [Google Scholar] [CrossRef] [PubMed]
- Sahn, D.J.; De Maria, A.; Kisslo, J.; Weyman, A. Recommendations Regarding Quantitation in M-mode Echocardiography: Results of a Survey of Echocardiographic Measurement. Circulation 1978, 58, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Devereux, R.B.; Alonso, D.R.; Lutas, E.M.; Gottlieb, G.J.; Campo, E.; Sachs, I.; Reichek, N. Echocardiograph Assessment of Left Ventricular Hypertrophy: Comparison to Necropsy Findings. Am. J. Cardiol. 1986, 57, 450–458. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Yang, W.; Parikh, R.V.; Anderson, A.H.; Chen, T.K.; Cohen, D.L.; He, J.; Mohanty, M.J.; Lash, J.P.; Mills, K.T.; et al. CRIC Study Investigators. Race, Genetic Ancestry, and Estimating Kidney Function in CKD. N. Engl. J. Med. 2021, 385, 1750–1760. [Google Scholar] [CrossRef]
- Xiao, D.; Sun, H.; Chen, L.; Li, X.; Huo, H.; Zhou, G.; Zhang, M.; He, B. Assessment of Six Surrogate Insulin Resistance Indexes for Predicting Cardiometabolic Multimorbidity Incidence in Chinese Middle-aged and Older Populations: Insights from the China Health and Retirement Longitudinal Study. Diabetes. Metab. Res. Rev. 2024, 40, e3764. [Google Scholar] [CrossRef]
- Kianu Phanzu, B.; Nkodila Natuhoyila, A.; Kintoki Vita, E.; M’Buyamba Kabangu, J.R.; Longo-Mbenza, B. Association Between Insulin Resistance and Left Ventricular Hypertrophy in Asymptomatic, Black, Sub-Saharan African, Hypertensive Patients: A Case-control Study. BMC Cardiovasc. Disord. 2021, 21, 1. [Google Scholar] [CrossRef]
- Ellman, N.; Keswell, D.; Collins, M.; Tootla, M.; Goedecke, J.H. Ethnic Differences in the Association Between Lipid Metabolism Genes and Lipid Levels in Black and White South African Women. Atherosclerosis 2015, 240, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Kalk, W.; Joffe, B. The Metabolic Syndrome, Insulin Resistance and its Surrogates in African and White Subjects with Type 2 Diabetes in South Africa. Metab. Syndr. Relat. Disord. 2008, 6, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Baneu, P.; Văcărescu, C.; Drăgan, S.R.; Cirin, L.; Lazăr-Höcher, A.I.; Cozgarea, A.; Faur-Grigori, A.A.; Crișan, S.; Gaiță, D.; Luca, C.T.; et al. The Triglyceride/HDL Ratio as a Surrogate Biomarker for Insulin Resistance. Biomedicines 2024, 12, 1493. [Google Scholar] [CrossRef]
- Goedecke, J.H.; Mendham, A.E. Pathophysiology of Type 2 Diabetes in Sub-Saharan Africans. Diabetologia 2022, 65, 1967–1980. [Google Scholar] [CrossRef]
- Russo, C.; Jin, Z.; Homma, S.; Rundek, T.; Elkind, M.S.; Sacco, R.L.; Di Tullio, M.R. Effect of Obesity and Overweight on Left Ventricular Diastolic Function: A Community-Based Study in an Elderly Cohort. J. Am. Coll. Cardiol. 2011, 57, 1368–1374. [Google Scholar] [CrossRef] [PubMed]
- Kosmas, C.E.; Silverio, D.; Tsomidou, C.; Salcedo, M.D.; Montan, P.D.; Guzman, E. The Impact of Insulin Resistance and Chronic Kidney Disease on Inflammation and Cardiovascular Disease. Clin. Med. Insights. Endocrinol. Diabetes. 2018, 11, 1–6. [Google Scholar] [CrossRef]
- Falkner, B. Insulin Resistance in African Americans. Kidney Int. 2003, 63 (Suppl. 83), S27–S30. [Google Scholar] [CrossRef]
- Addo, J.; Agyemang, C.; de-Graft Aikins, A.; Beune, E.; Schulze, M.B.; Danquah, I.; Galbete, C.; Nicolaou, M.; Meeks, K.; Klipstein-Grobusch, K.; et al. Association Between Socioeconomic Position and the Prevalence of Type 2 Diabetes in Ghanaians in Different Geographic Locations: The RODAM study. J. Epidemiol. Community Health 2017, 71, 633–639. [Google Scholar] [CrossRef]
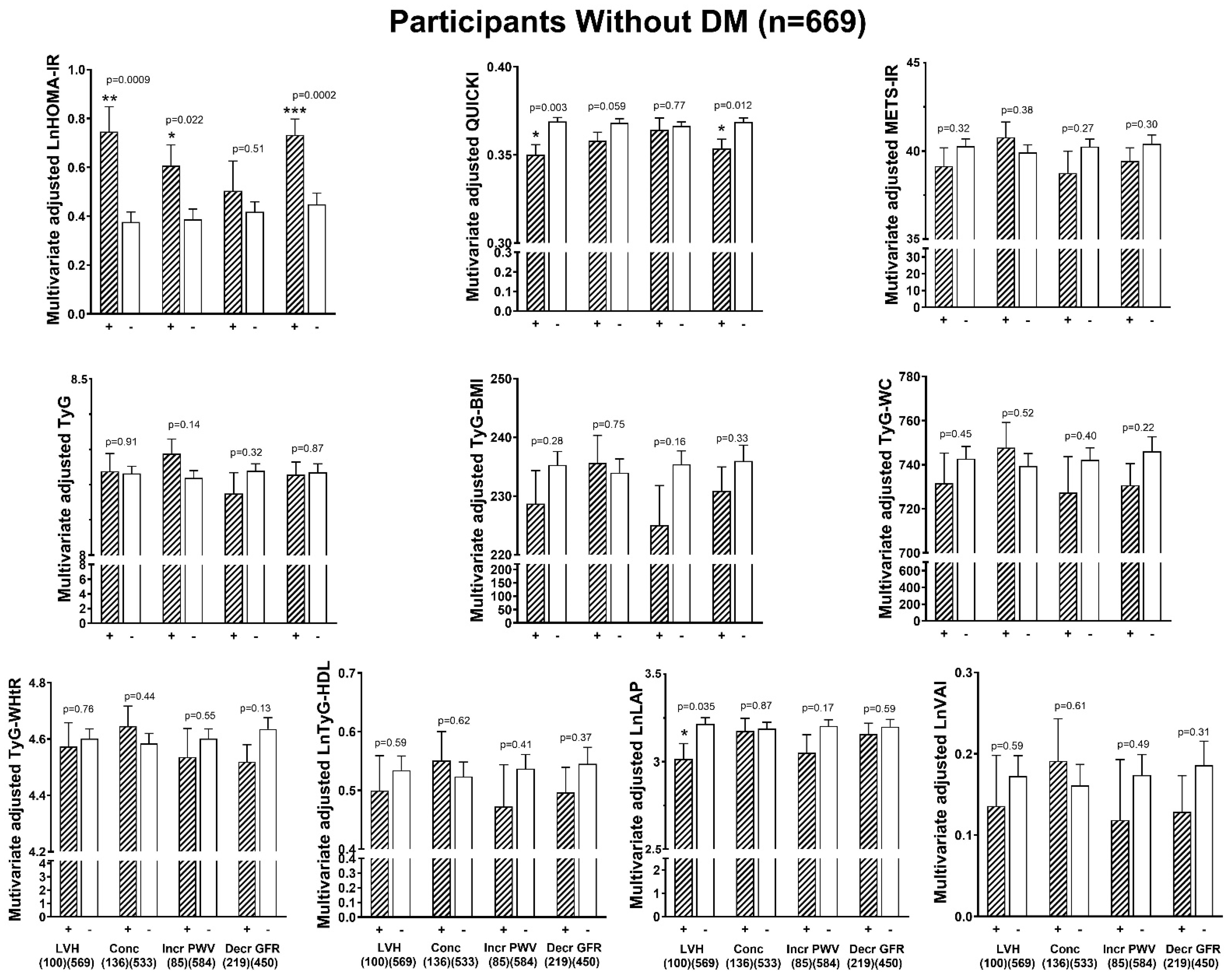
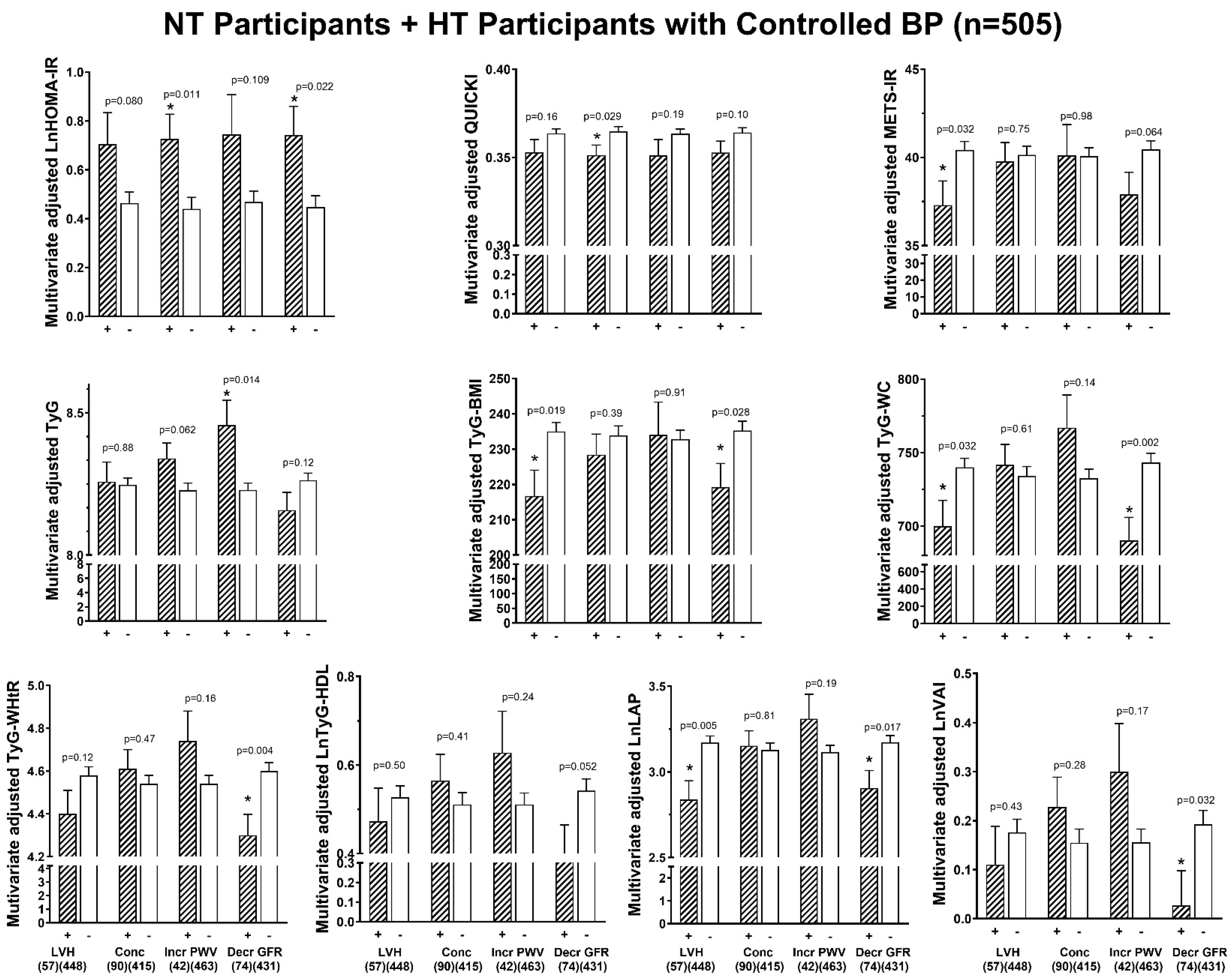
| All | Without DM | |
|---|---|---|
| Sample size | 779 | 669 |
| % Female (n) | 65.1 (508) | 64.3 (430) |
| Age (years) | 45.0 ± 18.3 | 42.3 ± 17.8 |
| % <65 years of age (n) | 82.8 (645) | 86.1 (576) |
| Body mass index (kg/m2) | 29.0 ± 7.4 | 28.3 ± 7.3 |
| Waist circumference (cm) | 91.7 ± 16.7 | 89.6 ± 16.2 |
| % Overweight (n) | 25.3 (197) | 25.3 (169) |
| % Obese (n) | 41.3 (322) | 37.2 (249) |
| Brachial SBP (mm Hg) | 128 ± 22 | 126 ± 22 |
| Brachial DBP (mm Hg) | 83 ± 12 | 82 ± 12 |
| % Hypertension (n) | 46.7 (364) | 40.1 (268) |
| % Uncontrolled BP (n) | 35.2 (274) | 32.6 (218) |
| % HT with uncontrolled BP (n) | 75.3 (274/364) | 81.3 (218/268) |
| % Treated for hypertension (n) | 26.6 (207) | 19.6 (131) |
| % HT treated for HT (n) | 56.9 (207/364) | 48.9 (131/268) |
| Heart rate (beats/min) | 65.9 ± 11.5 | 65.1 ± 11.1 |
| % Regular tobacco intake (n) | 15.4 (120) | 16.4 (110) |
| % Regular alcohol intake (n) | 21.2 (165) | 22.7 (152) |
| % Diabetes mellitus (n) | 14.2 (110) | 0 |
| Fasting plasma concentrations | ||
| Glucose (mmol/L) | 4.60 (4.20 to 5.30) | 4.50 (4.20 to 5.00) *** |
| Glycated haemoglobin (%) | 5.80 (5.50 to 6.10) | 5.70 (5.50 to 5.96) *** |
| Insulin (μU/mL) | 7.66 (4.02 to 14.00) | 7.03 (3.77 to 13.30) |
| Total cholesterol (mmol/L) | 4.60 (4.00 to 5.21) | 4.50 (4.00 to 5.16) |
| LDL cholesterol (mmol/L) | 2.60 (2.20 to 3.19) | 2.57 (2.15 to 3.18) |
| HDL cholesterol (mmol/L) | 1.40 (1.20 to 1.50) | 1.40 (1.24 to 1.60) |
| Triglycerides (mmol/L) | 1.07 (0.80 to 1.51) | 1.00 (0.70 to 1.47) |
| Insulin resistance indices | ||
| HOMA-IR | 1.61 (0.83 to 3.42) | 1.43 (0.76 to 2.80) |
| QUICKI | 0.36 ± 0.06 | 0.37 ± 0.06 * |
| METS-IR | 41.9 ± 12.3 | 40.1 ± 11.6 ** |
| TyG | 8.36 ± 0.67 | 8.23 ± 0.55 *** |
| TyG-BMI | 244.2 ± 69.5 | 234.3 ± 66.0 * |
| TyG-WC | 771.1 ± 174.4 | 741.1 ± 161.2 ** |
| TyG-WHtR | 4.79 ± 1.14 | 4.60 ± 1.06 ** |
| TyG-HDL | 1.72 (1.21 to 2.67) | 1.57 (1.14 to 2.56) |
| LAP | 32.2 (13.5 to 60.9) | 27.2 (12.0 to 52.5) * |
| VAI | 1.27 (0.80 to 2.03) | 1.16 (0.76 to 1.83) |
| End-organ measures | ||
| LVMI (g/m2) | 75.3 ± 29.6 | 74.3 ± 29.1 |
| % LV hypertrophy (n) | 16.1 (125) | 15.0 (100) |
| LV relative wall thickness | 0.38 ± 0.08 | 0.37 ± 0.07 |
| % Concentricity of LV | 22.1 (172) | 20.3 (136) |
| LV midwall fractional shortening | 0.26 ± 0.08 | 0.26 ± 0.08 |
| EF (%) | 67.2 ± 8.8 | 67.2 ± 8.6 |
| E/A | 1.29 ± 0.50 | 1.33 ± 0.49 |
| E/e’ (n) | 7.38 ± 4.04 (516) | 7.03 ± 3.68 (414) |
| e’ (n) | 11.48 ± 4.04 (516) | 12.02 ± 3.98 (414) * |
| GFR (ml/min per 1.73 m2) | 98.13 ± 21.29 | 100.47 ± 20.36 * |
| % Decreased GFR (n) | 35.2 (274) | 32.7 (219) |
| Pulse wave velocity (m/s) | 6.09 ± 2.65 | 5.77 ± 2.36 * |
| % Increased PWV (n) | 17.1 (133) | 12.7 (85) |
| lnHOMA-IR | QUICKI | METS-IR | TyG | TyG-BMI | TyG-WC | TyG-WHtR | lnTyG-HDL | lnLAP | ||
|---|---|---|---|---|---|---|---|---|---|---|
| QUICKI | −0.982 | Pearson’s Correlation | ||||||||
| METS-IR | 0.334 | −0.340 | ||||||||
| TyG | 0.402 | −0.375 | 0.498 | 0.501 to 1.000 | ||||||
| TyG-BMI | 0.313 | −0.321 | 0.965 | 0.510 | 0.001 to 0.500 | |||||
| TyG-WC | 0.349 | −0.348 | 0.847 | 0.690 | 0.874 | 0 | ||||
| TyG-WHtR | 0.331 | −0.335 | 0.860 | 0.651 | 0.894 | 0.974 | −0.001 to −0.500 | |||
| lnTyG-HDL | 0.283 | −0.269 | 0.512 | 0.856 | 0.437 | 0.612 | 0.565 | −0.501 to −1.000 | ||
| lnLAP | 0.304 | −0.306 | 0.769 | 0.717 | 0.804 | 0.912 | 0.894 | 0.683 | ||
| lnVAI | 0.304 | -0.297 | 0.600 | 0.839 | 0.541 | 0.715 | 0.695 | 0.955 | 0.789 | p < 0.0001 for all |
| r (95%CI) | p-Value | r (95%CI) | p-Value | r (95%CI) | p-Value | |
|---|---|---|---|---|---|---|
| LVMI | LV RWT | EF | ||||
| lnHOMA-IR | 0.140 (0.070 to 0.208) | <0.0001 | 0.204 (0.136 to 0.270) | <0.0001 | 0.002 (−0.068 to 0.072) | =0.9553 |
| QUICKI | −0.110 (−0.179 to −0.040) | =0.0020 | −0.182 (−0.249 to −0.114) | <0.0001 | 0.005 (−0.066 to 0.075) | =0.8984 |
| METS-IR | 0.076 (0.005 to 0.145) | =0.0347 | 0.176 (0.107 to 0.243) | <0.0001 | −0.008 (−0.078 to 0.063) | =0.8327 |
| TyG | 0.125 (0.055 to 0.193) | =0.0005 | 0.175 (0.106 to 0.242) | <0.0001 | 0.023 (−0.047 to 0.093) | =0.5221 |
| TyG-BMI | 0.088 (0.018 to 0.158) | =0.0136 | 0.179 (0.110 to 0.246) | <0.0001 | −0.002 (−0.072 to 0.068) | =0.9567 |
| TyG-WC | 0.145 (0.076 to 0.213) | <0.0001 | 0.185 (0.116 to 0.252) | <0.0001 | −0.002 (−0.072 to 0.068) | =0.9577 |
| TyG-WHtR | 0.142 (0.072 to 0.210) | <0.0001 | 0.212 (0.144 to 0.278) | <0.0001 | 0.013 (−0.057 to 0.084) | =0.7098 |
| lnTyG-HDL | 0.087 (0.017 to 0.156) | =0.0150 | 0.110 (0.040 to 0.179) | =0.0021 | 0.025 (−0.046 to 0.095) | =0.4890 |
| lnLAP | 0.104 (0.033 to 0.173) | =0.0039 | 0.167 (0.098 to 0.235) | <0.0001 | 0.009 (−0.062 to 0.079) | =0.8120 |
| lnVAI | 0.058 (−0.012 to 0.128) | =0.1046 | 0.131 (0.061 to 0.199) | =0.0002 | 0.034 (−0.037 to 0.104) | =0.3457 |
| mFS | E/A | E/e’ (n = 515) | ||||
| lnHOMA-IR | −0.054 (−0.124 to 0.016) | =0.1292 | −0.198 (−0.264 to −0.129) | <0.0001 | 0.182 (0.097 to 0.264) | <0.0001 |
| QUICKI | 0.035 (−0.035 to 0.105) | =0.3282 | 0.181 (0.112 to 0.248) | <0.0001 | −0.184 (−0.266 to −0.099) | <0.0001 |
| METS-IR | −0.013 (−0.083 to 0.058) | =0.7256 | −0.389 (−0.447 to −0.327) | <0.0001 | 0.290 (0.208 to 0.366) | <0.0001 |
| TyG | −0.045 (−0.115 to 0.025) | =0.2067 | −0.384 (−0.442 to −0.322) | <0.0001 | 0.175 (0.090 to 0.257) | <0.0001 |
| TyG-BMI | −0.036 (−0.106 to 0.035) | =0.3194 | −0.417 (−0.473 to −0.357) | <0.0001 | 0.308 (0.227 to 0.384) | <0.0001 |
| TyG-WC | −0.052 (−0.122 to 0.018) | =0.1476 | −0.483 (−0.534 to −0.427) | <0.0001 | 0.296 (0.215 to 0.372) | <0.0001 |
| TyG-WHtR | −0.054 (−0.123 to 0.017) | =0.1357 | −0.501 (−0.552 to −0.446) | <0.0001 | 0.337 (0.258 to 0.411) | <0.0001 |
| lnTyG-HDL | 0.021 (−0.049 to 0.091) | =0.5601 | −0.301 (−0.363 to −0.236) | <0.0001 | 0.111 (0.025 to 0.195) | =0.0115 |
| lnLAP | −0.061 (−0.131 to 0.009) | =0.0891 | −0.470 (−0.523 to −0.413) | <0.0001 | 0.262 (0.179 to 0.341) | <0.0001 |
| lnVAI | 0.013 (−0.057 to 0.083) | =0.7192 | −0.355 (−0.415 to −0.292) | <0.0001 | 0.171 (0.086 to 0.253) | <0.0001 |
| e’ (n = 515) | PWV | GFR | ||||
| lnHOMA-IR | −0.219 (−0.299 to −0.135) | <0.0001 | 0.149 (0.080 to 0.217) | <0.0001 | −0.253 (−0.318 to −0.186) | <0.0001 |
| QUICKI | 0.205 (0.121 to 0.286) | <0.0001 | −0.127 (−0.196 to −0.057) | = 0.0004 | 0.235 (0.167 to 0.300) | < 0.0001 |
| METS-IR | −0.449 (−0.515 to −0.377) | <0.0001 | 0.250 (0.183 to 0.314) | <0.0001 | −0.322 (−0.384 to −0.258) | <0.0001 |
| TyG | −0.430 (−0.497 to −0.357) | <0.0001 | 0.353 (0.290 to 0.413) | <0.0001 | −0.346 (−0.406 to −0.282) | <0.0001 |
| TyG-BMI | −0.475 (−0.539 to −0.405) | <0.0001 | 0.279 (0.213 to 0.343) | <0.0001 | −0.347 (−0.407 to −0.283) | <0.0001 |
| TyG-WC | −0.533 (−0.591 to −0.467) | <0.0001 | 0.379 (0.317 to 0.437) | <0.0001 | −0.404 (−0.461 to −0.344) | <0.0001 |
| TyG-WHtR | −0.555 (−0.612 to −0.492) | <0.0001 | 0.385 (0.323 to 0.443) | <0.0001 | −0.412 (−0.468 to −0.352) | <0.0001 |
| lnTyG-HDL | −0.355 (−0.428 to −0.277) | <0.0001 | 0.240 (0.173 to 0.305) | <0.0001 | −0.256 (−0.320 to −0.189) | <0.0001 |
| lnLAP | −0.499 (−0.562 to −0.430) | <0.0001 | 0.368 (0.305 to 0.427) | <0.0001 | −0.417 (−0.474 to −0.357) | <0.0001 |
| lnVAI | −0.407 (−0.476 to −0.332) | <0.0001 | 0.266 (0.199 to 0.330) | <0.0001 | −0.299 (−0.362 to −0.234) | <0.0001 |
| OR (95%CI) | p-Value | OR (95%CI) | p-Value | OR (95%CI) | p-Value | |
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||||
| HOMA-IR vs. | All Participants (n = 779) | |||||
| LVH-BSA | 1.219 (1.029–1.439) | =0.0192 | 1.261 (1.055–1.506) | =0.0099 | 1.260 (1.055–1.506) | =0.0100 |
| Concentricity | 1.189 (1.018–1.392) | =0.0281 | 1.199 (1.020–1.411) | =0.0272 | 1.199 (1.020–1.412) | =0.0268 |
| Incr PWV | 1.197 (0.994–1.442) | =0.0557 | 1.147 (0.947–1.391) | =0.1574 | 1.153 (0.951–1.397) | =0.1426 |
| Decr GFR | 1.272 (1.075–1.513) | =0.0054 | 1.374 (1.148–1.655) | =0.0006 | 1.367 (1.141–1.647) | =0.0008 |
| QUICKI vs. | ||||||
| LVH-BSA | 0.772 (0.622–0.952) | =0.0169 | 0.737 (0.587–0.919) | =0.0073 | 0.737 (0.587–0.919) | =0.0073 |
| Concentricity | 0.813 (0.676–0.974) | =0.0262 | 0.802 (0.661–0.970) | =0.0240 | 0.800 (0.660–0.967) | =0.0224 |
| Incr PWV | 0.829 (0.655–1.045) | =0.1149 | 0.874 (0.680–1.120) | =0.2872 | 0.865 (0.674–1.108) | =0.2526 |
| Decr GFR | 0.739 (0.597–0.909) | =0.0047 | 0.660 (0.525–0.824) | =0.0003 | 0.668 (0.532–0.834) | =0.0004 |
| HOMA-IR vs. | Participants without DM (n = 669) | |||||
| LVH-BSA | 1.306 (1.092–1.564) | =0.0032 | 1.343 (1.114–1.627) | =0.0019 | 1.342 (1.113–1.625) | =0.0019 |
| Concentricity | 1.205 (1.017–1.426) | =0.0284 | 1.225 (1.026–1.463) | =0.0230 | 1.227 (1.028–1.464) | =0.0217 |
| Incr PWV | 1.110 (0.873–1.364) | =0.3468 | 1.040 (0.802–1.299) | =0.7448 | 1.048 (0.809–1.309) | =0.6929 |
| Decr GFR | 1.290 (1.076–1.552) | =0.0060 | 1.407 (1.160–1.718) | =0.0006 | 1.398 (1.151–1.709) | =0.0008 |
| QUICKI vs. | ||||||
| LVH-BSA | 0.683 (0.537–0.863) | =0.0017 | 0.657 (0.510–0.839) | =0.0009 | 0.654 (0.508–0.835) | =0.0008 |
| Concentricity | 0.823 (0.674–1.003) | =0.0549 | 0.814 (0.660–0.999) | =0.0508 | 0.810 (0.657–0.995) | =0.0464 |
| Incr PWV | 0.899 (0.686–1.177) | =0.4376 | 0.976 (0.729–1.308) | =0.8712 | 0.965 (0.721–1.293) | =0.8112 |
| Decr GFR | 0.744 (0.587–0.937) | =0.0130 | 0.663 (0.514–0.849) | =0.0013 | 0.669 (0.519–0.857) | =0.0017 |
| HOMA-IR vs. | NT Participants + HT Participants with Controlled BP (n = 505) | |||||
| LVH-BSA | 1.205 (0.959–1.490) | =0.0892 | 1.215 (0.955–1.520) | =0.0934 | 1.204 (0.949–1.504) | =0.1059 |
| Concentricity | 1.267 (1.039–1.547) | =0.0176 | 1.252 (1.021–1.535) | =0.0278 | 1.249 (1.020–1.531) | =0.0294 |
| Incr PWV | 1.195 (0.898–1.546) | =0.1865 | 1.119 (0.823–1.468) | =0.4343 | 1.124 (0.827–1.473) | =0.4167 |
| Decr GFR | 1.426 (1.152–1.779) | =0.0011 | 1.626 (1.295–2.072) | <0.0001 | 1.619 (1.288–2.065) | <0.0001 |
| QUICKI vs. | ||||||
| LVH-BSA | 0.796 (0.590–1.065) | =0.1258 | 0.775 (0.562–1.058) | =0.1126 | 0.774 (0.563–1.055) | =0.1095 |
| Concentricity | 0.763 (0.596–0.971) | =0.0298 | 0.771 (0.597–0.989) | =0.0431 | 0.756 (0.585–0.971) | =0.0302 |
| Incr PWV | 0.777 (0.518–1.149) | =0.2114 | 0.905 (0.590–1.377) | =0.6443 | 0.881 (0.575–1.337) | =0.5538 |
| Decr GFR | 0.776 (0.580–1.031) | =0.0844 | 0.654 (0.473–0.891) | =0.0083 | 0.667 (0.484–0.908) | =0.0114 |
| Wald Χ2 | p-Value | Wald Χ2 | p-Value | Wald Χ2 | p-Value | |
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||||
| LVH-BSA vs. | ||||||
| HOMA-IR | 5.48 | =0.0192 | 6.65 | =0.0099 | 6.63 | =0.0100 |
| Age | 12.72 | =0.0004 | 13.17 | =0.0003 | 13.62 | =0.0002 |
| BP * | 13.31 | =0.0003 | 11.65 | =0.0006 | 11.70 | =0.0006 |
| HR | 6.05 | =0.0139 | 5.86 | =0.0155 | 5.93 | =0.0148 |
| WC | — | — | −0.13 | =0.7225 | — | — |
| BMI | — | — | — | — | −0.12 | =0.7242 |
| Triglycerides | — | — | −3.45 | =0.0631 | −3.62 | =0.0571 |
| HDL cholesterol | — | — | −0.06 | =0.8104 | −0.05 | =0.8171 |
| LVH-BSA vs. | ||||||
| QUICKI | −5.71 | =0.0169 | −7.19 | =0.0073 | −7.20 | =0.0073 |
| Age | 13.06 | =0.0003 | 13.82 | =0.0002 | 14.17 | =0.0002 |
| BP * | 14.22 | =0.0002 | 12.63 | =0.0004 | 12.71 | =0.0004 |
| HR | −6.26 | =0.0123 | −6.00 | =0.0143 | −6.12 | =0.0134 |
| WC | — | — | −0.30 | =0.5852 | — | — |
| BMI | — | — | — | — | −0.32 | =0.5720 |
| Triglycerides | — | — | −3.38 | =0.0660 | −3.61 | =0.0573 |
| HDL cholesterol | — | — | −0.01 | =0.9088 | −0.01 | =0.9166 |
| Concentricity vs. | ||||||
| HOMA-IR | 4.82 | =0.0281 | 4.88 | =0.0272 | 4.90 | =0.0268 |
| Age | 11.51 | =0.0007 | 10.96 | =0.0009 | 11.77 | =0.0006 |
| WC | — | −0.13 | =0.7225 | — | — | |
| BMI | — | — | — | — | −0.34 | =0.5621 |
| Triglycerides | −0.96 | =0.3265 | −1.00 | =0.3168 | −0.96 | |
| HDL cholesterol | — | — | −0.22 | =0.6357 | −0.26 | =0.6109 |
| Concentricity vs. | ||||||
| QUICKI | −4.94 | =0.0262 | −5.09 | =0.0240 | −5.21 | =0.0224 |
| Age | 11.88 | =0.0006 | 11.58 | =0.0007 | 12.31 | =0.0004 |
| WC | — | −0.27 | =0.6013 | — | — | |
| BMI | — | — | — | — | −0.58 | =0.4449 |
| Triglycerides | −0.88 | =0.3467 | −0.94 | =0.3316 | ||
| HDL cholesterol | — | — | −0.11 | =0.7376 | −0.13 | =0.7151 |
| Incr PWV vs. | ||||||
| HOMA-IR | 3.66 | =0.0557 | 2.00 | =0.1574 | 2.15 | =0.1426 |
| Age | 65.59 | <0.0001 | 60.85 | <0.0001 | 63.87 | <0.0001 |
| BP * | 20.11 | <0.0001 | 15.78 | <0.0001 | 15.88 | <0.0001 |
| HR | 8.14 | =0.0043 | 6.45 | =0.0111 | 6.63 | =0.0100 |
| WC | — | — | 0.32 | =0.5716 | — | — |
| BMI | — | — | — | — | 0.01 | =0.9165 |
| Triglycerides | — | — | 0.17 | =0.6793 | 0.20 | =0.6563 |
| HDL cholesterol | — | — | −0.16 | =0.6918 | −0.25 | =0.6172 |
| Incr PWV vs. | ||||||
| QUICKI | −2.48 | =0.1149 | −1.13 | =0.2872 | −1.31 | =0.2526 |
| Age | 66.19 | <0.0001 | 61.09 | <0.0001 | 64.11 | <0.0001 |
| BP * | 20.17 | <0.0001 | 15.47 | <0.0001 | 15.59 | <0.0001 |
| HR | 7.83 | =0.0051 | 6.32 | =0.0119 | 6.46 | =0.0110 |
| WC | — | — | 0.27 | =0.5999 | — | — |
| BMI | — | — | — | — | 0.002 | =0.9658 |
| Triglycerides | — | — | 0.21 | =0.6449 | 0.24 | =0.6264 |
| HDL cholesterol | — | — | −0.14 | =0.7062 | −0.22 | =0.6368 |
| Decr GFR vs. | ||||||
| HOMA-IR | 7.74 | =0.0054 | 11.75 | =0.0006 | 11.23 | =0.0008 |
| Age | 41.26 | <0.0001 | 52.07 | <0.0001 | 49.59 | <0.0001 |
| WC | — | — | −4.56 | =0.0327 | — | — |
| BMI | — | — | — | — | −1.77 | =0.1839 |
| Triglycerides | — | — | −3.48 | =0.0621 | −4.32 | =0.0377 |
| HDL cholesterol | — | — | −0.05 | =0.8311 | 0.001 | =0.9959 |
| Decr GFR vs. | ||||||
| QUICKI | −8.00 | =0.0047 | −13.08 | =0.0003 | −12.40 | =0.0004 |
| Age | 41.74 | <0.0001 | 53.11 | <0.0001 | 50.31 | <0.0001 |
| WC | — | — | −5.69 | =0.0171 | — | — |
| BMI | — | — | — | — | −2.56 | =0.1096 |
| Triglycerides | — | — | −3.23 | =0.0724 | −4.13 | =0.0420 |
| HDL cholesterol | — | — | 0.001 | =0.9807 | 0.049 | =0.8242 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woodiwiss, A.J.; Norton, G.R.; Libhaber, C.D.; Sareli, P.; Dessein, P.H.C. Differential Association Between Ten Indices of Insulin Resistance and End-Organ Damage in a Community of African Ancestry in Africa. J. Clin. Med. 2025, 14, 2703. https://doi.org/10.3390/jcm14082703
Woodiwiss AJ, Norton GR, Libhaber CD, Sareli P, Dessein PHC. Differential Association Between Ten Indices of Insulin Resistance and End-Organ Damage in a Community of African Ancestry in Africa. Journal of Clinical Medicine. 2025; 14(8):2703. https://doi.org/10.3390/jcm14082703
Chicago/Turabian StyleWoodiwiss, Angela J., Gavin R. Norton, Carlos D. Libhaber, Pinhas Sareli, and Patrick H. C. Dessein. 2025. "Differential Association Between Ten Indices of Insulin Resistance and End-Organ Damage in a Community of African Ancestry in Africa" Journal of Clinical Medicine 14, no. 8: 2703. https://doi.org/10.3390/jcm14082703
APA StyleWoodiwiss, A. J., Norton, G. R., Libhaber, C. D., Sareli, P., & Dessein, P. H. C. (2025). Differential Association Between Ten Indices of Insulin Resistance and End-Organ Damage in a Community of African Ancestry in Africa. Journal of Clinical Medicine, 14(8), 2703. https://doi.org/10.3390/jcm14082703






