Milestones in Mandibular Bone Tissue Engineering: A Systematic Review of Large Animal Models and Critical-Sized Defects
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Databases and Search Criteria
2.4. Data Collection Process
2.5. Data Extraction
2.6. Quality and Risk of Bias Assessment
3. Results
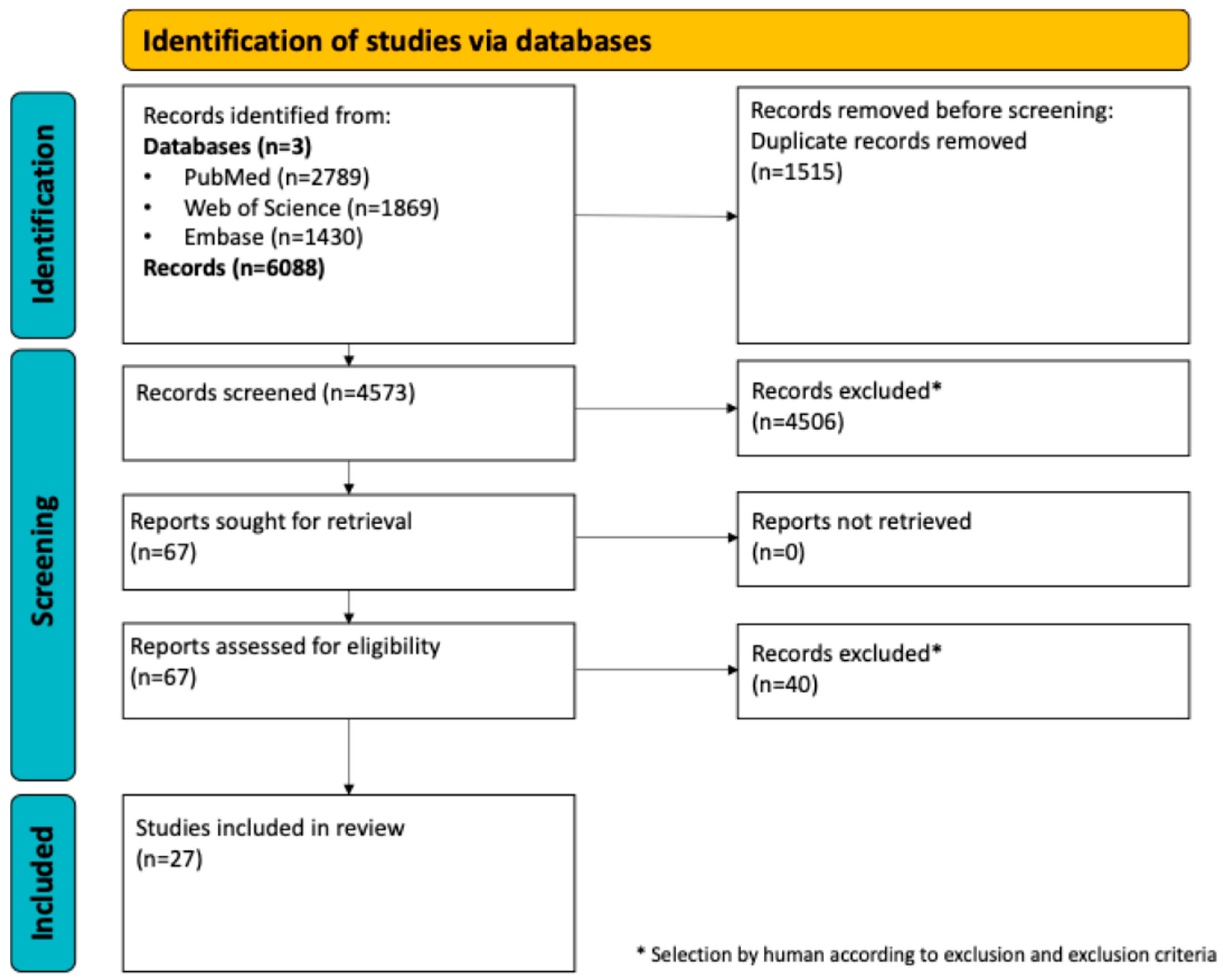
3.1. Search Results
3.2. Study Design, Animal Model, and Defect Characteristics
3.3. Bone Tissue Engineering Strategies
3.4. Outcome Measures
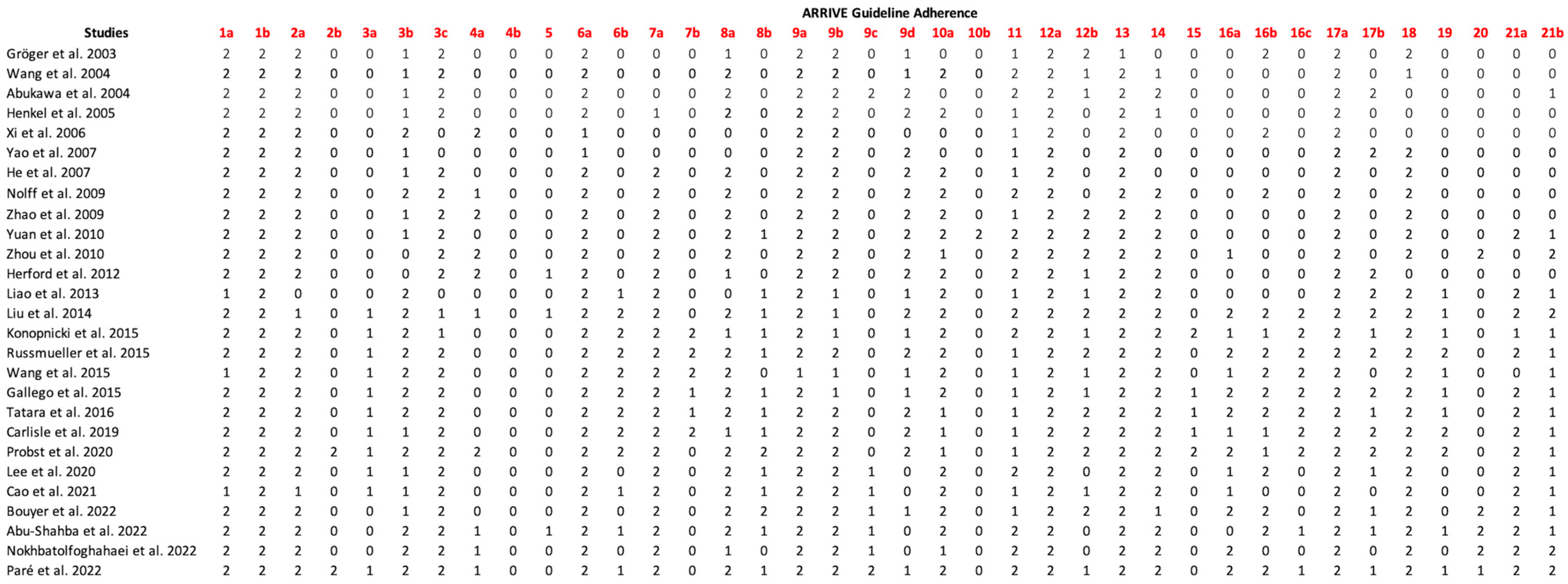
3.5. Assessment of Adherence to the ARRIVE Guidelines
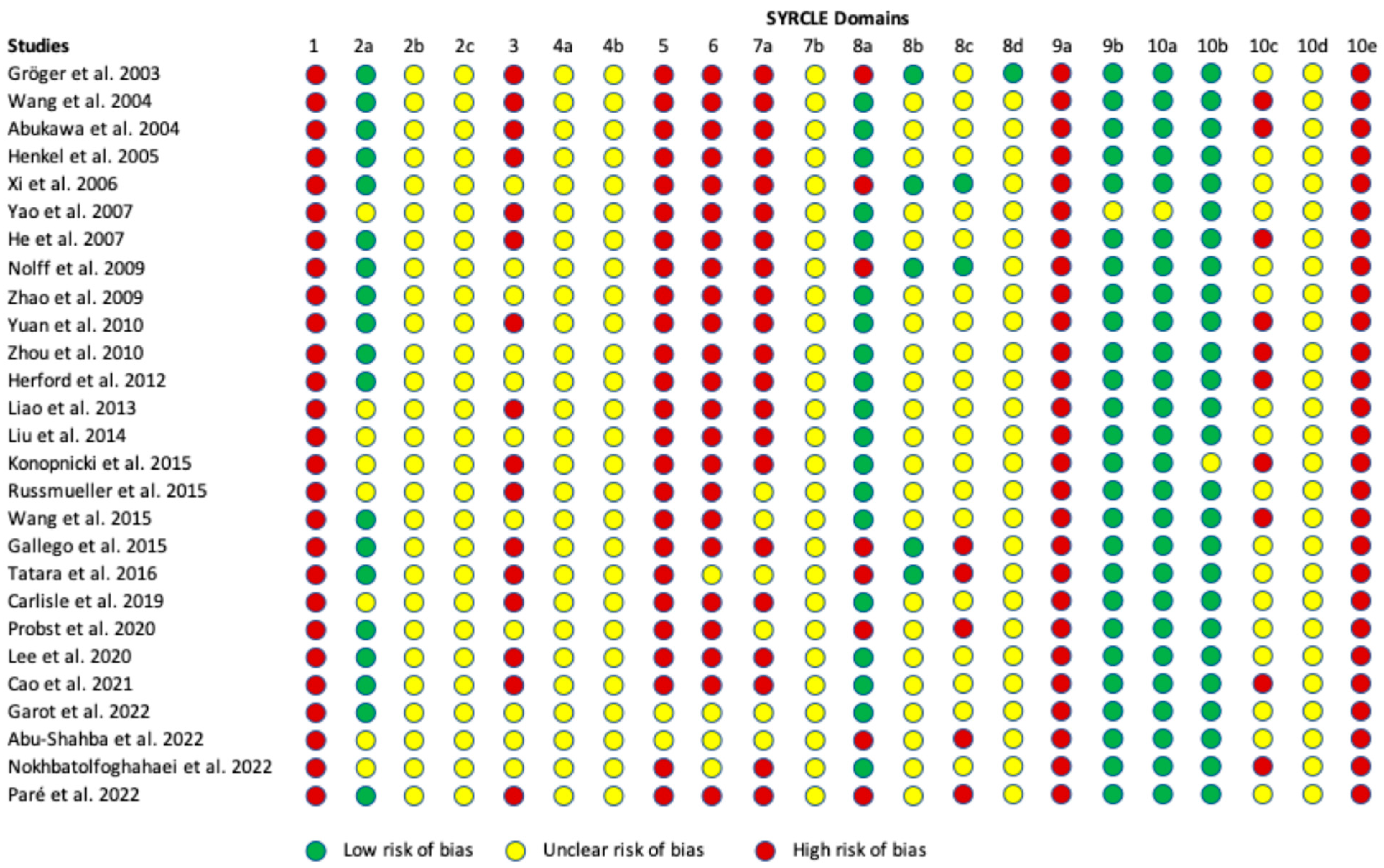
3.6. Risk of Bias Assessment
3.7. Conclusions Drawn by the Included Studies
4. Discussion
4.1. Historical Perspective: The Evolution of Mandibular Bone Tissue Engineering
4.1.1. Early Efforts and Initial Challenges
4.1.2. Integration of Cellular Components
4.1.3. Emergence of Growth Factor Strategies
4.1.4. Technological Advancements
4.2. Current State of Mandibular Bone Tissue Engineering
4.2.1. Key Achievements
4.2.2. Persistent Challenges
4.2.3. Animal Models
4.3. Future Outlook and Directions
4.3.1. Technological Innovations
4.3.2. Translational and Clinical Applications
4.3.3. Interdisciplinary Approaches
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chandu, A.; Sun, K.C.V.; DeSilva, R.N.; Smith, A.C.H. The Assessment of Quality of Life in Patients Who Have Undergone Surgery for Oral Cancer: A Preliminary Report. J. Oral Maxillofac. Surg. 2005, 63, 1606–1612. [Google Scholar] [CrossRef] [PubMed]
- Molteni, G.; Gazzini, L.; Sacchetto, A.; Nocini, R.; Comini, L.V.; Arietti, V.; Locatello, L.G.; Mannelli, G. Mandibular Reconstruction in Head and Neck Cancer: Which Is the Gold Standard? Eur. Arch. Otorhinolaryngol. 2023, 280, 3953–3965. [Google Scholar] [CrossRef]
- McGovern, J.A.; Griffin, M.; Hutmacher, D.W. Animal Models for Bone Tissue Engineering and Modelling Disease. Dis. Models Mech. 2018, 11, dmm033084. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.-L.; Ottensmeyer, M.P.; Thamm, J.R.; Schmelzeisen, R.; Troulis, M.J.; Guastaldi, F.P.S. Increased Osteogenic Activity of Dynamic Cultured Composite Bone Scaffolds: Characterization and In Vitro Study. J. Oral Maxillofac. Surg. 2022, 80, 303–312. [Google Scholar] [CrossRef]
- Sakkas, A.; Schramm, A.; Winter, K.; Wilde, F. Risk Factors for Post-Operative Complications after Procedures for Autologous Bone Augmentation from Different Donor Sites. J. Cranio-Maxillofac. Surg. 2018, 46, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Emara, A.; Shah, R. Recent Update on Craniofacial Tissue Engineering. J. Tissue Eng. 2021, 12, 204173142110037. [Google Scholar] [CrossRef]
- Deng, Y.; Jiang, C.; Li, C.; Li, T.; Peng, M.; Wang, J.; Dai, K. 3D Printed Scaffolds of Calcium Silicate-Doped β-TCP Synergize with Co-Cultured Endothelial and Stromal Cells to Promote Vascularization and Bone Formation. Sci. Rep. 2017, 7, 5588. [Google Scholar] [CrossRef]
- Kang, Y.; Kim, S.; Fahrenholtz, M.; Khademhosseini, A.; Yang, Y. Osteogenic and Angiogenic Potentials of Monocultured and Co-Cultured Human-Bone-Marrow-Derived Mesenchymal Stem Cells and Human-Umbilical-Vein Endothelial Cells on Three-Dimensional Porous Beta-Tricalcium Phosphate Scaffold. Acta Biomater. 2013, 9, 4906–4915. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, N.; Liang, H.; Tan, B.; Huang, F.; Hu, H.; Chen, Y.; Wang, G.; Ling, Z.; Liu, C.; et al. Bone Tissue Engineering Scaffolds with HUVECs/hBMSCs Cocultured on 3D-Printed Composite Bioactive Ceramic Scaffolds Promoted Osteogenesis/Angiogenesis. J. Orthop. Transl. 2022, 37, 152–162. [Google Scholar] [CrossRef]
- Rossi, N.; Hadad, H.; Bejar-Chapa, M.; Peretti, G.M.; Randolph, M.A.; Redmond, R.W.; Guastaldi, F.P.S. Bone Marrow Stem Cells with Tissue-Engineered Scaffolds for Large Bone Segmental Defects: A Systematic Review. Tissue Eng. Part B Rev. 2023, 29, 457–472. [Google Scholar] [CrossRef]
- Bi, M.; Yang, K.; Yu, T.; Wu, G.; Li, Q. Cell-Based Mechanisms and Strategies of Co-Culture System Both in Vivo and Vitro for Bone Tissue Engineering. Biomed. Pharmacother. 2023, 169, 115907. [Google Scholar] [CrossRef] [PubMed]
- Simunovic, F.; Finkenzeller, G. Vascularization Strategies in Bone Tissue Engineering. Cells 2021, 10, 1749. [Google Scholar] [CrossRef]
- Unger, R.E.; Ghanaati, S.; Orth, C.; Sartoris, A.; Barbeck, M.; Halstenberg, S.; Motta, A.; Migliaresi, C.; Kirkpatrick, C.J. The Rapid Anastomosis between Prevascularized Networks on Silk Fibroin Scaffolds Generated in Vitro with Cocultures of Human Microvascular Endothelial and Osteoblast Cells and the Host Vasculature. Biomaterials 2010, 31, 6959–6967. [Google Scholar] [CrossRef]
- Nauth, A.; Schemitsch, E.; Norris, B.; Nollin, Z.; Watson, J.T. Critical-Size Bone Defects: Is There a Consensus for Diagnosis and Treatment? J. Orthop. Trauma 2018, 32, S7–S11. [Google Scholar] [CrossRef]
- Schemitsch, E.H. Size Matters: Defining Critical in Bone Defect Size! J. Orthop. Trauma 2017, 31, S20–S22. [Google Scholar] [CrossRef]
- Schmitz, J.P.; Hollinger, J.O. The Critical Size Defect as an Experimental Model for Craniomandibulofacial Nonunions. Clin. Orthop. Relat. Res. 1986, 205, 299–308. [Google Scholar] [CrossRef]
- Marei, H.F.; Mahmood, K.; Almas, K. Critical Size Defects for Bone Regeneration Experiments in the Dog Mandible: A Systematic Review. Implant. Dent. 2018, 27, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Dalfino, S.; Savadori, P.; Piazzoni, M.; Connelly, S.T.; Giannì, A.B.; Del Fabbro, M.; Tartaglia, G.M.; Moroni, L. Regeneration of Critical-Sized Mandibular Defects Using 3D-Printed Composite Scaffolds: A Quantitative Evaluation of New Bone Formation in In Vivo Studies. Adv. Healthc. Mater. 2023, 12, 2300128. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Chen, X.; Xu, Y.; Shi, L.; Zhang, M.; Nie, M.; Liu, X. Significance and Considerations of Establishing Standardized Critical Values for Critical Size Defects in Animal Models of Bone Tissue Regeneration. Heliyon 2024, 10, e33768. [Google Scholar] [CrossRef]
- Moher, D. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Intern. Med. 2009, 151, 264. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]
- Schwarz, F.; Iglhaut, G.; Becker, J. Quality Assessment of Reporting of Animal Studies on Pathogenesis and Treatment of Peri-implant Mucositis and Peri-implantitis. A Systematic Review Using the ARRIVE Guidelines. J. Clin. Periodontol. 2012, 39, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Hooijmans, C.R.; Rovers, M.M.; De Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s Risk of Bias Tool for Animal Studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Nolff, M.C.; Gellrich, N.-C.; Hauschild, G.; Fehr, M.; Bormann, K.-H.; Rohn, K.; Spalthoff, S.; Rücker, M.; Kokemüller, H. Comparison of Two Beta-Tricalcium Phosphate Composite Grafts Used for Reconstruction of Mandibular Critical Size Bone Defects. Vet. Comp. Orthop. Traumatol. 2009, 22, 96–102. [Google Scholar] [PubMed]
- Zhao, J.; Zhang, Z.; Wang, S.; Sun, X.; Zhang, X.; Chen, J.; Kaplan, D.L.; Jiang, X. Apatite-Coated Silk Fibroin Scaffolds to Healing Mandibular Border Defects in Canines. Bone 2009, 45, 517–527. [Google Scholar] [CrossRef]
- Abukawa, H.; Shin, M.; Williams, W.B.; Vacanti, J.P.; Kaban, L.B.; Troulis, M.J. Reconstruction of Mandibular Defects with Autologous Tissue-Engineered Bone. J. Oral Maxillofac. Surg. 2004, 62, 601–606. [Google Scholar] [CrossRef]
- Gallego, L.; Pérez-Basterrechea, M.; García-Consuegra, L.; Álvarez-Viejo, M.; Megías, J.; Novoa, A.; Costilla, S.; Meana, Á.; Junquera, L. Repair of Segmental Mandibular Bone Defects in Sheep Using Bone Marrow Stromal Cells and Autologous Serum Scaffold: A Pilot Study. J. Clin. Periodontol. 2015, 42, 1143–1151. [Google Scholar] [CrossRef]
- Bouyer, M.; Garot, C.; Machillot, P.; Vollaire, J.; Fitzpatrick, V.; Morand, S.; Boutonnat, J.; Josserand, V.; Bettega, G.; Picart, C. 3D-Printed Scaffold Combined to 2D Osteoinductive Coatings to Repair a Critical-Size Mandibular Bone Defect. Mater. Today Bio 2021, 11, 100113. [Google Scholar] [CrossRef]
- Carlisle, P.; Guda, T.; Silliman, D.T.; Burdette, A.J.; Talley, A.D.; Alvarez, R.; Tucker, D.; Hale, R.G.; Guelcher, S.A.; BrownBaer, P.R. Localized Low-dose rhBMP-2 Is Effective at Promoting Bone Regeneration in Mandibular Segmental Defects. J. Biomed. Mater. Res. 2019, 107, 1491–1503. [Google Scholar] [CrossRef]
- Gröger, A.; Kläring, S.; Merten, H.-A.; Holste, J.; Kaps, C.; Sittinger, M. Tissue Engineering of Bone for Mandibular Augmentation in Immunocompetent Minipigs: Preliminary Study. Scand. J. Plast. Reconstr. Surg. Hand Surg. 2003, 37, 129–133. [Google Scholar] [CrossRef]
- Henkel, K.-O.; Gerber, T.; Dörfling, P.; Gundlach, K.K.H.; Bienengräber, V. Repair of Bone Defects by Applying Biomatrices with and without Autologous Osteoblasts. J. Cranio-Maxillofac. Surg. 2005, 33, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Konopnicki, S.; Sharaf, B.; Resnick, C.; Patenaude, A.; Pogal-Sussman, T.; Hwang, K.-G.; Abukawa, H.; Troulis, M.J. Tissue-Engineered Bone With 3-Dimensionally Printed β-Tricalcium Phosphate and Polycaprolactone Scaffolds and Early Implantation: An In Vivo Pilot Study in a Porcine Mandible Model. J. Oral Maxillofac. Surg. 2015, 73, 1016.e1–1016.e11. [Google Scholar] [CrossRef]
- Liao, H.-T.; Chen, J.-P.; Lee, M.-Y. Bone Tissue Engineering with Adipose-Derived Stem Cells in Bioactive Composites of Laser-Sintered Porous Polycaprolactone Scaffolds and Platelet-Rich Plasma. Materials 2013, 6, 4911–4929. [Google Scholar] [CrossRef]
- Probst, F.A.; Fliefel, R.; Burian, E.; Probst, M.; Eddicks, M.; Cornelsen, M.; Riedl, C.; Seitz, H.; Aszódi, A.; Schieker, M.; et al. Bone Regeneration of Minipig Mandibular Defect by Adipose Derived Mesenchymal Stem Cells Seeded Tri-Calcium Phosphate-Poly(D,L-Lactide-Co-Glycolide) Scaffolds. Sci. Rep. 2020, 10, 2062. [Google Scholar] [CrossRef]
- Wang, H.; Springer, I.N.G.; Schildberg, H.; Acil, Y.; Ludwig, K.; Rueger, D.R.; Terheyden, H. Carboxymethylcellulose-stabilized Collagenous rhOP-1 Device—A Novel Carrier Biomaterial for the Repair of Mandibular Continuity Defects. J. Biomed. Mater. Res. 2004, 68A, 219–226. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhang, Z.; Zhu, H.; Qiu, W.; Jiang, X.; Guo, W. Experimental Study on Reconstruction of Segmental Mandible Defects Using Tissue Engineered Bone Combined Bone Marrow Stromal Cells With Three-Dimensional Tricalcium Phosphate. J. Craniofac. Surg. 2007, 18, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Choi, D.; Shim, J.-H.; Nam, W. Efficacy of Three-Dimensionally Printed Polycaprolactone/Beta Tricalcium Phosphate Scaffold on Mandibular Reconstruction. Sci. Rep. 2020, 10, 4979. [Google Scholar] [CrossRef]
- Liu, C.; Tan, X.; Luo, J.; Liu, H.; Hu, M.; Yue, W. Reconstruction of Beagle Hemi-Mandibular Defects with Allogenic Mandibular Scaffolds and Autologous Mesenchymal Stem Cells. PLoS ONE 2014, 9, e105733. [Google Scholar] [CrossRef]
- Nokhbatolfoghahaei, H.; Bastami, F.; Farzad-Mohajeri, S.; Rezai Rad, M.; Dehghan, M.M.; Bohlouli, M.; Farajpour, H.; Nadjmi, N.; Khojasteh, A. Prefabrication Technique by Preserving a Muscular Pedicle from Masseter Muscle as an in Vivo Bioreactor for Reconstruction of Mandibular Critical-sized Bone Defects in Canine Models. J. Biomed. Mater. Res. 2022, 110, 1675–1686. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, J.; Zhang, W.; Ye, D.; Zhang, X.; Zou, D.; Zhang, X.; Sun, X.; Sun, S.; Zhang, W.; et al. Comprehensive Evaluation of Cryopreserved Bone-Derived Osteoblasts for the Repair of Segmental Mandibular Defects in Canines. Clin. Implant. Dent. Relat. Res. 2015, 17, 798–810. [Google Scholar] [CrossRef]
- Yao, J.F.; Zhang, Y.Z.; Bao, C.Y.; Sun, L.Y.; Hao, X.M.; Fan, H.S.; Zhang, X.D. Reconstructing Box-Like Bone Defect of Mandible with In Vivo Tissue Engineering Bone. Key Eng. Mater. 2007, 330–332, 1165–1168. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, W.J.; Liu, G.; Wei, M.; Qi, Z.L.; Liu, W.; Cui, L.; Cao, Y.L. Repair of Canine Mandibular Bone Defects with Bone Marrow Stromal Cells and Coral. Tissue Eng. Part A 2010, 16, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- Abu-Shahba, A.G.; Wilkman, T.; Kornilov, R.; Adam, M.; Salla, K.M.; Lindén, J.; Lappalainen, A.K.; Björkstrand, R.; Seppänen-Kaijansinkko, R.; Mannerström, B. Periosteal Flaps Enhance Prefabricated Engineered Bone Reparative Potential. J. Dent. Res. 2022, 101, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Paré, A.; Charbonnier, B.; Veziers, J.; Vignes, C.; Dutilleul, M.; De Pinieux, G.; Laure, B.; Bossard, A.; Saucet-Zerbib, A.; Touzot-Jourde, G.; et al. Standardized and Axially Vascularized Calcium Phosphate-Based Implants for Segmental Mandibular Defects: A Promising Proof of Concept. Acta Biomater. 2022, 154, 626–640. [Google Scholar] [CrossRef]
- Russmueller, G.; Moser, D.; Spassova, E.; Plasenzotti, R.; Poeschl, P.W.; Seemann, R.; Becker, S.; Pirklbauer, K.; Eder-Czembirek, C.; Czembirek, C.; et al. Tricalcium Phosphate-Based Biocomposites for Mandibular Bone Regeneration—A Histological Study in Sheep. J. Cranio-Maxillofac. Surg. 2015, 43, 696–704. [Google Scholar] [CrossRef]
- Tatara, A.M.; Koons, G.L.; Watson, E.; Piepergerdes, T.C.; Shah, S.R.; Smith, B.T.; Shum, J.; Melville, J.C.; Hanna, I.A.; Demian, N.; et al. Biomaterials-Aided Mandibular Reconstruction Using in Vivo Bioreactors. Proc. Natl. Acad. Sci. USA 2019, 116, 6954–6963. [Google Scholar] [CrossRef]
- Cao, S.; Li, S.; Geng, Y.; Kapat, K.; Liu, S.; Perera, F.H.; Li, Q.; Terheyden, H.; Wu, G.; Che, Y.; et al. Prefabricated 3D-Printed Tissue-Engineered Bone for Mandibular Reconstruction: A Preclinical Translational Study in Primate. ACS Biomater. Sci. Eng. 2021, 7, 5727–5738. [Google Scholar] [CrossRef]
- Herford, A.S.; Lu, M.; Buxton, A.N.; Kim, J.; Henkin, J.; Boyne, P.J.; Caruso, J.M.; Rungcharassaeng, K.; Hong, J. Recombinant Human Bone Morphogenetic Protein 2 Combined With an Osteoconductive Bulking Agent for Mandibular Continuity Defects in Nonhuman Primates. J. Oral Maxillofac. Surg. 2012, 70, 703–716. [Google Scholar] [CrossRef]
- Zhou, M.; Peng, X.; Mao, C.; Xu, F.; Hu, M.; Yu, G. Primate Mandibular Reconstruction with Prefabricated, Vascularized Tissue-Engineered Bone Flaps and Recombinant Human Bone Morphogenetic Protein-2 Implanted in Situ. Biomaterials 2010, 31, 4935–4943. [Google Scholar] [CrossRef]
- Xi, Q.; Bu, R.-F.; Liu, H.-C.; Mao, T.-Q. Reconstruction of Caprine Mandibular Segmental Defect by Tissue Engineered Bone Reinforced by Titanium Reticulum. Chin. J. Traumatol. 2006, 9, 67–71. [Google Scholar]
- Basyuni, S.; Ferro, A.; Santhanam, V.; Birch, M.; McCaskie, A. Systematic Scoping Review of Mandibular Bone Tissue Engineering. Br. J. Oral Maxillofac. Surg. 2020, 58, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Fishero, B.; Kohli, N.; Das, A.; Christophel, J.; Cui, Q. Current Concepts of Bone Tissue Engineering for Craniofacial Bone Defect Repair. Craniomaxillofac. Trauma Reconstr. 2015, 8, 23–30. [Google Scholar] [CrossRef]
- Hatt, L.P.; Thompson, K.; Helms, J.A.; Stoddart, M.J.; Armiento, A.R. Clinically Relevant Preclinical Animal Models for Testing Novel Cranio-maxillofacial Bone 3D-printed Biomaterials. Clin. Transl. Med. 2022, 12, e690. [Google Scholar] [CrossRef] [PubMed]
- Hurley, C.M.; McConn Walsh, R.; Shine, N.P.; O’Neill, J.P.; Martin, F.; O’Sullivan, J.B. Current Trends in Craniofacial Reconstruction. Surgeon 2023, 21, e118–e125. [Google Scholar] [CrossRef]
- Sillmann, Y.M.; Monteiro, J.L.G.C.; Haugstad, M.; Burris, B.; Keith, D.A.; Handa, S.; Guastaldi, F.P.S. Intra-Articular Injection of Orthobiologics for Temporomandibular Joint Osteoarthritis: A Systematic Review of Randomized Controlled Trials. Int. J. Oral Maxillofac. Surg. 2025; Epub ahead of print. [Google Scholar] [CrossRef]
- Guastaldi, F.P.S. Bone Tissue Engineering: Bench to Bedside Using 3D Printing; Springer International Publishing AG: Cham, Switzerland, 2022; ISBN 978-3-030-92014-2. [Google Scholar]
- Guastaldi, F.P.S.; Matheus, H.R.; Faloni, A.P.D.S.; De Almeida-Filho, E.; Cominotte, M.A.; Moretti, L.A.C.; Verzola, M.H.A.; Marcantonio, E.; De Almeida, J.M.; Guastaldi, A.C.; et al. A New Multiphase Calcium Phosphate Graft Material Improves Bone Healing—An in Vitro and in Vivo Analysis. J. Biomed. Mater. Res. 2022, 110, 2686–2704. [Google Scholar] [CrossRef] [PubMed]
- Guastaldi, F.P.S.; Takusagawa, T.; Monteiro, J.L.G.C.; He, Y.; Ye, Q.; Troulis, M.J. 3D Printing for Oral and Maxillofacial Regeneration. In Bone Tissue Engineering; Guastaldi, F.P.S., Mahadik, B., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 93–119. ISBN 978-3-030-92013-5. [Google Scholar]
- Hadad, H.; Boos Lima, F.B.; Shirinbak, I.; Porto, T.S.; Chen, J.E.; Guastaldi, F.P. The Impact of 3D Printing on Oral and Maxillofacial Surgery. J. 3D Print. Med. 2023, 7, 3DP007. [Google Scholar] [CrossRef]
- Matheus, H.R.; Hadad, H.; Guastaldi, F.P.S. The State of 3D Printing of Bioengineered Customized Maxillofacial Bone Scaffolds: A Narrative Review. Front. Oral Maxillofac. Med. 2024, 6, 5. [Google Scholar] [CrossRef]
- Bolander, M.E. Regulation of Fracture Repair by Growth Factors. Exp. Biol. Med. 1992, 200, 165–170. [Google Scholar] [CrossRef]
- Guillaume, O.; Geven, M.A.; Varjas, V.; Varga, P.; Gehweiler, D.; Stadelmann, V.A.; Smidt, T.; Zeiter, S.; Sprecher, C.; Bos, R.R.M.; et al. Orbital Floor Repair Using Patient Specific Osteoinductive Implant Made by Stereolithography. Biomaterials 2020, 233, 119721. [Google Scholar] [CrossRef]
- Lemperle, S.M.; Calhoun, C.J.; Curran, R.W.; Holmes, R.E. Bony Healing of Large Cranial and Mandibular Defects Protected from Soft-Tissue Interposition: A Comparative Study of Spontaneous Bone Regeneration, Osteoconduction, and Cancellous Autografting in Dogs. Plast. Reconstr. Surg. 1998, 101, 660–672. [Google Scholar] [CrossRef]
- Li, Y.; Chen, S.-K.; Li, L.; Qin, L.; Wang, X.-L.; Lai, Y.-X. Bone Defect Animal Models for Testing Efficacy of Bone Substitute Biomaterials. J. Orthop. Transl. 2015, 3, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Pilawski, I.; Tulu, U.S.; Ticha, P.; Schüpbach, P.; Traxler, H.; Xu, Q.; Pan, J.; Coyac, B.R.; Yuan, X.; Tian, Y.; et al. Interspecies Comparison of Alveolar Bone Biology, Part I: Morphology and Physiology of Pristine Bone. JDR Clin. Transl. Res. 2021, 6, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Spicer, P.P.; Kretlow, J.D.; Young, S.; Jansen, J.A.; Kasper, F.K.; Mikos, A.G. Evaluation of Bone Regeneration Using the Rat Critical Size Calvarial Defect. Nat. Protoc. 2012, 7, 1918–1929. [Google Scholar] [CrossRef]
- Aerssens, J.; Boonen, S.; Lowet, G.; Dequeker, J. Interspecies Differences in Bone Composition, Density, and Quality: Potential Implications for in Vivo Bone Research*. Endocrinology 1998, 139, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.; Nakamura, M.; Miclau, T.; Marcucio, R. Effects of Aging on Fracture Healing. Curr. Osteoporos. Rep. 2017, 15, 601–608. [Google Scholar] [CrossRef]
- Stegen, S.; Van Gastel, N.; Carmeliet, G. Bringing New Life to Damaged Bone: The Importance of Angiogenesis in Bone Repair and Regeneration. Bone 2015, 70, 19–27. [Google Scholar] [CrossRef]
- Wancket, L.M. Animal Models for Evaluation of Bone Implants and Devices: Comparative Bone Structure and Common Model Uses. Vet. Pathol. 2015, 52, 842–850. [Google Scholar] [CrossRef]
- Akhter, M.P.; Fan, Z.; Rho, J.Y. Bone Intrinsic Material Properties in Three Inbred Mouse Strains. Calcif. Tissue Int. 2004, 75, 416–420. [Google Scholar] [CrossRef]
- Begg, D.J.; Purdie, A.C.; De Silva, K.; Dhand, N.K.; Plain, K.M.; Whittington, R.J. Variation in Susceptibility of Different Breeds of Sheep to Mycobacterium Avium Subspecies Paratuberculosis Following Experimental Inoculation. Vet. Res. 2017, 48, 36. [Google Scholar] [CrossRef]
- Barnes, G.L.; Kostenuik, P.J.; Gerstenfeld, L.C.; Einhorn, T.A. Growth Factor Regulation of Fracture Repair. J. Bone Miner. Res. 1999, 14, 1805–1815. [Google Scholar] [CrossRef]
- Li, F.; Yu, F.; Liao, X.; Wu, C.; Wang, Y.; Li, C.; Lou, F.; Li, B.; Yin, B.; Wang, C.; et al. Efficacy of Recombinant Human BMP2 and PDGF-BB in Orofacial Bone Regeneration: A Systematic Review and Meta-Analysis. Sci. Rep. 2019, 9, 8073. [Google Scholar] [CrossRef] [PubMed]
- Haffner-Luntzer, M.; Kovtun, A.; Rapp, A.E.; Ignatius, A. Mouse Models in Bone Fracture Healing Research. Curr. Mol. Biol. Rep. 2016, 2, 101–111. [Google Scholar] [CrossRef]
- Monteiro, J.L.G.C.; Sillmann, Y.M.; Kambakhsh, T.M.; Bei, M.; Guastaldi, F.P.S. Molecular Mechanisms of Temporomandibular Joint Degeneration in Large Animal Models. Int. J. Oral Maxillofac. Surg. 2025; Epub ahead of print. [Google Scholar] [CrossRef]
- Sillmann, Y.M.; Monteiro, J.L.G.C.; Eber, P.; Baggio, A.M.P.; Peacock, Z.S.; Guastaldi, F.P.S. Empowering Surgeons: Will Artificial Intelligence Change Oral and Maxillofacial Surgery? Int. J. Oral Maxillofac. Surg. 2025, 54, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Eber, P.; Sillmann, Y.M.; Guastaldi, F.P.S. The Transformative Potential of Artificial Intelligence in Oral and Maxillofacial Surgery. J. Oral Maxillofac. Surg. 2025, 83, 402–405. [Google Scholar] [CrossRef]
- Gharehdaghi, N.; Nokhbatolfoghahaei, H.; Khojasteh, A. 4D Printing of Smart Scaffolds for Bone Regeneration: A Systematic Review. Biomed. Mater. 2025, 20, 012003. [Google Scholar] [CrossRef]
- Kolomenskaya, E.; Butova, V.; Poltavskiy, A.; Soldatov, A.; Butakova, M. Application of Artificial Intelligence at All Stages of Bone Tissue Engineering. Biomedicines 2023, 12, 76. [Google Scholar] [CrossRef]
- Mackay, B.S.; Marshall, K.; Grant-Jacob, J.A.; Kanczler, J.; Eason, R.W.; Oreffo, R.O.C.; Mills, B. The Future of Bone Regeneration: Integrating AI into Tissue Engineering. Biomed. Phys. Eng. Express 2021, 7, 052002. [Google Scholar] [CrossRef]
- Wu, Y.; Ding, X.; Wang, Y.; Ouyang, D. Harnessing the Power of Machine Learning into Tissue Engineering: Current Progress and Future Prospects. Burn. Trauma 2024, 12, tkae053. [Google Scholar] [CrossRef]
- De Carvalho, A.B.G.; Rahimnejad, M.; Oliveira, R.L.M.S.; Sikder, P.; Saavedra, G.S.F.A.; Bhaduri, S.B.; Gawlitta, D.; Malda, J.; Kaigler, D.; Trichês, E.S.; et al. Personalized Bioceramic Grafts for Craniomaxillofacial Bone Regeneration. Int. J. Oral Sci. 2024, 16, 62. [Google Scholar] [CrossRef]
- Rosa, A.; Ronsivalle, V.; Fiorillo, L.; Arcuri, C. Different Uses of Conscious Sedation for Managing Dental Anxiety During Third-Molar Extraction: Clinical Evidence and State of the Art. J. Craniofac. Surg. 2024, 35, 2524–2530. [Google Scholar] [CrossRef]
| Studies | Animal Model | Strain/Breed | Age (Years) | Gender | Weight | Size of Defect | Defect Location | Period of Analysis |
|---|---|---|---|---|---|---|---|---|
| Gröger et al., 2003 | Porcine | Gottingen Minipig | 5–6 months | N/A | 28–32 kg | 20 mm × 10 mm, box shape | Body of mandible | 90 and 180 days |
| Wang et al., 2004 | Porcine | Gottingen Minipig | 18 months | Female | Ø 43.5 kg | 5 cm, box shape | Body of mandible | 4, 8, and 12 weeks |
| Abukawa et al., 2004 | Porcine | Yucatan Minipig | 6 months | Female | Ø 25 kg | 2 cm × 2 cm, box shape | Body and ramus of mandible | 6 weeks |
| Henkel et al., 2005 | Porcine | Ellegard Gottingen Minipig | 1 year | N/A | Ø 27.4 kg | >5 cm3, box shape | Anterior mandible | 5 weeks |
| Xi et al., 2006 | Caprine | Guanzhong | 1 year | N/A | 15–20 kg | 25 mm, segmental defect | Body of mandible | 4, 8, and 16 weeks |
| Yao et al., 2007 | Canine | N/A | N/A | N/A | N/A | 20 mm × 10 mm, box shape | Body of mandible | 8 and 12 weeks |
| He et al., 2007 | Canine | Beagle | 1–2 years | N/A | 10–16 kg | 3 cm, segmental defect | Body of mandible | 1 and 3 months |
| Nolff et al., 2009 | Ovine | German Blackhead | 2–4 years (mean: 3.75) | Female | 72.5 ± 7.4 kg | 2.7 cm × 1.5 cm, triangular | Body of mandible | 12 weeks |
| Zhao et al., 2009 | Canine | Mongrel | 18 months | Male | 15–20 kg | 20 mm × 10 mm, box shape | Body of mandible | 1, 3, 6, and 12 months |
| Yuan et al., 2010 | Canine | Mongrel | 16 months | N/A | 19.7 kg | 3 cm, segmental | Body of mandible | 4, 12, 26, and 32 weeks |
| Zhou et al., 2010 | Primate | Rhesus | 6–9 years | Male | 6–12 kg | 20 mm × 10 mm × 15 mm (3 cm3) | Body of mandible | 26 weeks |
| Herford et al., 2012 | Primate | Rhesus Macaque | “Skeletally mature” | Male | N/A | 2.5 cm | Body of mandible | 6 months |
| Liao et al., 2013 | Porcine | N/A | N/A | N/A | N/A | 3 cm × 3 cm | Body of mandible | 6 months |
| Liu et al., 2014 | Canine | Beagle | 2 years | Female | 10 ± 3 kg | Full condylectomy | Body of mandible, ramus, and condyle | 4, 12, 24, and 48 weeks |
| Konopnicki et al., 2015 | Porcine | Yucatan Minipig | N/A | N/A | N/A | 20 mm × 20 mm | Body of mandible | 8 weeks |
| Russmueller et al., 2015 | Ovine | N/A | Skeletally nature | Female | 60–80 kg | 30 mm × 30 mm | Angle of mandible | 12 weeks |
| Wang et al., 2015 | Canine | Beagle | 12–18 months | Female | Average 12.5 kg | 30 mm, segmental defect | Body of mandible | 12 months |
| Gallego et al., 2015 | Ovine | Laxta Asturian | 12–15 months | Female | 57.3–64.8 kg | 30 mm, segmental defect | Parasymphysis | 12 and 32 weeks |
| Tatara et al., 2019 | Ovine | Dorper | 4–6 months | Female | 40.4 ± 7.3 kg | 2 cm, box shaped | Body of mandible | 9 and 21 weeks |
| Carlisle et al., 2019 | Porcine | Sinclair Minipig | >12 months | Female | N/A | 2 cm, segmental defect | Body of mandible | 4, 8, and 12 weeks |
| Probst et al., 2020 | Porcine | Munchener Trollschweine | 12–14 months | Mixed gender | Mean: 85 kg | 3 cm × 1 cm × 2 cm, box shape | Body and angle of mandible | 12 weeks |
| Lee et al., 2020 | Canine | Beagle | 12–15 months | Male | 12.5 kg | 20 mm × 10 mm × 10 mm, box shaped | Body of mandible | 12 weeks |
| Cao et al., 2021 | Primate | Rhesus | 6–9 years | Male | 6–12 kg | 20 mm × 15 mm × 10 mm, segmental defect | Body of mandible | 12 weeks |
| Bouyer et al., 2022 | Porcine | Minipigs | >24 months | Female | 43–69.5 kg | 4 cm × 3 cm, Box shaped | Body and angle of mandible | 16, 30, 51, and 91 days |
| Abu-Shahba et al., 2022 | Ovine | Texel and Crossbred | 24–35 months | Female | 51–65 kg | 29 (±2) mm × 18 (±1) mm | Angle of mandible | 13 and 23 weeks |
| Nokhbatolfoghahaei et al., 2022 | Canine | Mongrel | N/A | N/A | 15–25 kg | 25 mm × 10 mm × 8 mm | Posterior mandible | 12 weeks |
| Paré et al., 2022 | Ovine | Vendean | 4–7 years | Female | 75–84 kg | 35 mm × 55 mm, segmental defect | Angle of mandible | 3, 5, and 12 months |
| Tissue Engineering Approach | ||||
|---|---|---|---|---|
| Studies | Groups | Biomaterial | Cells | Growth Factors/Bioreactors |
| Gröger et al., 2003 | Treated vs. Non-treated Groups 1 (n = 4) and 2 (n = 2) | 3D polymer Fibrin-fleece scaffold (Ethisorb 510®,, Ethicon, Raritan, NJ, USA) | Unspecified bone tissue derived cells, treated with osteogenic media | N/A |
| Wang et al., 2004 | Group 1: Placebo-treated (n = 1). Group 2: RhOP-1 treated (n = 4) | Type I collagen bone matrix (ground) | N/A | Recombinant human osteogenic protein-1 (RhOP-1) |
| Abukawa et al., 2004 | Constructs (n = 2), Control (n = 1), Empty (n = 1) | Poly-lactic-co-glycolic (PGLA) scaffold | Autologous BMSCs | Osteogenic differentiation media + ROBS |
| Henkel et al., 2005 | Groups with control/osteoblasts/scaffold alone/scaffold + osteoblasts (n = 4 each) | 60% Hydroxyapatite, 40% β-TCP matrix | Autologous osteoblasts | N/A |
| Xi et al., 2006 | Experimental vs. Control (scaffold alone) (n = 10 in total) | Coral scaffold | Autologous BMSCs | N/A |
| Yao et al., 2007 | Animals received in vivo tissue-engineered bone/no control group (n = 3) | Ca-P ceramic | N/A | In vivo bioreactor (femoral muscle) |
| He et al., 2007 | β-TCP scaffold + BMSCs (n = 3) β-TCP scaffold alone (n = 3) | β-TCP | 3rd generation BMSCs | Osteogenic differentiation medium |
| Nolff et al., 2009 | Group 1: β-TCP composite (n = 6); Group 2: β-TCP composite with bone marrow and cancellous bone (n = 6) | β-TCP cylinders | Bone marrow stromal cells (BMSCs) + mozelized cancellous bone | N/A |
| Zhao et al., 2009 | 5 groups (n = 4 each), SS/mSS with/without BMSCs | Silk fibroin scaffold; apatite-coated silk fibroin scaffold; | BMSCs (2–3 passage) | N/A |
| Yuan et al., 2010 | 1. Group: BMSCs + coral cuboids (n = 12) 2. Group: coral cuboid alone (n = 12) | Coral cuboids (natural coral) | BMSCs | Osteogenic differentiation medium |
| Zhou et al., 2010 | DFDBA-BMP, CHA-BMP, DFDBA, CHA (n = 3 per group) | Demineralized freeze-dried bone allograft or coralline hydroxyapatite | N/A (latissimus dorsi auto-bioreactor) | rhBMP-2 |
| Herford et al., 2012 | 5 groups with varying rhBMP-2/ACS + CRM (n = varies/26 defects total) | Absorbable collagen sponge (ACS), compression-resistant matrix (CRM) (HA + β-TCP) | N/A | rhBMP-2 |
| Liao et al., 2013 | Group A: PCL alone Group B: PCL/PRP/PASCs | Laser-sintered porous polycaprolactone (PCL) scaffold | PASCs | PRP |
| Liu et al., 2014 | Control/Experimental groups (n = 30 total) | Allogenic freeze-dried scaffold | BMSCs | N/A |
| Konopnicki et al., 2015 | Seeded Scaffold (n = 3) Unseeded scaffold (n = 3) Empty defects (n = 3) | 50% β-TCP/50% PCL scaffold | Autologous bone marrow-derived osteoblasts | N/A |
| Russmueller et al., 2015 | ChronOS/bone marrow (n = 6) ChronOS/factor XIII (n = 6) ChonOS/venous blood (n = 6) | Polylactide scaffold containing a tricalcium phosphate biomaterial (chronOS®, DePuy Synthes, Warsaw, IN, USA) | BMSCs | Factor XIII |
| Wang et al., 2015 | CBOs/β-TCP (n = 4) FBOs/β-TCP (n = 4) β-TCP (n = 4) Autogenous mandibular segment (n = 4) | β-TCP scaffold | Cryo-preserved osteoblasts (CBOs)and fresh bone-derived osteoblasts (FBOs) | N/A |
| Gallego et al., 2015 | Scaffold alone (n = 5) Scaffold/BMSCs (n = 8) | Serum-based, custom mold | BMSCs | N/A |
| Tatara et al., 2019 | Autograft (AG) (n = 15) Synthetic graft (SG) (n = 9) | PMMA, carboxymethylcellulose-gel | N/A | In vivo bioreactor (rib periosteum) |
| Carlisle et al., 2019 | Polyurethane (PUR) +rhBMP-2 (n = 6); Untreated (n = 6) | Polyurethane (PUR) + HA/β-tricalcium phosphate scaffold | N/A | rhBMP-2 |
| Probst et al., 2020 | Scaffold + pADCs (n = 8) Empty scaffold (n = 8) | Tri-calcium phosphate infiltrated with polymer (TCP-PLGA) | Pig adipose-derived stem cells (pADSCs) | N/A |
| Lee et al., 2020 | No treatment (n = 4) PCL/β-TCP (n = 4) PCL/β-TCP/rhBMP-2 (n = 4) PCL/β-TCP/ABPs (n = 4) | PCL/β-TCP | Autologous bone particles (ABPs) | rhBMP-2 |
| Cao et al., 2021 | 5 groups (n = 3 each), TCP/PLGA variations | TCP, PLGA/TCP scaffolds | N/A | rhBMP-2; autologous bioreactor (Latissimus dorsi) |
| Bouyer et al., 2022 | Autologous bone graft (n = 4) PLA scaffold BMP-2 coating (varying dose) (n = varies) | PLA scaffold, 24 alternative polyelectrolyte films (HA and PLL) | N/A | rhBMP-2 |
| Abu-Shahba et al., 2022 | Control, M, MP, MVP (n = 5 per group) | Bovine-derived mineral matrix, reinforced with resorbable poly(lactic-co-caprolactone) copolymer and RGD-exposing collagen fragments for surface activation | N/A | Autologous bioreactor (M, MP, MVP) |
| Nokhbatolfoghahaei et al., 2022 | βTCP, βTCP/rhBMP2, βTCP/MSCs, PCL/βTCP, PCL/βTCP/rhBMP2, and PCL/βTCP/MSCs (n = 4 each) | FDM 3D-printed PCL/βTCP; foam-cast βTCP | Adipose-derived MSCs | rhBMP-2; autologous bioreactor (Masseter) |
| Paré et al., 2022 | BCP + TBM (n = 6) VBT control (n = 6) | BCP bioceramic implants | Total bone marrow (TBM) | Perfused by an arteriovenous loop (in loco bioreactor) |
| Outcome Parameters | ||||
|---|---|---|---|---|
| Studies | Imaging | Biomechanical Testing | Histology/Histomorphometry | Immunohistochemistry/Molecular Biology |
| Gröger et al., 2003 | X-ray radiography | N/A | Masson’s trichrome staining | N/A |
| Wang et al., 2004 | X-ray radiography, CT scan | Three-point bending test | Toluidine blue staining | N/A |
| Abukawa et al., 2004 | X-ray radiography | N/A | Hematoxylin and eosin staining | N/A |
| Henkel et al., 2005 | X-ray radiography | N/A | Hematoxylin and eosin staining | N/A |
| Xi et al., 2006 | X-ray radiography, scanning electron microscope | N/A | Hematoxylin and eosin staining | N/A |
| Yao et al., 2007 | X-ray radiography | N/A | Hematoxylin and eosin staining, Masson’s trichrome staining | Tetracycline fluorescence labeling for histomorphometry analysis |
| He et al., 2007 | X-ray radiography, CT scan | Compression, stress, and energy tests | Hematoxylin and Masson’s staining | N/A |
| Nolff et al., 2009 | N/A | N/A | Alizarin and methylene blue staining, histomorphometry analysis performed on histology sections | N/A |
| Zhao et al., 2009 | X-ray radiography, CT scan, DXA scan | N/A | Hematoxylin and eosin staining, histomorphometry analysis performed on histology sections | N/A |
| Yuan et al., 2010 | X-ray radiography, micro-CT scan | Three-point bending test | Van Gieson’s picrofuchsine stain | N/A |
| Zhou et al., 2010 | X-ray radiography, angiography | N/A | Hematoxylin and eosin staining | Alizarin complexion, tetracycline, xylenol orange, and calcein for histomorphometric analysis |
| Herford et al., 2012 | X-ray radiography, micro CT | N/A | Hematoxylin and eosin staining, Masson’s trichrome staining | Histomorphometry analysis performed on histology sections, toluidine blue to evaluate new bone formation |
| Liao et al., 2013 | 3D-CT | Compressive Young’s modulus test | Masson’s trichrome staining | Immunohistochemistry with collagen type I and osteocalcin, qRT-PCR assessment for alkaline phosphatase activity |
| Liu et al., 2014 | 3D-CT, micro-CT, 36-XR dual energy X-ray absorptiometry scan | N/A | Hematoxylin and eosin staining | Histomorphometry analysis performed on histology sections |
| Konopnicki et al., 2015 | CD 31 immunofluorescence | N/A | Hematoxylin and eosin staining, nuclear staining with 4′,6-diamidino-2-phenylindole stain | Histomorphometry analysis performed on histology sections, primary anti-pig CD31 anti- body immunohistochemistry stain (for angiogenesis) |
| Russmueller et al., 2015 | X-ray radiography | N/A | 1% thionine stain | Histomorphometry analysis performed on histology sections |
| Wang et al., 2015 | X-ray radiography, CT | N/A | Fluorescent labeling under confocal laser scanning microscope, tetracycline hydrochloride, calcein, alizarin, and calcein blue | Tetracycline hydrochloride, calcein, alizarin, and calcein blue for histomorphometry, diaminobenzidine substrate counterstained with hematoxylin for immunohistochem |
| Gallego 2015 | CT, micro-CT | N/A | Hematoxylin and eosin staining, Masson’s trichrome staining | N/A |
| Tatara et al., 2019 | Micro-CT, | Compression load test | Methylene blue/basic fuchsin stain | Histomorphometry analysis performed on histology sections |
| Carlisle et al., 2019 | Micro-CT | N/A | van Gieson’s picrofuchsin stain | Osteogenesis and rhBMP-2 release cytokine profile analysis |
| Probst et al., 2020 | Micro-CT | N/A | Hematoxylin and eosin staining | Osteocalcin immunostaining |
| Lee et al., 2020 | Micro-CT | N/A | Hematoxylin and eosin staining, Masson’s trichrome staining | Histomorphometry analysis performed on histology sections |
| Cao et al., 2021 | PET and CT imaging, angiography | Uniaxial compressive testing | Hematoxylin and eosin staining | Histomorphometry analysis performed on histology sections |
| Bouyer et al., 2022 | CT scan, micro-CT analysis | N/A | Sanderson’s rapid stain and Van Gieson’s stain | Histomorphometry analysis performed on histology sections |
| Abu-Shahba et al., 2022 | CT, CT angiography, micro-CT | N/A | Hematoxylin and eosin staining, Masson’s trichrome staining, picrosirius red, reticulin, and Movat’s pentachrome staining | Immunohistochemical (IHC) staining using anti-von Willebrand factor (vWF) antibody to assess vascularization, histomorphometry analysis performed on histology sections |
| Nokhbatolfoghahaei et al., 2022 | CT scan | N/A | Hematoxylin and eosin staining | Histomorphometry analysis performed on histology sections |
| Paré et al., 2022 | CT scan, micro-CT, scanning electron microscope | N/A | Hematoxylin and eosin stain, Safranin stain, Movat’s pentachrome stain | N/A |
| Studies | Take-Home-Massages |
|---|---|
| Gröger et al., 2003 | Enhanced Radiodensity and Tissue Integration: Increased radiodensity and calcification were observed in defects filled with cell-fibrin-fleece constructs compared to untreated controls after 90 and 180 days, with complete integration at defect borders and seamless host–implant transitions. Promising Approach for Mandibular Augmentation: The combination of periosteal cells and polymer fleece facilitated membranous bone formation without acute inflammation, suggesting clinical potential for mandibular augmentation. Phenotype Shift and Improved Healing: Light microscopy showed a shift to cuboid osteoblast-like cells, along with vascularization and calcification over time, attributed to the short degradation time and optimized fleece structures. |
| Wang et al., 2004 | Effective Bone Regeneration with CMC-Stabilized Collagen Matrix: The study demonstrated successful regeneration of a 5 cm mandibular defect in Göttingen miniature pigs using rhOP-1 delivered with a CMC-stabilized collagen type I matrix, filling the defect with sufficient bone volume without foreign body reaction. Enhanced Bone Formation and Space-Keeping Properties: The rhOP-1-treated group showed increased bone volume, density, and mineralization, with complete defect filling and good plasticity, although mechanical stress resistance was 25% lower than the control side. Species-Specific Optimization and Potential Limitations: The optimal rhOP-1 concentration varies by species and defect, with cartilage and fibrous tissue observed under reconstruction plates potentially due to impaired rhOP-1 interactions with stem cells in surrounding tissues. |
| Abukawa et al., 2004 | Successful Reconstruction with Tissue-Engineered Constructs: Porcine mandibular defects were effectively reconstructed using autologous mesenchymal stem cells (MSCs) cultured on a biodegradable polymer scaffold, resulting in hard, noncompressible tissue that was indistinguishable from native bone, with complete defect bridging. Bone Formation and Vascularization: The tissue-engineered constructs promoted uniform radiodensity, osteoblast and osteocyte presence, bone trabeculae, and primitive blood vessels throughout the defects, contrasting with fibrous tissue observed in controls. Integration and Structural Similarity: Bone reconstructed with tissue-engineered constructs showed seamless integration with adjacent native bone, with indistinct margins and character comparable to natural bone, demonstrating the potential of scaffold designs with varied pore sizes for effective jaw reconstruction. |
| Henkel et al., 2005 | Superior Bone Formation with Biomatrix Alone: The biomatrix group without osteoblasts showed the highest new bone formation rate, filling 73% of the defect, with biodegradation matching the pace of new bone deposition, outperforming groups with osteoblast transplantation. Effective Osteoconductive Properties: The HA-bTCP matrix (60% hydroxyapatite, 40% beta-tricalcium phosphate) demonstrated high bioactivity and osteoconductive capabilities, surpassing conventional hydroxyapatite ceramics as a temporary bone replacement material. Limited Impact of Osteoblast Transplantation: Adding autologous osteoblasts to the biomatrix did not enhance bone production compared to controls. |
| Xi et al., 2006 | Successful Bone Regeneration with Coral and Titanium Reinforcement: Histological analysis showed new bone formation on the surface and within the pores of natural coral, with grafts fully restored after 16 weeks. Titanium reticulum reinforcement enhanced mandibular defect restoration with high biocompatibility and minimal stress shielding. Coral Microstructure and Bone Healing: Natural coral’s trabecular-like microstructure supported osteogenesis, with smooth, red bone tissue covering graft surfaces in the cell seeding group, although bone healing was limited to areas outside the titanium reticulum due to periosteal proliferation. Enhanced Osteogenic Phenotype with Supplements: Adding bone morphogenetic protein, β-glycerophosphate sodium, and dexamethasone to the medium improved the osteogenic properties of the cells, aiding in reconstructing segmental mandibular defects with tissue-engineered bone. |
| Yao et al., 2007 | Successful Integration and Bone Regeneration: The in vivo tissue-engineered (TE) bone graft integrated with host bone, supported active bone regeneration, and restored mandibular shape without infection or inflammation, demonstrating feasibility for box-like mandibular defect reconstruction. Enhanced Bone Properties with Ca-P Ceramics: Calcium phosphate ceramics (60% HA/40% α-TCP, 60% porosity) facilitated osteoconductivity and osteoinduction, with new bone forming into ceramic pores, maturing with trabeculae and Haversian systems, and modifying ceramic’s poor biomechanical properties. Bioactivity and Host Participation: The TE bone graft, carrying living cells and a good blood supply, participated in host bone metabolism, achieving biomechanical properties and bioactivity comparable to autografts. |
| He et al., 2007 | Enhanced Bone Regeneration and Biomechanical Strength: Tissue-engineered constructs combining bone marrow stromal cells and a 3D β-tricalcium phosphate scaffold significantly improved bone formation, radiodensity, and biomechanical properties, minimizing donor site morbidity while offering a customizable solution for complex 3D mandibular defects. Histological and Structural Advantages: New bone formation, osteoblast activity, and cartilage were observed in the scaffold’s central sections after 3 months, with the engineered graft precisely shaped to mimic the lost bone using computer-aided design. |
| Nolff et al., 2009 | Effective Bone Healing with B-TCP Composite: The beta-TCP composite loaded with bone marrow and cancellous bone (B-TCPB/BM/CB) effectively healed critical-sized mandibular defects, showing significantly higher bone formation and osteointegration compared to B-TCP alone, with dense lamellar bone bridging the defects. Clinical Potential and Surgical Advantages: The B-TCP composite offers a promising alternative to autografts for mandibular reconstruction, enabling table-side preparation with the patient’s own cells, avoiding cell culture or expansion, and showing potential for various clinical applications. |
| Zhao et al., 2009 | Effective Repair of Mandibular Defects: The combination of bMSCs with an apatite-coated silk scaffold (bMSCs/mSS) completely repaired canine mandibular border defects within 12 months, achieving bone mineral densities comparable to normal mandibles and reconstructing the mandibular contour seamlessly with native bone. Enhanced Osteoconductive Environment: The premineralized silk fibroin scaffold provided an osteoconductive matrix for bMSCs, promoting differentiation into osteoblasts, extracellular matrix secretion, and preventing fibrillar tissue infiltration, resulting in substantial new bone formation and vascularization. |
| Yuan et al., 2010 | Effective Repair and Bone Integration: GFP-labeled BMSCs on beta-TCP coral scaffolds successfully repaired 30 mm critical-sized mandibular defects in 32 weeks, achieving bony union, smooth remodeling, and biomechanical properties comparable to normal mandibles, ensuring long-term stability and function. Scaffold Degradation and Osteogenesis: Coral scaffolds demonstrated an ideal degradation rate, with reduced residual volumes over time, facilitating new bone formation as BMSCs differentiated into osteoblast-like cells and integrated with endogenous MSCs. |
| Zhou et al., 2010 | Prefabricated Bone Flaps with rhBMP-2 Show Superior Regeneration: Combining prefabricated tissue-engineered bone flaps with an rhBMP-2-incorporated CHA scaffold effectively reconstructs mandibular critical-sized defects, achieving robust bone regeneration and structural integrity. Key Role of rhBMP-2 in Osteoinduction: The integration of rhBMP-2 enhances osteoinductive properties, promoting homogeneous bone formation, vascularization, and functional remodeling in mandibular reconstructions. Long-Term Structural and Functional Success: Mandibular defects reconstructed with rhBMP-2-incorporated CHA scaffolds demonstrated bone morphology, density, and mechanical properties comparable to native mandibles, emphasizing the potential for clinical applications. |
| Herford et al., 2012 | Superior Bone Formation with CRM and rhBMP-2: The combination of CRM and rhBMP-2 achieved significantly greater bone density and reduced voids, leading to effective mandibular defect repair compared to ACS-based carriers. Effective Space Maintenance: The compression-resistant properties of CRM preserved the defect structure, supporting consistent and robust bone regeneration. Critical Role of rhBMP-2 and Carrier Synergy: The optimized release and high-dose delivery of rhBMP-2 facilitated early osteoinduction, highlighting the importance of carrier properties in enhancing bone regeneration outcomes. |
| Liao et al., 2013 | Enhanced Bone Formation: The PCL/PRP/PASCs construct supported robust new bone formation with increased density and compact structure, crucial for successful mandibular defect repair. Synergistic Osteoinduction: The combination of PRP and PASCs within the PCL scaffold promoted osteogenic differentiation, contributing to effective bone regeneration. Effective Scaffold Integration: The construct’s interconnected porous structure facilitated seamless integration with surrounding bone tissue, promoting uniform bone growth throughout the scaffold. |
| Liu et al., 2014 | Enhanced and Accelerated Bone Remodeling: Autologous MSCs accelerated the absorption of allogenic scaffolds and facilitated their complete replacement with new bone within 48 weeks, promoting trabecular bone formation, Haversian canal expansion, and significantly improving bone mineral density and micro-architecture. Need for Optimization: The prevalence of fibrous ossification and postoperative infections highlights the need for additional growth factors and strategies to improve bone quality and reduce complications. |
| Konopnicki et al., 2015 | Effective Bone Formation: 3D-printed b-TCP and PCL scaffolds seeded with pBMPCs demonstrated robust bone penetration, with significantly higher bone formation in the center of constructs compared to unseeded scaffolds. Angiogenesis and Scaffold Resorption: Enhanced CD31 expression and vascularization were observed in the constructs, particularly in areas of new bone, facilitating scaffold resorption and bone integration. Critical Role of Early Implantation: Early implantation supports efficient cell penetration, collagen deposition, and extracellular matrix formation, optimizing the healing process and promoting bony architecture development. |
| Russmueller et al., 2015 | Superior Performance of Autologous Bone Marrow: Polylactide scaffolds combined with tricalcium phosphate biomaterial and autologous bone marrow demonstrated robust bone regeneration across all regions of interest, preserving scaffold structure and achieving consistent osteoconductive bone formation. Limited Efficacy of Factor XIII: Contrary to prior studies, coagulation factor XIII failed to enhance bone regeneration, showing performance comparable to blood-based controls and significant scaffold deformation. |
| Wang et al., 2015 | Effective Bone Regeneration with CBOs and β-TCP: Tissue-engineered bone using cryopreserved bone-derived osteoblasts (CBOs) combined with β-TCP successfully repaired critical-sized segmental mandibular defects, promoting bone mineralization and deposition comparable to fresh bone-derived osteoblasts (FBOs). Clinical Potential of CBOs: The use of CBOs offers a practical solution for large-volume bone regeneration, addressing limitations of tissue banking and enabling reconstruction even in cases of reduced regenerative capacity due to aging. Immunohistochemical Validation: Intensive osteocalcin expression in the bone matrix of CBO and FBO groups confirmed active bone formation and integration, further supporting the osteogenic potential of these constructs. |
| Gallego et al., 2015 | Improved Bone Quality with BM-MSCs: Segmental mandibular defects repaired using the serum scaffold seeded with autologous BM-MSCs exhibited significantly enhanced bone quality, with BMD, BV/TV, trabecular thickness (TbTh), and trabecular number (TbN) all significantly higher than in the control group at 32 weeks. The newly formed bone in the experimental group was similar to native bone Localized Bone Formation: Ossification was most advanced in the central area of the defect in the BM-MSCs-seeded scaffold group, demonstrating a high degree of mineralization and osteon formation Faster and More Consistent Bone Union: The BM-MSCs-seeded scaffold group achieved earlier and more consistent bony union compared to the control group. |
| Tatara et al., 2019 | Successful Use of 3D-Printed Bioreactors for Mandibular Reconstruction: A total of 83% of 3D-printed in vivo bioreactors generated mineralized tissue suitable for reconstructing large mandibular defects in sheep, demonstrating their potential for creating autologous vascularized bone free tissue flaps. Superior Bone Quality with AG Scaffolds: AG scaffolds supported the formation of more mature bone tissue with mechanical properties closer to native bone, outperforming SG scaffolds in generating bone suitable for reconstructive purposes. Effective Space Maintenance: Space maintainers integrated well with local soft tissue and successfully preserved the defect area, allowing for the generation of customized bone grafts that matched the geometry of the mandibular defect. |
| Carlisle et al., 2019 | Enhanced Bone Regeneration with Low-Dose rhBMP-2: Treatment with low-dose rhBMP-2 delivered through a PUR composite with calcium phosphate granules significantly enhanced bone regeneration, leading to complete bone bridging in mandibular continuity defects within 12 weeks, as evidenced by increased bone volume and mineral density. Localized Healing Response: The regenerative treatment elicited a localized inflammatory response with increased levels of cytokines such as IL-1ra and IL-6 at early time points, without systemic inflammation or excessive cytokine levels, indicating a controlled and safe therapeutic effect. Localized Delivery and Safety: The PUR scaffold enabled localized delivery of rhBMP-2 without detectable systemic absorption, reducing the risk of systemic side effects and ensuring a controlled release for effective bone regeneration. |
| Probst et al., 2020 | Enhanced Bone Regeneration with ADSCs: ADSCs-seeded TCP-PLGA scaffolds demonstrated significantly improved bone volume and osteocalcin deposition compared to non-seeded scaffolds after 12 weeks, indicating the osteogenic potential of ADSCs in large mandibular defect repair. Challenges with Hypoxia in Scaffold Centers: Despite the interconnected macroporous design of the scaffold facilitating vascular ingrowth, hypoxic conditions in the scaffold center hindered complete bone regeneration. Scaffold Integration and Stability: The TCP-PLGA scaffold was well integrated into the defect area and fixable with titanium screws, although brittleness and the need for improved mechanical properties remain limitations. |
| Lee et al., 2020 | Enhanced Bone Regeneration with Additives: PCL/β-TCP scaffolds loaded with rhBMP-2 or autogenous bone particles (ABP) significantly improved bone regeneration compared to the control or PCL/β-TCP scaffolds alone. Micro-CT analysis revealed that the rhBMP-2-loaded scaffold group generated the highest bone volume among the groups, followed by the ABP-loaded scaffold group. Limitations in Clinical Application: Despite improved bone formation in experimental groups, the volume of newly formed bone was insufficient for clinical application. Periosteal resection and the lower dose of rhBMP-2 contributed to these suboptimal outcomes, highlighting the need for further optimization of growth factor dosage and scaffold design Scaffold Design and Stability: The 3D-printed PCL/β-TCP scaffold, with its heterogeneous pore sizes and additional wing structures, provided stable screw fixation and allowed for proper integration within mandibular defects |
| Cao et al., 2021 | Superior Stability and Osteoconductivity of TCP Scaffolds: β-TCP scaffolds demonstrated significantly superior in vivo stability, mechanical strength, and osteoconductivity compared to PLGA/TCP scaffolds, retaining their 3D architecture and porous structure even after prolonged implantation. Enhanced Bone Regeneration with rhBMP-2 Coating: TCP scaffolds coated with rhBMP-2 showed a notable increase in bone volume and mineralization at both ectopic and orthotopic implantation sites. Prefabricated rhBMP-2-coated TCP scaffolds (P-TCP-BMP) achieved significantly better outcomes in bone regeneration and structural integration than directly implanted rhBMP-2-coated TCP scaffolds (S-TCP-BMP). Utility of 18F-FDG PET/CT in Monitoring Regeneration: 18F-FDG PET/CT provided a reliable method for tracking bone regeneration and vascularization, highlighting higher uptake in the rhBMP-2-coated TCP scaffold, correlating with enhanced scaffold performance. |
| Bouyer et al., 2022 | BMP-2 Dose-Dependent Bone Regeneration and Maturity: BMP-2 doses significantly influenced the rate, quality, and maturity of bone regeneration. Higher BMP-2 doses (e.g., BMP110) led to significantly greater bone volume and mineralization, producing mature bone with Haversian canals and robust integration between host and regenerated bone. These natural bone-like connections enhanced mechanical stability, mimicking the structure of native bone. Repair kinetics were also dose dependent, with slower repair observed for higher BMP-2 doses. Enhanced Performance of EDC30 Films: EDC30 films provided superior BMP-2 adsorption and sustained release compared to EDC70 films, leading to more robust and predictable bone regeneration. Comparable Outcomes and Safety with BMP-2-Loaded 3D-Printed Scaffolds: BMP-2-loaded 3D-printed PLA scaffolds demonstrated bone regeneration results comparable to gold standard autografts, with uniformly distributed bone growth and limited ectopic bone formation (~28–35%), even at high BMP-2 doses. The BMP-2 coating not only promoted osteogenesis and angiogenesis but also ensured safety by effectively controlling ectopic bone formation and maintaining scaffold durability. |
| Abu-Shahba et al., 2022 | Superior Bone Regeneration and Remodeling with MVP Group: The MVP group (muscle + vascularized periosteal flap) achieved the highest bone formation and lowest residual biomaterial volume among all groups. Periosteal flaps significantly enhanced vascularization, bone regeneration, and biomaterial remodeling. Role of Periosteal Flaps in Bone Regeneration: Vascularized periosteal flaps demonstrated predictable pro-vascularization and osteogenic potential. Bone regeneration was dependent on interaction with the mechanically stimulated local bony microenvironment post-transplantation, rather than on the periosteum’s intrinsic vascular supply during prefabrication. Biomechanical and Histological Features: Newly formed bone in the MVP group showed organized lamellar structures, integration with Sharpey’s fiber-like formations, and vascularized fibrovascular stroma, further boosting remodeling efficiency Influence of Recipient Periosteum: The preserved recipient site periosteum in the control group contributed significantly to bone regeneration, emphasizing the regenerative capacity of the periosteum. |
| Nokhbatolfoghahaei et al., 2022 | Enhanced Bone Formation with β-TCP Scaffolds: β-TCP scaffolds demonstrated significantly higher rates of new bone formation compared to PCL/βTCP scaffolds, highlighting their potential as an effective solution for reconstructing critical-sized mandibular defects while simplifying surgical procedures and minimizing risks. Role of rhBMP2 and MSCs: Treatments with rhBMP2 and MSCs significantly promoted bone formation, vascularization, and osteogenesis, while reducing scaffold residues. Among all groups, the rhBMP2-treated pedicled β-TCP scaffold group achieved the highest bone regeneration rates, demonstrating the synergy of scaffold composition and biological enhancements. Masseter Muscle as an In Vivo Bioreactor: The masseter muscle served as an effective in vivo bioreactor, supporting scaffold prefabrication with minimal surgical complexity. This approach demonstrated promising outcomes for mandibular reconstruction, leveraging the muscle’s natural vascular and osteogenic properties. |
| Paré et al., 2022 | Bone Regeneration with Customized Constructs: The calcium phosphate-based implant achieved successful regeneration of segmental mandibular defects (SMD) with full osseointegration and vascularization within 3 months. By 12 months, implants were entirely encased in lamellar bone, and healthy yellow marrow filled the remaining spaces, demonstrating long-term functional restoration. Low Biodegradation Rate of BCP Scaffolds: While biphasic calcium phosphate (BCP) scaffolds demonstrated excellent biocompatibility, their slow degradation left 75% of the initial ceramic volume intact after 12 months, limiting complete bone replacement and highlighting the need for improved scaffold materials with faster biodegradation. Advanced Imaging with Deep Learning: Deep learning algorithms significantly enhance segmentation accuracy for micro-CT analysis of bioceramic scaffolds, enabling a more precise assessment of bone formation, implant integration, and material performance. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sillmann, Y.M.; Eber, P.; Orbeta, E.; Wilde, F.; Gross, A.J.; Guastaldi, F.P.S. Milestones in Mandibular Bone Tissue Engineering: A Systematic Review of Large Animal Models and Critical-Sized Defects. J. Clin. Med. 2025, 14, 2717. https://doi.org/10.3390/jcm14082717
Sillmann YM, Eber P, Orbeta E, Wilde F, Gross AJ, Guastaldi FPS. Milestones in Mandibular Bone Tissue Engineering: A Systematic Review of Large Animal Models and Critical-Sized Defects. Journal of Clinical Medicine. 2025; 14(8):2717. https://doi.org/10.3390/jcm14082717
Chicago/Turabian StyleSillmann, Yannick M., Pascal Eber, Elizabeth Orbeta, Frank Wilde, Andrew J. Gross, and Fernando P. S. Guastaldi. 2025. "Milestones in Mandibular Bone Tissue Engineering: A Systematic Review of Large Animal Models and Critical-Sized Defects" Journal of Clinical Medicine 14, no. 8: 2717. https://doi.org/10.3390/jcm14082717
APA StyleSillmann, Y. M., Eber, P., Orbeta, E., Wilde, F., Gross, A. J., & Guastaldi, F. P. S. (2025). Milestones in Mandibular Bone Tissue Engineering: A Systematic Review of Large Animal Models and Critical-Sized Defects. Journal of Clinical Medicine, 14(8), 2717. https://doi.org/10.3390/jcm14082717








