Artificial Intelligence in Thoracic Surgery: A Review Bridging Innovation and Clinical Practice for the Next Generation of Surgical Care
Abstract
:1. Introduction
2. Materials and Methods
2.1. Critical Analysis of Bias
2.2. Inclusion and Exclusion Criteria
2.2.1. Inclusion Criteria
- Peer-reviewed publications on the application of AI in thoracic surgery.
- English-language articles.
- Research examining AI applications in thoracic surgery for diagnostic, surgical, therapeutic, or postoperative care purposes.
- Systematic reviews, meta-analyses, or original research presenting primary data.
- Studies with clearly defined methodologies, AI models, and measurable outcomes relevant to thoracic surgery.
2.2.2. Exclusion Criteria
- Articles focusing exclusively on preclinical or animal models.
- Studies unrelated to minimally invasive techniques or thoracic surgery.
- Abstracts, conference proceedings, editorials, opinion pieces, or gray literature.
- Research that did not explicitly apply AI or lacked sufficient methodological detail.
- Study design.
- AI methodology and model architecture.
- Application focus (e.g., diagnosis, surgery, postoperative care, treatment).
- Sample size and patient demographics.
- Outcome measures and performance metrics.
- Study limitations and potential biases.
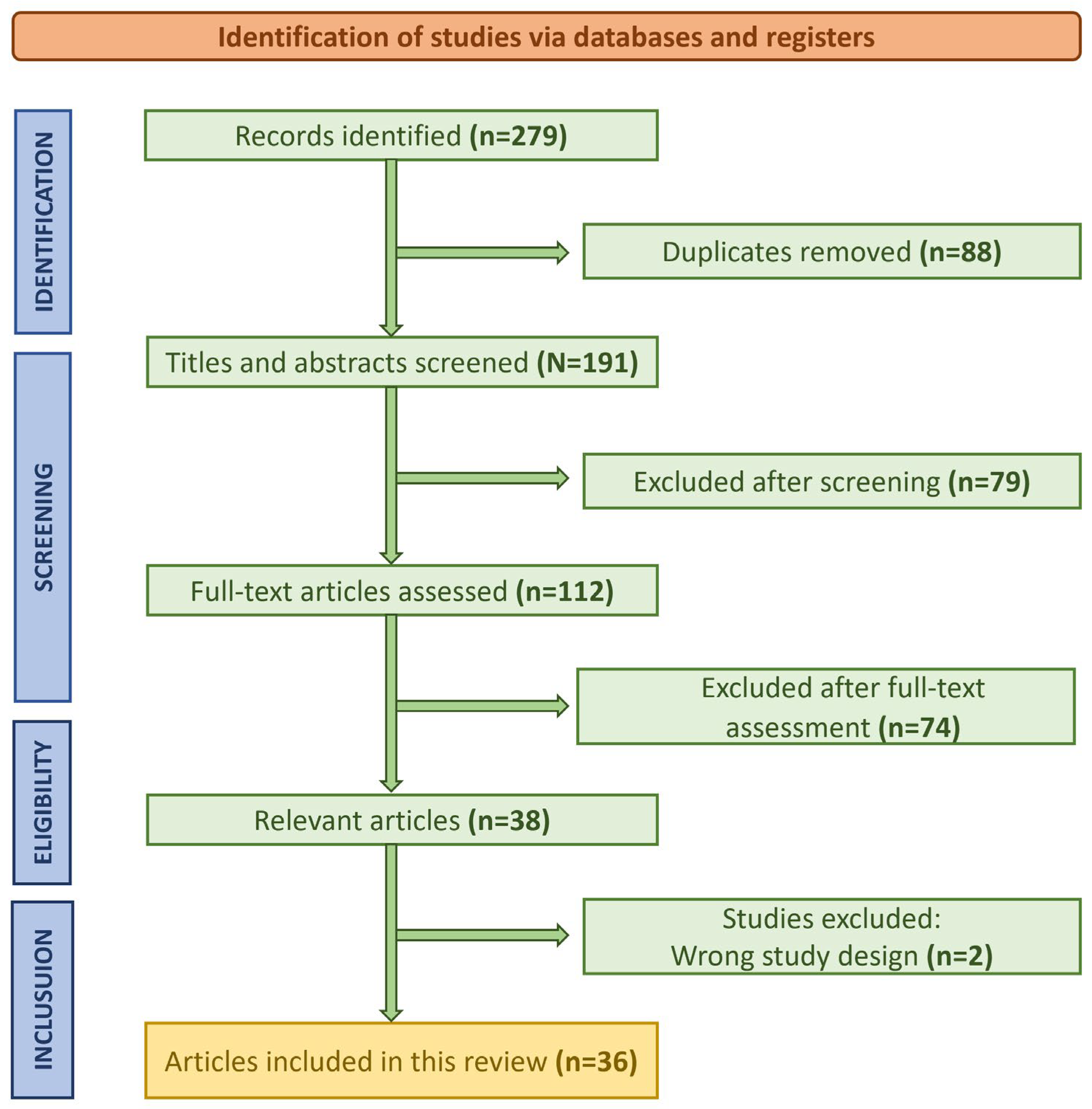
2.3. Study Selection Process
2.4. Discussion and Gaps in the Literature
3. Results
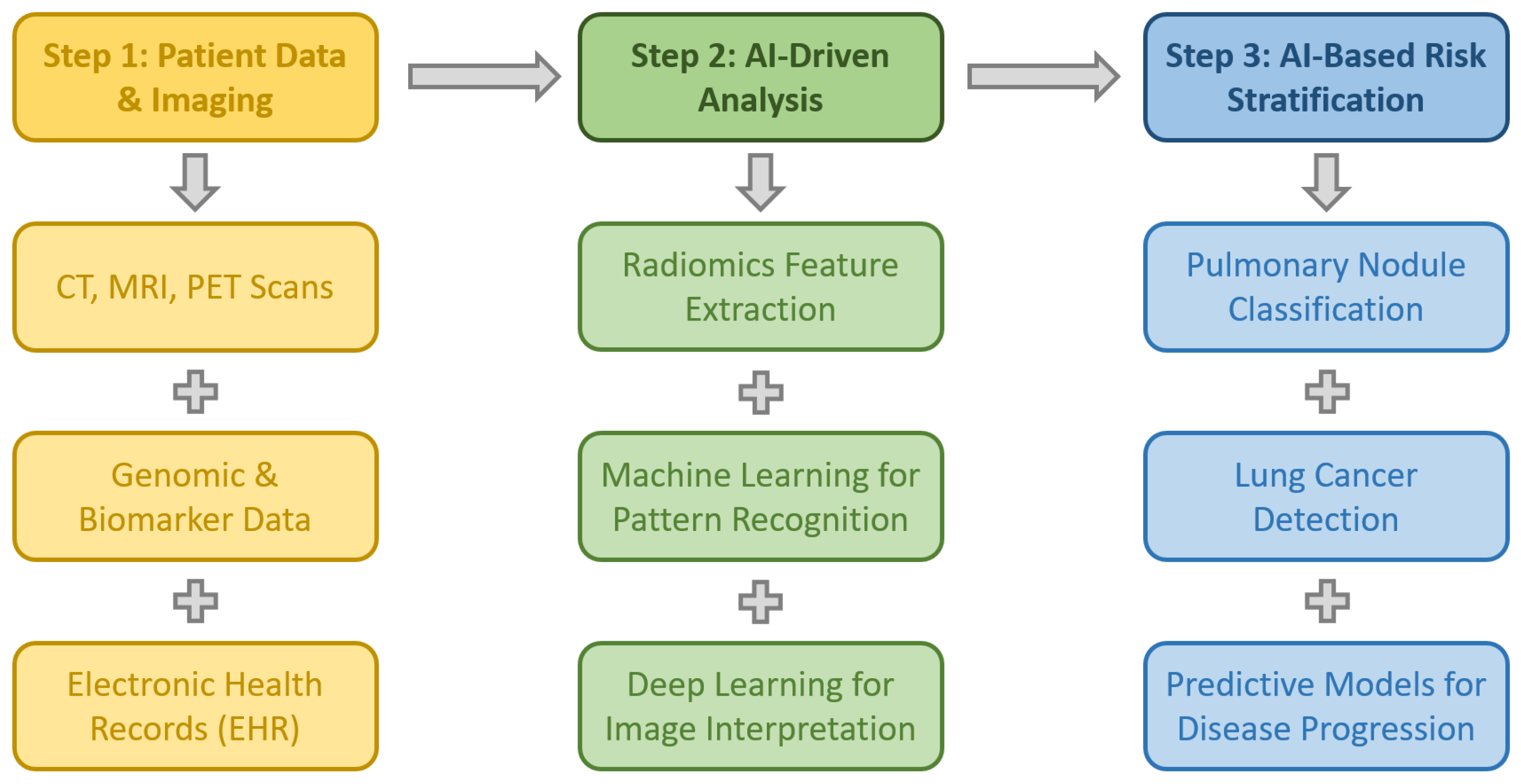
3.1. Early Diagnosis of Thoracic Pathologies
3.1.1. Screening and Early Detection and Evaluation of Pulmonary Nodules
AI in Lung Cancer Screening and Nodule Detection
AI-Assisted Detection and Follow-Up of Indeterminate Pulmonary Nodules (IPNs)
Advancements in AI for Lung Nodule Characterization
AI in Lung Cancer Screening Programs and Risk Stratification
AI in Lung Nodule Diagnosis and Staging
3.1.2. Radiomics and AI in Lung Cancer Diagnosis
3.1.3. Bronchoscopy, Ultrasound, and Endobronchial Ultrasound (EBUS)
AI in Bronchoscopy and Lung Cancer Diagnosis
AI in Lung Ultrasound for Thoracic Surgery
AI in Endobronchial Ultrasound (EBUS) for Lymph Node Metastasis
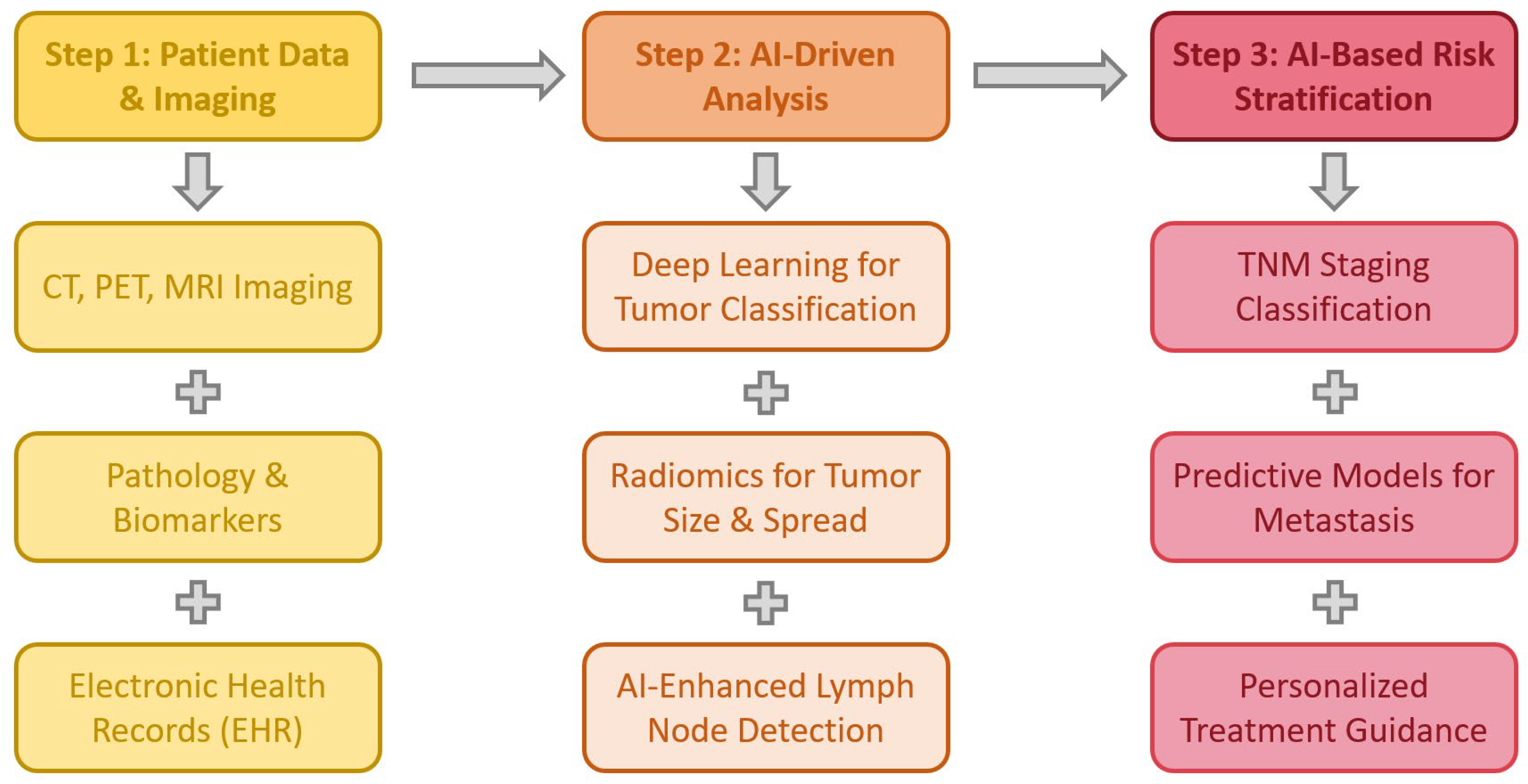
3.2. AI in Lung Cancer Staging
3.2.1. AI in Imaging-Based Staging
3.2.2. AI-Based Prediction of Lymph Node Metastases
3.2.3. AI in Multidisciplinary Lung Cancer Management
3.3. Risk Assessment
3.3.1. AI in Preoperative Risk Assessment
3.3.2. AI Models for Postoperative Mortality and Survival Prediction
3.3.3. AI in Surgical Risk Stratification
3.3.4. AI in Personalized Prognostic Assessment
3.4. AI in Predicting Outcomes for Lung Transplantation
3.4.1. AI for Lung Size Estimation and Donor Matching
3.4.2. AI in Predicting Post-Transplant Complications and Survival
3.4.3. AI for Non-Invasive Rejection Monitoring
3.5. AI in Genomics, Proteomics, and Novel Therapeutic Targeting
3.5.1. AI in Drug Discovery and Target Identification
3.5.2. Deep Learning for Gene Mutation Detection
3.5.3. AI-Driven Prognostic Biomarkers for Immunotherapy
3.6. AI in Predicting Outcomes for Infectious Thoracic Diseases
3.6.1. AI in Diagnosing and Managing Pleural Empyema
3.6.2. Machine Learning in Parapneumonic Effusions
3.6.3. Predicting Intrapleural Therapy Failure in Pleural Infections
3.7. AI in Oncological Therapies
3.7.1. AI in Chemotherapy Optimization
3.7.2. AI in Immunotherapy and Targeted Therapy
AI in Immunotherapy Response Prediction
AI in Biomarker Discovery and Digital Pathology
Radiomics and AI for Immunotherapy Precision
3.7.3. AI in Radiotherapy
AI for Prognostic Prediction in Radiotherapy
AI in Radiation-Induced Toxicity Prediction
AI-Assisted Contouring in Radiotherapy Planning
3.8. AI-Driven Robotic-Assisted Techniques in Thoracic Surgery
3.8.1. The Increasing Role of Robotic-Assisted Techniques in Thoracic Surgery
Robotic Thoracic Day Surgery and Enhanced Recovery Protocols
Robotic Lobectomy: Expanding Adoption and Optimizing Patient Outcomes
Evolution of Robotic Thoracic Surgery and Technological Milestones
Robotic Surgery for Mediastinal Disease
Challenges and Learning Curve in Robotic Thoracic Surgery
Perioperative Considerations in Robotic Thoracic Surgery
Hybrid “Fusion Surgery”: Combining Robotic Assistance with Manual Techniques
Institutional Experience in Scaling Robotic Surgery Programs
Future Directions and Research in Robotic Thoracic Surgery
Building Sustainable Robotic Surgery Programs
3.8.2. Implications of AI in Intraoperative Support
AI-Based 3D Reconstruction
Machine Learning in Intraoperative Decision Support and Surgical Skill Assessment
- Enhanced Diagnosis—AI-driven image analysis and clinical data interpretation can improve early disease detection and intraoperative navigation, leading to better surgical decision-making.
- Surgical Skill Assessment—ML models can provide real-time performance reviews, offering personalized feedback to surgeons for skill enhancement and optimized outcomes.
- Postoperative Prognostication—AI-powered risk prediction models can assess individualized patient recovery trajectories, allowing for tailored postoperative care strategies.
- Intraoperative Performance Optimization—ML algorithms can provide real-time analysis of patient data, alerting surgeons to potential complications or deviations from optimal surgical techniques.
- Accelerating Translational Research—AI can analyze large-scale surgical datasets, identifying patterns that inform new techniques, improve surgical efficiency, and develop novel therapeutic approaches.
3.8.3. Augmented Cognition and Computer Vision in the Operating Room
3.9. Postoperative Care and Follow-Up in Thoracic Surgery
3.9.1. AI-Assisted Postoperative Recovery in Robotic Thoracic Surgery
3.9.2. AI-Enhanced Lung Ultrasound for Postoperative Monitoring
- Improving diagnostic accuracy by detecting postoperative complications such as pleural effusions, pneumothorax, and atelectasis with greater precision.
- Standardizing ultrasound interpretations, reducing interobserver variability and improving diagnostic consistency.
- Accelerating learning curves, making LUS a more accessible tool for thoracic surgeons and critical care teams.
- Minimizing inconclusive results, ensuring that imaging-based decisions are more reliable and reproducible.
3.9.3. AI in Predicting Postoperative Complications and Remote Patient Monitoring
- Analyzing patient data to forecast postoperative risks such as pulmonary infections, delayed recovery, and respiratory complications, enabling timely medical interventions.
- Facilitating continuous remote monitoring through wearable devices and AI-driven telemedicine platforms, ensuring early detection of adverse events and reducing hospital readmissions.
- Optimizing resource allocation in hospitals by providing real-time recovery predictions, allowing personalized follow-up strategies tailored to each patient’s needs.
3.10. AI in Education and Research in Thoracic Surgery
3.10.1. AI in Surgical Training and Skill Development
3.10.2. AI in Medical Education and Exam Performance
3.10.3. AI-Driven Research and Predictive Analytics
4. Discussion
4.1. AI and Robotic-Assisted Thoracic Surgery: Opportunities and Challenges
- High upfront costs associated with robotic platforms pose a financial barrier, particularly in low-resource healthcare settings [114].
- A steep learning curve requires specialized training and credentialing, delaying the adoption of robotic-assisted techniques [115].
- Limited accessibility in certain institutions due to a lack of infrastructure and high maintenance costs [109].
4.2. AI’s Role in Diagnostics, Perioperative Care, and Prognostic Modeling
4.3. AI in Perioperative Management and Postoperative Monitoring
4.4. Standardizing AI Integration into Thoracic Surgery
4.5. Real-World Barriers: Economic, Regulatory, Ethical, and Implementation Challenges
5. Future Perspectives
5.1. Advancing AI in Thoracic Surgery
5.2. Ethical and Practical Challenges in AI Implementation
- Data Security and Privacy—AI’s reliance on large-scale patient data raises concerns regarding confidentiality, cybersecurity, and regulatory compliance [109]. Implementing federated learning approaches, where AI models are trained across multiple institutions without sharing raw data, may help mitigate privacy risks while preserving data integrity.
- Regulatory and Legal Considerations—Clear guidelines for AI validation, liability, and clinical responsibility must be established before autonomous AI systems can be fully trusted in surgical settings [117]. Policymakers must develop legal frameworks to address AI-driven clinical decision-making and define accountability measures for AI errors or misdiagnoses.
- Integration into Clinical Practice—AI-driven solutions must be seamlessly incorporated into existing surgical workflows, requiring user-friendly interfaces, clinician training, and robust clinical validation [49]. AI tools should function as augmentative systems, complementing rather than replacing surgeon expertise.
5.3. Further Expanding AI’s Role in Thoracic Surgery
- Digital Twin Technology—creating virtual patient models to simulate surgical outcomes, predict complications, and personalize treatment planning based on real-time physiological data.
- Explainable AI (XAI)—developing transparent AI algorithms that provide interpretable, clinician-friendly insights to enhance trust and adoption in surgical settings.
- Multicenter AI Validation—conducting large-scale, multi-institutional trials to assess the generalizability and clinical utility of AI models across diverse patient populations and healthcare systems.
- Autonomous Surgical Assistance—advancing AI-powered robotic platforms capable of providing real-time intraoperative decision support, including predictive analytics for bleeding risks, automated suturing, and augmented visualization through AI-enhanced imaging systems.
- AI-Augmented Training Programs—implementing AI-driven virtual reality (VR) and simulation-based training modules to accelerate surgeon proficiency in robotic-assisted techniques while ensuring standardized, high-quality education.
- Integration of Multi-Omics Data—combining genomic, proteomic, radiomic, and clinical data to develop comprehensive AI models for thoracic oncology, improving precision in diagnosis, prognosis, and treatment selection.
6. Limitations
7. Conclusions
Funding
Conflicts of Interest
References
- Lee, D.; Yoon, S.N. Application of Artificial Intelligence-Based Technologies in the Healthcare Industry: Opportunities and Challenges. Int. J. Environ. Res. Public Health 2021, 18, 271. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ahmed, A.; Hameed, I. Artificial intelligence in academic cardiothoracic surgery. J. Cardiovasc. Surg. 2024, 65, 177–178. [Google Scholar] [CrossRef] [PubMed]
- Cusumano, G.; D’Arrigo, S.; Terminella, A.; Lococo, F. Artificial Intelligence Applications for Thoracic Surgeons: “The Phenomenal Cosmic Powers of the Magic Lamp”. J. Clin. Med. 2024, 13, 3750. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bellini, V.; Valente, M.; Del Rio, P.; Bignami, E. Artificial intelligence in thoracic surgery: A narrative review. J. Thorac. Dis. 2021, 13, 6963–6975. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pan, J.M.; Watkins, A.A.; Stock, C.T.; Moffatt-Bruce, S.D.; Servais, E.L. The Surgical Renaissance: Advancements in Video-Assisted Thoracoscopic Surgery and Robotic-Assisted Thoracic Surgery and Their Impact on Patient Outcomes. Cancers 2024, 16, 3086. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, L.; Ge, L.; Song, S.; Ren, Y. Clinical applications of minimally invasive uniportal video-assisted thoracic surgery. J. Cancer Res. Clin. Oncol. 2023, 149, 10235–10239. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Li, R.; Ma, Z.; Han, J.; Yue, W.; Aigner, C.; Casiraghi, M.; Tian, H. Comparison of the perioperative outcomes between robotic-assisted thoracic surgery and video-assisted thoracic surgery in non-small cell lung cancer patients with different body mass index ranges. Transl. Lung Cancer Res. 2022, 11, 1108–1118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moglia, A.; Georgiou, K.; Georgiou, E.; Satava, R.M.; Cuschieri, A. A systematic review on artificial intelligence in robot-assisted surgery. Int. J. Surg. 2021, 95, 106151. [Google Scholar] [CrossRef] [PubMed]
- Resio, B.J.; Dhanasopon, A.P.; Blasberg, J.D. Big data, big contributions: Outcomes research in thoracic surgery. J. Thorac. Dis. 2019, 11 (Suppl. S4), S566–S573. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Taha, A.; Flury, D.V.; Enodien, B.; Taha-Mehlitz, S.; Schmid, R.A. The development of machine learning in lung surgery: A narrative review. Front. Surg. 2022, 9, 914903. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Etienne, H.; Hamdi, S.; Le Roux, M.; Camuset, J.; Khalife-Hocquemiller, T.; Giol, M.; Debrosse, D.; Assouad, J. Artificial intelligence in thoracic surgery: Past, present, perspective and limits. Eur. Respir. Rev. 2020, 29, 200010. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hanna, M.G.; Pantanowitz, L.; Jackson, B.; Palmer, O.; Visweswaran, S.; Pantanowitz, J.; Deebajah, M.; Rashidi, H.H. Ethical and Bias Considerations in Artificial Intelligence/Machine Learning. Mod. Pathol. 2024, 38, 100686. [Google Scholar] [CrossRef] [PubMed]
- Cavique, L. Implications of causality in artificial intelligence. Front. Artif. Intell. 2024, 7, 1439702. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nair, A.S. Publication bias—Importance of studies with negative results! Ind. J. Anaesth. 2019, 63, 505–507. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guni, A.; Sounderajah, V.; Whiting, P.; Bossuyt, P.; Darzi, A.; Ashrafian, H. Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies Using AI (QUADAS-AI): Protocol for a Qualitative Study. JMIR Res. Protoc. 2024, 13, e58202. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. Open Med. 2009, 3, e123–e130. [Google Scholar] [PubMed] [PubMed Central]
- Muka, T.; Glisic, M.; Milic, J.; Verhoog, S.; Bohlius, J.; Bramer, W.; Chowdhury, R.; Franco, O.H. A 24-step guide on how to design, conduct, and successfully publish a systematic review and meta-analysis in medical research. Eur. J. Epidemiol. 2020, 35, 49–60. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Lai, X.; Pan, Y.; Zheng, H.; Vera, J.; Liu, S.; Deng, S.; Zhang, L. Artificial intelligence in cancer target identification and drug discovery. Signal Transduct. Target. Ther. 2022, 7, 156. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, K.; Chen, K. Artificial intelligence: Opportunities in lung cancer. Curr. Opin. Oncol. 2022, 34, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Števík, M.; Malík, M.; Vetešková, Š.; Trabalková, Z.; Hliboký, M.; Kolárik, M.; Magyar, J.; Bundzel, M.; Szabóová, M.; Babič, F.; et al. Hybrid Artificial Intelligence Solution Combining Convolutional Neural Network and Analytical Approach Showed Higher Accuracy in A-lines Detection on Lung Ultrasound in Thoracic Surgery Patients Compared with Radiology Resident. Neuro Endocrinol. Lett. 2024, 45, 229–237. [Google Scholar] [PubMed]
- Woodhouse, P.; Paez, R.; Meyers, P.; Lentz, R.J.; Shoajaee, S.; Sharp, K.; Baldi, N.; Maldonado, F.; Grogan, E.L. Leveraging Artificial Intelligence as a Safety Net for Incidentally Identified Lung Nodules at a Tertiary Center. J. Am. Coll. Surg. 2025, 240, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Yang, T.; Liu, Z.; Jian, W.; Chen, Y.; Li, B.; Yan, Z.; Xu, W.; Chen, L.; Qi, Y.; et al. LungDiag: Empowering artificial intelligence for respiratory diseases diagnosis based on electronic health records, a multicenter study. Med. Comm. 2025, 6, e70043. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zumla, A.; Ahmed, R.; Bakhri, K. The role of artificial intelligence in the diagnosis, imaging, and treatment of thoracic empyema. Curr. Opin. Pulm. Med. 2024, 31, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, R.; Endo, Y.; Akashi, T.; Deguchi, H.; Tomoyasu, M.; Shigeeda, W.; Kaneko, Y.; Saito, H. Diagnostic artificial intelligence model predicts lymph node status in non-small cell lung cancer using simplified examination. J. Thorac. Dis. 2024, 16, 7320–7328. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ishiwata, T.; Inage, T.; Aragaki, M.; Gregor, A.; Chen, Z.; Bernards, N.; Kafi, K.; Yasufuku, K. Deep learning-based prediction of nodal metastasis in lung cancer using endobronchial ultrasound. JTCVS Tech. 2024, 28, 151–161. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, Y.; Xiong, S.; Ren, Q.; Wang, J.; Li, M.; Yang, L.; Wu, D.; Tang, K.; Pan, X.; Chen, F.; et al. Deep learning using histological images for gene mutation prediction in lung cancer: A multicentre retrospective study. Lancet Oncol. 2025, 26, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.K.; Araki, T.; Gefter, W.B.; Suzuki, Y.; Raevsky, A.; Saleh, A.; Yusuf, S.; Marquis, A.; Alcudia, A.; Duncan, I.; et al. Artificial intelligence-driven automated lung sizing from chest radiographs. Am. J. Transplant. 2025, 25, 198–203. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sargiotis, G.C.; Sergentanis, T.N.; Pavi, E.; Athanasakis, K. Predictive Performance of Artificial intelligence Models on Heart and Lung Posttransplant Health Outcomes: A Systematic Review. Exp. Clin. Transplant. 2024, 22, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Yan, H.J.; Huang, H.; Zuo, Y.J.; Liu, M.Z.; Zhao, J.; Wu, B.; Shi, L.Z.; Chen, J.Y. Machine Learning-Based Prognostic Model for Patients After Lung Transplantation. JAMA Netw. Open. 2023, 6, e2312022. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aleem, M.U.; Khan, J.A.; Younes, A.; Sabbah, B.N.; Saleh, W.; Migliore, M. Enhancing Thoracic Surgery with AI: A Review of Current Practices and Emerging Trends. Curr. Oncol. 2024, 31, 6232–6244. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shigemura, N. Transforming Diagnostics in Lung Transplantation: From Bronchoscopy to an Artificial Intelligence-driven Approach. Am. J. Respir. Crit. Care Med. 2020, 202, 486–488. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mitzman, B.; Smith, B.K.; Varghese, T.K., Jr. Resident Training in Robotic Thoracic Surgery. Thorac. Surg. Clin. 2023, 33, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Todd, J.L.; Neely, M.L.; Kopetskie, H.; Sever, M.L.; Kirchner, J.; Frankel, C.W.; Snyder, L.D.; Pavlisko, E.N.; Martinu, T.; Tsuang, W.; et al. Risk Factors for Acute Rejection in the First Year after Lung Transplant. A Multicenter Study. Am. J. Respir. Crit. Care Med. 2020, 202, 576–585. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hu, X.; He, X. Evaluation of the Postoperative Nursing Effect of Thoracic Surgery Assisted by Artificial Intelligence Robot. Contrast Media Mol. Imaging 2021, 2021, 3941600. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, X.; Liu, Y.; Zhou, Y.; Gao, Y.; Duan, C.; Zhang, C. Day surgery unit robotics thoracic surgery: Feasibility and management. J. Cancer Res. Clin. Oncol. 2023, 149, 7831–7836. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fairbairn, K.; Rice, J.; Worrell, S.G. Robotic Lobectomy. Thorac. Surg. Clin. 2023, 33, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Lazar, J.F.; Hwalek, A.E. A Review of Robotic Thoracic Surgery Adoption and Future Innovations. Thorac. Surg. Clin. 2023, 33, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Seastedt, K.P.; Watkins, A.A.; Kent, M.S.; Stock, C.T. Robotic Mediastinal Surgery. Thorac. Surg. Clin. 2023, 33, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, G. Robotic thoracic surgery: Technical considerations and learning curve for pulmonary resection. Thorac. Surg. Clin. 2014, 24, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Steenwyk, B.; Lyerly, R., 3rd. Advancements in robotic-assisted thoracic surgery. Anesthesiol. Clin. 2012, 30, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Tane, S.; Tanaka, Y.; Nishikubo, M.; Doi, T.; Hokka, D.; Maniwa, Y. Console and bedside surgeon fused robot-assisted thoracic surgery. Gen. Thorac. Cardiovasc. Surg. 2023, 71, 730–732. [Google Scholar] [CrossRef] [PubMed]
- Ostberg, N.P.; Zafar, M.A.; Elefteriades, J.A. Machine learning: Principles and applications for thoracic surgery. Eur. J. Cardiothorac. Surg. 2021, 60, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Herrera, L.; Escalon, J.; Johnston, M.; Sanchez, A.; Sanchez, R.; Mogollon, I. Development of a robot-assisted thoracic surgery (RATS) program. Lessons learned after 2500 cases. J. Robot. Surg. 2023, 17, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, B. Current status and prospect of robot-assisted thoracic surgery: A bibliometric analysis. Asian J. Surg. 2023, 46, 6037–6038. [Google Scholar] [CrossRef] [PubMed]
- Cold, K.M.; Xie, S.; Nielsen, A.O.; Clementsen, P.F.; Konge, L. Artificial Intelligence Improves Novices’ Bronchoscopy Performance: A Randomized Controlled Trial in a Simulated Setting. Chest 2024, 165, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Seastedt, K.P.; Moukheiber, D.; Mahindre, S.A.; Thammineni, C.; Rosen, D.T.; Watkins, A.A.; Hashimoto, D.A.; Hoang, C.D.; Kpodonu, J.; Celi, L.A. A scoping review of artificial intelligence applications in thoracic surgery. Eur. J. Cardiothorac. Surg. 2022, 61, 239–248. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Esteva, H.; Núñez, T.G.; Rodríguez, R.O. Neural networks and artificial intelligence in thoracic surgery. Thorac. Surg. Clin. 2007, 17, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Gencer, A.; Aydin, S. Can. ChatGPT pass the thoracic surgery exam? Am. J. Med. Sci. 2023, 366, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.; Reed, B.; Hayanga, J.A. Autonomously Driven: Artificial Intelligence in Cardiothoracic Surgery. Ann. Thorac. Surg. 2020, 110, 373. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- González-Gonzalo, C.; Thee, E.F.; Klaver, C.C.W.; Lee, A.Y.; Schlingemann, R.O.; Tufail, A.; Verbraak, F.; Sánchez, C.I. Trustworthy AI: Closing the gap between development and integration of AI systems in ophthalmic practice. Prog. Retin. Eye Res. 2022, 90, 101034. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abgrall, G.; Holder, A.L.; Chelly Dagdia, Z.; Zeitouni, K.; Monnet, X. Should AI models be explainable to clinicians? Crit. Care 2024, 28, 301. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ahmed, M.I.; Spooner, B.; Isherwood, J.; Lane, M.; Orrock, E.; Dennison, A. A Systematic Review of the Barriers to the Implementation of Artificial Intelligence in Healthcare. Cureus 2023, 15, e46454. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rahman, A.; Hossain, M.S.; Muhammad, G.; Kundu, D.; Debnath, T.; Rahman, M.; Khan, M.S.I.; Tiwari, P.; Band, S.S. Federated learning-based AI approaches in smart healthcare: Concepts, taxonomies, challenges and open issues. Cluster Comput. 2022, 26, 2271–2311. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chassagnon, G.; De Margerie-Mellon, C.; Vakalopoulou, M.; Marini, R.; Hoang-Thi, T.N.; Revel, M.P.; Soyer, P. Artificial intelligence in lung cancer: Current applications and perspectives. Jpn. J. Radiol. 2023, 41, 235–244. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zolfaghari, E.J.; Kuehne, A.M.; Antonoff, M.B. Silent Signals: Advancing Incidental Lung Nodule Screening Through Artificial Intelligence Innovation. Ann. Thorac. Surg. 2024, 19, S0003–S4975. [Google Scholar] [CrossRef] [PubMed]
- Cellina, M.; De Padova, G.; Caldarelli, N.; Libri, D.; Cè, M.; Martinenghi, C.; Alì, M.; Papa, S.; Carrafiello, G. Artificial Intelligence in Lung Cancer Imaging: From Data to Therapy. Crit. Rev. Oncog. 2024, 29, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Feng, X.; Chi, W.; Li, Z.; Duan, W.; Liu, H.; Liang, W.; Wang, W.; Chen, P.; He, J.; et al. Deep learning aided decision support for pulmonary nodules diagnosing: A review. J. Thorac. Dis. 2018, 10 (Suppl. S7), S867–S875. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Adams, S.J.; Stone, E.; Baldwin, D.R.; Vliegenthart, R.; Lee, P.; Fintelmann, F.J. Lung cancer screening. Lancet 2023, 401, 390–408. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.J.; Mikhael, P.; Wohlwend, J.; Barzilay, R.; Sequist, L.V.; Fintelmann, F.J. Artificial Intelligence and Machine Learning in Lung Cancer Screening. Thorac. Surg. Clin. 2023, 33, 401–409. [Google Scholar] [CrossRef] [PubMed]
- de Margerie-Mellon, C.; Chassagnon, G. Artificial intelligence: A critical review of applications for lung nodule and lung cancer. Diagn. Interv. Imaging 2023, 104, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wang, F.; Cao, W.; Qin, C.; Dong, X.; Yang, Z.; Zheng, Y.; Luo, Z.; Zhao, L.; Yu, Y.; et al. Lung cancer risk prediction models based on pulmonary nodules: A systematic review. Thorac. Cancer 2022, 13, 664–677. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Quanyang, W.; Yao, H.; Sicong, W.; Linlin, Q.; Zewei, Z.; Donghui, H.; Hongjia, L.; Shijun, Z. Artificial intelligence in lung cancer screening: Detection, classification, prediction, and prognosis. Cancer Med. 2024, 13, e7140. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, M.; Copley, S.J.; Viola, P.; Lu, H.; Aboagye, E.O. Radiomics and artificial intelligence for precision medicine in lung cancer treatment. Semin. Cancer Biol. 2023, 93, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Jochems, A.; Refaee, T.; Ibrahim, A.; Yan, C.; Sanduleanu, S.; Woodruff, H.C.; Lambin, P. Structural and functional radiomics for lung cancer. Eur. J. Nucl. Med. Mol. Imaging. 2021, 48, 3961–3974. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Avanzo, M.; Stancanello, J.; Pirrone, G.; Sartor, G. Radiomics and deep learning in lung cancer. Strahlenther. Onkol. 2020, 196, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Tunali, I.; Gillies, R.J.; Schabath, M.B. Application of Radiomics and Artificial Intelligence for Lung Cancer Precision Medicine. Cold Spring Harb. Perspect. Med. 2021, 11, a039537. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, A.; Wang, Z.; Yang, Y.; Wang, J.; Dai, X.; Wang, L.; Lu, Y.; Xue, F. Preoperative diagnosis of malignant pulmonary nodules in lung cancer screening with a radiomics nomogram. Cancer Commun. 2020, 40, 16–24. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mehta, V. Artificial intelligence augmentation raises questions about the future of bronchoscopy. ERJ Open Res. 2025, 11, 00931-2024. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, X.; Yu, Z. Pathological analysis of hesperetin-derived small cell lung cancer by artificial intelligence technology under fiberoptic bronchoscopy. Math. Biosci. Eng. 2021, 18, 8538–8558. [Google Scholar] [CrossRef] [PubMed]
- Churchill, I.F.; Gatti, A.A.; Hylton, D.A.; Sullivan, K.A.; Patel, Y.S.; Leontiadis, G.I.; Farrokhyar, F.; Hanna, W.C. An Artificial Intelligence Algorithm to Predict Nodal Metastasis in Lung Cancer. Ann. Thorac. Surg. 2022, 114, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Patel, Y.S.; Gatti, A.A.; Farrokhyar, F.; Xie, F.; Hanna, W.C. Clinical utility of artificial intelligence-augmented endobronchial ultrasound elastography in lymph node staging for lung cancer. JTCVS Tech. 2024, 27, 158–166. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zheng, X.; He, B.; Hu, Y.; Ren, M.; Chen, Z.; Zhang, Z.; Ma, J.; Ouyang, L.; Chu, H.; Gao, H.; et al. Diagnostic Accuracy of Deep Learning and Radiomics in Lung Cancer Staging: A Systematic Review and Meta-Analysis. Front. Public Health 2022, 10, 938113. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhong, Y.; She, Y.; Deng, J.; Chen, S.; Wang, T.; Yang, M.; Ma, M.; Song, Y.; Qi, H.; Wang, Y.; et al. Deep Learning for Prediction of N2 Metastasis and Survival for Clinical Stage I Non-Small Cell Lung Cancer. Radiology 2022, 302, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Ladbury, C.; Amini, A.; Govindarajan, A.; Mambetsariev, I.; Raz, D.J.; Massarelli, E.; Williams, T.; Rodin, A.; Salgia, R. Integration of artificial intelligence in lung cancer: Rise of the machine. Cell Rep. Med. 2023, 4, 100933. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Poullis, M. The transformation of risk modelling in cardiac and thoracic surgery through artificial intelligence. Eur. J. Cardiothorac. Surg. 2024, 65, ezae013. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.; Matthews, J. Artificial Intelligence: Predicting Perioperative Problems. Br. J. Hosp. Med. 2024, 85, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Raghu, V.K.; Moonsamy, P.; Sundt, T.M.; Ong, C.S.; Singh, S.; Cheng, A.; Hou, M.; Denning, L.; Gleason, T.G.; Aguirre, A.D.; et al. Deep Learning to Predict Mortality After Cardiothoracic Surgery Using Preoperative Chest Radiographs. Ann. Thorac. Surg. 2023, 115, 257–264. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lynch, C.M.; Abdollahi, B.; Fuqua, J.D.; de Carlo, A.R.; Bartholomai, J.A.; Balgemann, R.N.; van Berkel, V.H.; Frieboes, H.B. Prediction of lung cancer patient survival via supervised machine learning classification techniques. Int. J. Med. Inform. 2017, 108, 1–8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- She, Y.; Jin, Z.; Wu, J.; Deng, J.; Zhang, L.; Su, H.; Jiang, G.; Liu, H.; Xie, D.; Cao, N.; et al. Development and Validation of a Deep Learning Model for Non-Small Cell Lung Cancer Survival. JAMA Netw. Open. 2020, 3, e205842. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhong, Y.; Cai, C.; Chen, T.; Gui, H.; Deng, J.; Yang, M.; Yu, B.; Song, Y.; Wang, T.; Sun, X.; et al. PET/CT based cross-modal deep learning signature to predict occult nodal metastasis in lung cancer. Nat. Commun. 2023, 14, 7513. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lococo, F.; Ghaly, G.; Chiappetta, M.; Flamini, S.; Evangelista, J.; Bria, E.; Stefani, A.; Vita, E.; Martino, A.; Boldrini, L.; et al. Implementation of Artificial Intelligence in Personalized Prognostic Assessment of Lung Cancer: A Narrative Review. Cancers 2024, 16, 1832. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shu, J.; Jiang, J.; Zhao, G. Identification of novel gene signature for lung adenocarcinoma by machine learning to predict immunotherapy and prognosis. Front. Immunol. 2023, 14, 1177847. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ost, D.E. Machine Learning for Treating Complicated Parapneumonic Effusions: The Fundamentals Still Apply. Chest 2018, 154, 471–473. [Google Scholar] [CrossRef] [PubMed]
- Khemasuwan, D.; Wilshire, C.; Reddy, C.; Gilbert, C.; Gorden, J.; Balwan, A.; Sanchez, T.M.; Bixby, B.; Sorensen, J.S.; Shojaee, S. Machine Learning Model Predictors of Intrapleural Tissue Plasminogen Activator and DNase Failure in Pleural Infection: A Multicenter Study. Ann. Am. Thorac. Soc. 2025, 22, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Torok, R.; Meszaros, B.; Gombas, V.; Vathy-Fogarassy, A.; Szabo, M.; Csanky, E.; Jarvas, G.; Guttman, A. Predicting the effectiveness of chemotherapy treatment in lung cancer utilizing artificial intelligence-supported serum N-glycome analysis. Comput. Biol. Med. 2025, 186, 109681. [Google Scholar] [CrossRef] [PubMed]
- She, Y.; He, B.; Wang, F.; Zhong, Y.; Wang, T.; Liu, Z.; Yang, M.; Yu, B.; Deng, J.; Sun, X.; et al. Deep learning for predicting major pathological response to neoadjuvant chemoimmunotherapy in non-small cell lung cancer: A multicentre study. EBioMedicine 2022, 86, 104364. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gu, Q.; Li, S. Computed Tomography Image under Artificial Intelligence Algorithm to Evaluate the Nursing and Treatment Effect of Pemetrexed Combined Platinum-Based Chemotherapy on Elderly Lung Cancer. Contrast Media Mol. Imaging 2022, 2022, 2574451. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mei, C.; Zhang, L.; Zhang, Z. Vomiting Management and Effect Prediction after Early Chemotherapy of Lung Cancer with Diffusion-Weighted Imaging under Artificial Intelligence Algorithm and Comfort Care Intervention. Comput. Math. Methods Med. 2022, 2022, 1056910. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lyman, G.H.; Kuderer, N.M. Artificial Intelligence and Cancer Clinical Research: III Risk Prediction Models for Febrile Neutropenia in Patients Receiving Cancer Chemotherapy. Cancer Investig. 2024, 42, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Darsey, J.A.; Ghosh, A.; Li, H.Y.; Yang, M.Q.; Wang, S. Artificial Intelligence and Cancer Drug Development. Recent. Pat. Anticancer. Drug Discov. 2022, 17, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, X.; Yang, P.; Jiang, G.; Luo, Y. Machine Learning for Lung Cancer Diagnosis, Treatment, and Prognosis. Genom. Proteom. Bioinform. 2022, 20, 850–866. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, N.; Zhang, H.; Liu, Z.; Dai, Z.; Wu, W.; Zhou, R.; Li, S.; Wang, Z.; Liang, X.; Wen, J.; et al. An artificial intelligence network-guided signature for predicting outcome and immunotherapy response in lung adenocarcinoma patients based on 26 machine learning algorithms. Cell Prolif. 2023, 56, e13409. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saad, M.B.; Hong, L.; Aminu, M.; Vokes, N.I.; Chen, P.; Salehjahromi, M.; Qin, K.; Sujit, S.J.; Lu, X.; Young, E.; et al. Predicting benefit from immune checkpoint inhibitors in patients with non-small-cell lung cancer by CT-based ensemble deep learning: A retrospective study. Lancet Digit. Health 2023, 5, e404–e420. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yin, X.; Liao, H.; Yun, H.; Lin, N.; Li, S.; Xiang, Y.; Ma, X. Artificial intelligence-based prediction of clinical outcome in immunotherapy and targeted therapy of lung cancer. Semin. Cancer Biol. 2022, 86 Pt 2, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Yang, L.; Lu, M.; Jin, R.; Ye, H.; Ma, T. The artificial intelligence and machine learning in lung cancer immunotherapy. J. Hematol. Oncol. 2023, 16, 55. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Park, S.; Ock, C.Y.; Kim, H.; Pereira, S.; Park, S.; Ma, M.; Choi, S.; Kim, S.; Shin, S.; Aum, B.J.; et al. Artificial Intelligence-Powered Spatial Analysis of Tumor-Infiltrating Lymphocytes as Complementary Biomarker for Immune Checkpoint Inhibition in Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2022, 40, 1916–1928. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheng, G.; Zhang, F.; Xing, Y.; Hu, X.; Zhang, H.; Chen, S.; Li, M.; Peng, C.; Ding, G.; Zhang, D.; et al. Artificial Intelligence-Assisted Score Analysis for Predicting the Expression of the Immunotherapy Biomarker PD-L1 in Lung Cancer. Front. Immunol. 2022, 13, 893198. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roisman, L.C.; Kian, W.; Anoze, A.; Fuchs, V.; Spector, M.; Steiner, R.; Kassel, L.; Rechnitzer, G.; Fried, I.; Peled, N.; et al. Radiological artificial intelligence—Predicting personalized immunotherapy outcomes in lung cancer. NPJ Precis. Oncol. 2023, 7, 125. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mei, T.; Wang, T.; Zhou, Q. Multi-omics and artificial intelligence predict clinical outcomes of immunotherapy in non-small cell lung cancer patients. Clin. Exp. Med. 2024, 24, 60. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Walls, G.M.; Osman, S.O.S.; Brown, K.H.; Butterworth, K.T.; Hanna, G.G.; Hounsell, A.R.; McGarry, C.K.; Leijenaar, R.T.H.; Lambin, P.; Cole, A.J.; et al. Radiomics for Predicting Lung Cancer Outcomes Following Radiotherapy: A Systematic Review. Clin. Oncol. 2022, 34, e107–e122. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Gao, W.; Lv, X.; Zhao, Z.; Xu, X.; Wu, Z.; Mao, G.; Chen, J. Artificial intelligence predicts lung cancer radiotherapy response: A meta-analysis. Artif. Intell. Med. 2023, 142, 102585. [Google Scholar] [CrossRef] [PubMed]
- Borghetti, P.; Costantino, G.; Santoro, V.; Mataj, E.; Singh, N.; Vitali, P.; Greco, D.; Volpi, G.; Sepulcri, M.; Guida, C.; et al. Artificial Intelligence-suggested Predictive Model of Survival in Patients Treated With Stereotactic Radiotherapy for Early Lung Cancer. Vivo 2024, 38, 1359–1366. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chan, S.T.; Ruan, D.; Shaverdian, N.; Raghavan, G.; Cao, M.; Lee, P. Effect of Radiation Doses to the Heart on Survival for Stereotactic Ablative Radiotherapy for Early-stage Non-Small-cell Lung Cancer: An Artificial Neural Network Approach. Clin. Lung Cancer 2020, 21, 136–144.e1. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, J.H.; Kim, H.J.; Park, C.M.; Wu, H.G.; Goo, J.M. Extended application of a CT-based artificial intelligence prognostication model in patients with primary lung cancer undergoing stereotactic ablative radiotherapy. Radiother. Oncol. 2021, 165, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Wang, Y.; Wang, W.; Zhang, T.; Wang, J.; Ma, X.; Men, K.; Shi, A.; Gao, Y.; Bi, N. Artificial intelligence-assisted delineation for postoperative radiotherapy in patients with lung cancer: A prospective, multi-center, cohort study. Front. Oncol. 2024, 14, 1388297. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hompe, E.D.; Furlow, P.W.; Schumacher, L.Y. Starting and Developing a Robotic Thoracic Surgery Program. Thorac. Surg. Clin. 2023, 33, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, S.; Luo, X.; Gao, G.; Luo, X.; Wang, S.; Li, S.; Zhao, D.; Wang, Y.; Cui, X.; et al. Accuracy and efficiency of an artificial intelligence-based pulmonary broncho-vascular three-dimensional reconstruction system supporting thoracic surgery: Retrospective and prospective validation study. EBioMedicine 2023, 87, 104422. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dias, R.D.; Shah, J.A.; Zenati, M.A. Artificial intelligence in cardiothoracic surgery. Minerva Cardioangiol. 2020, 68, 532–538. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Leivaditis, V.; Beltsios, E.; Papatriantafyllou, A.; Grapatsas, K.; Mulita, F.; Kontodimopoulos, N.; Baikoussis, N.G.; Tchabashvili, L.; Tasios, K.; Maroulis, I.; et al. Artificial Intelligence in Cardiac Surgery: Transforming Outcomes and Shaping the Future. Clin. Pract. 2025, 15, 17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zenati, M.A.; Kennedy-Metz, L.; Dias, R.D. Cognitive Engineering to Improve Patient Safety and Outcomes in Cardiothoracic Surgery. In Seminars in Thoracic and Cardiovascular Surgery; Spring: Berlin/Heidelberg, Germany, 2020; pp. 1–7. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mumtaz, H.; Saqib, M.; Ansar, F.; Zargar, D.; Hameed, M.; Hasan, M.; Muskan, P. The future of Cardiothoracic surgery in Artificial intelligence. Ann. Med. Surg. 2022, 80, 104251. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kennedy-Metz, L.R.; Mascagni, P.; Torralba, A.; Dias, R.D.; Perona, P.; Shah, J.A.; Padoy, N.; Zenati, M.A. Computer Vision in the Operating Room: Opportunities and Caveats. IEEE Trans. Med. Robot. Bionics. 2021, 3, 2–10. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Padoy, N. Machine and deep learning for workflow recognition during surgery. Minim. Invasive Ther. Allied Technol. 2019, 28, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Malík, M.; Dzian, A.; Števík, M.; Vetešková, Š.; Al Hakim, A.; Hliboký, M.; Magyar, J.; Kolárik, M.; Bundzel, M.; Babič, F. Lung Ultrasound Reduces Chest X-rays in Postoperative Care after Thoracic Surgery: Is There a Role for Artificial Intelligence?—Systematic Review. Diagnostics 2023, 13, 2995. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Raad, W.N.; Ayub, A.; Huang, C.Y.; Guntman, L.; Rehmani, S.S.; Bhora, F.Y. Robotic Thoracic Surgery Training for Residency Programs: A Position Paper for an Educational Curriculum. Innovations 2018, 13, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, A.; Sethi, S. Motivation and Learning: Leveraging Artificial Intelligence to Improve Bronchoscopy Performance. Chest 2024, 165, 243–245. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, D.; Oggiano, M.; Hecker, E. Einsatz künstlicher Intelligenz in der Thoraxchirurgie [Application of artificial intelligence in thoracic surgery]. Chirurg 2020, 91, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Olaye, I.M.; Seixas, A.A. The Gap Between AI and Bedside: Participatory Workshop on the Barriers to the Integration, Translation, and Adoption of Digital Health Care and AI Startup Technology Into Clinical Practice. J. Med. Internet Res. 2023, 25, e32962. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Esmaeilzadeh, P. Challenges and strategies for wide-scale artificial intelligence (AI) deployment in healthcare practices: A perspective for healthcare organizations. Artif. Intell. Med. 2024, 151, 102861. [Google Scholar] [CrossRef] [PubMed]
- Grayek, E.; Krishnamurti, T.; Hu, L.; Babich, O.; Warren, K.; Fischhoff, B. Collection and Analysis of Adherence Information for Software as a Medical Device Clinical Trials: Systematic Review. JMIR Mhealth Uhealth. 2023, 11, e46237. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Machal, M.L. Impact of Class I Software as Medical Devices (SaMDs) on Public Health. Stud. Health Technol. Inform. 2022, 295, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, E.; Mayer, C.C. Medical Device Regulation: Should We Care About It? Artery Res. 2022, 28, 55–60. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bretthauer, M.; Gerke, S.; Hassan, C.; Ahmad, O.F.; Mori, Y. The New European Medical Device Regulation: Balancing Innovation and Patient Safety. Ann. Intern. Med. 2023, 176, 844–848. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Hom, G.L.; Abramoff, M.D.; Campbell, J.P.; Chiang, M.F.; AAO Task Force on Artificial Intelligence. Current Challenges and Barriers to Real-World Artificial Intelligence Adoption for the Healthcare System, Provider, and the Patient. Transl. Vis. Sci. Technol. 2020, 9, 45. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mudgal, S.K.; Agarwal, R.; Chaturvedi, J.; Gaur, R.; Ranjan, N. Real-world application, challenges and implication of artificial intelligence in healthcare: An essay. Pan Afr. Med. J. 2022, 43, 3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Safdar, N.M.; Banja, J.D.; Meltzer, C.C. Ethical considerations in artificial intelligence. Eur. J. Radiol. 2020, 122, 108768. [Google Scholar] [CrossRef] [PubMed]
- Schwalbe, N.; Wahl, B. Artificial intelligence and the future of global health. Lancet 2020, 395, 1579–1586. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]



| Type of Bias | Description | Impact on Findings | Recommendations |
|---|---|---|---|
| Selection Bias | Overrepresentation of specific patient populations or disease stages due to non-randomized datasets. | Limits generalizability and external validity of AI model performance. | Promote inclusion of diverse, representative cohorts; use randomized or stratified sampling. |
| Reporting Bias | Incomplete or insufficient reporting of AI model development, training, and validation details. | Reduces transparency and reproducibility; impairs critical appraisal of findings. | Encourage adherence to AI-specific reporting guidelines (e.g., TRIPOD-AI, CONSORT-AI). |
| Publication Bias | Tendency to publish positive results, while negative or inconclusive findings are underreported. | Overestimates the perceived effectiveness of AI tools. | Register trials and encourage publication of all results regardless of outcome. |
| Performance Bias | Variability in the quality and size of training datasets used to develop AI models. | Increases risk of overfitting and poor generalization to external datasets. | Utilize larger, multicenter datasets; apply external validation methods. |
| Detection Bias | Inconsistent outcome measurement methods and validation techniques across studies. | Complicates study comparability; may distort conclusions about AI effectiveness. | Standardize outcome measures and validation protocols across studies. |
| Author(s) | Year | Title | Study Focus | AI Application | Key Findings |
|---|---|---|---|---|---|
| You et al. [18] | 2022 | Artificial intelligence in cancer target identification and drug discovery | Cancer target identification and drug discovery | Machine learning and network-based models for identifying anticancer targets and drug discovery | AI enables quantitative analysis of biological networks, identifying novel drug targets and promising drug candidates. |
| Zhang and Chen [19] | 2022 | Artificial intelligence: opportunities in lung cancer | Lung cancer management | AI in screening, diagnosis, and treatment, including clinical decision-support systems | AI shows significant potential across the lung cancer clinical pathway but requires advancements in interpretability. |
| Števík et al. [20] | 2024 | Hybrid AI solution combining CNN and analytical approaches in lung ultrasound for thoracic surgery | Lung ultrasound diagnosis | Convolutional neural networks (CNNs) combined with analytical approaches | Hybrid AI models demonstrated higher accuracy in detecting A-lines compared to radiology residents. |
| Woodhouse et al. [21] | 2025 | Leveraging AI as a safety net for incidental lung nodule identification | Lung nodule identification | AI-based safety systems for incidental findings in imaging | AI effectively reduces missed incidental nodules, enhancing detection rates. |
| Liang et al. [22] | 2025 | LungDiag: AI for respiratory disease diagnosis based on electronic health records | Diagnosis of respiratory diseases | AI analysis of electronic health records (EHRs) | AI models accurately diagnose respiratory diseases, demonstrating scalability across multiple centers. |
| Zumla et al. [23] | 2024 | The role of AI in the diagnosis, imaging, and treatment of thoracic empyema | Thoracic empyema | AI applications in imaging and treatment | AI offers significant diagnostic accuracy in imaging-based thoracic empyema management. |
| Yoshimura et al. [24] | 2024 | Diagnostic AI model predicts lymph node status in NSCLC | Lymph node status prediction in lung cancer | AI predictive models for lymph node involvement | AI demonstrated high accuracy in predicting lymph node status using simplified data inputs. |
| Ishiwata et al. [25] | 2024 | Deep learning-based prediction of nodal metastasis in lung cancer | Prediction of nodal metastasis | Deep learning applied to endobronchial ultrasound (EBUS) imaging | AI improved accuracy in detecting nodal metastasis compared to traditional methods. |
| Zhao et al. [26] | 2025 | Deep learning for gene mutation prediction in lung cancer | Gene mutation prediction | Deep learning applied to histological image analysis | AI achieved robust performance in predicting gene mutations associated with lung cancer. |
| Ismail et al. [27] | 2025 | AI-driven automated lung sizing from chest radiographs | Lung sizing pre-transplantation | Automated lung sizing from radiographs | AI significantly streamlined pre-transplantation workflows, reducing manual errors. |
| Sargiotis et al. [28] | 2024 | Predictive performance of AI models for post-transplant outcomes | Post-transplant outcomes | AI for prediction of heart and lung post-transplant health outcomes | AI accurately predicted post-transplant complications and recovery trajectories. |
| Tian et al. [29] | 2023 | ML-based prognostic models for post-lung transplantation | Prognostic modeling | Machine learning for long-term outcome prediction | AI-enabled models effectively identified patients at risk of adverse outcomes post-transplant. |
| Etienne et al. [11] | 2020 | Artificial intelligence in thoracic surgery: past, present, perspective, and limits | AI in thoracic surgery | Comprehensive review of AI applications | AI offers significant potential, but limitations in data integration and adoption persist. |
| Aleem et al. [30] | 2024 | Enhancing thoracic surgery with AI: A review of current practices and emerging trends | AI in thoracic surgery | Review of AI advancements and emerging trends | Identifies trends in AI applications and challenges for widespread integration. |
| Shigemura [31] | 2020 | Transforming diagnostics in lung transplantation with AI | Diagnostics for lung transplantation | AI applications for diagnostics, particularly in imaging | AI improves diagnostic accuracy in lung transplantation by incorporating real-time imaging analysis. |
| Mitzman et al. [32] | 2023 | Resident training in robotic thoracic surgery | Training in robotic thoracic surgery | AI for robotic surgery training | Highlights the role of AI in enhancing surgical training, particularly for complex robotic techniques. |
| Todd et al. [33] | 2020 | Risk Factors for Acute Rejection in the First Year after Lung Transplant | Lung transplant rejection | AI for identifying risk factors for acute rejection | Identified key risk factors for rejection using data from a multicenter cohort, improving predictive modeling. |
| Hu and He [34] | 2021 | Evaluation of the Postoperative Nursing Effect of Thoracic Surgery Assisted by AI Robot | Postoperative nursing care | AI robotic systems for monitoring and assistance | AI-assisted robotic systems improved postoperative nursing outcomes and reduced complications. |
| Li et al. [35] | 2023 | Day surgery unit robotics thoracic surgery: feasibility and management | Robotics in thoracic day surgery | Robotic systems for minimally invasive day surgery | Demonstrated the feasibility and benefits of robotic-assisted day surgeries, including reduced hospital stays. |
| Fairbairn et al. [36] | 2023 | Robotic Lobectomy | Robotic lobectomy | Robotic-assisted thoracic surgery | Highlighted the advantages of robotic lobectomy in terms of precision, safety, and recovery times. |
| Lazar and Hwalek [37] | 2023 | A Review of Robotic Thoracic Surgery Adoption and Future Innovations | Robotic thoracic surgery adoption | Overview of robotic surgery technologies and innovations | Reviewed the current adoption trends and potential future advancements in robotic thoracic surgery. |
| Seastedt et al. [38] | 2023 | Robotic Mediastinal Surgery | Robotic mediastinal surgery | Robotic platforms for mediastinal procedures | Discussed the effectiveness of robotic platforms for complex mediastinal surgeries, highlighting reduced complications. |
| Veronesi G [39] | 2014 | Robotic thoracic surgery: technical considerations and learning curve | Robotic thoracic surgery learning curve | Robotic-assisted surgery and surgeon training | Discussed the technical challenges and surgeon learning curves associated with robotic thoracic surgery. |
| Steenwyk and Lyerly [40] | 2012 | Advancements in robotic-assisted thoracic surgery | Robotic-assisted thoracic surgery | Innovations in robotic surgery | Provided a historical perspective on advancements in robotic-assisted thoracic surgery technologies. |
| Tane et al. [41] | 2023 | Console and bedside surgeon fused robot-assisted thoracic surgery | Robotic thoracic surgery | Fusion of console and bedside surgical roles in robotic thoracic procedures | Demonstrated improved workflow efficiency and precision by fusing roles of console and bedside surgeons. |
| Ostberg et al. [42] | 2021 | Machine learning: principles and applications for thoracic surgery | Machine learning applications in thoracic surgery | Principles and frameworks for machine learning in thoracic surgical settings | Highlighted practical applications and barriers to implementation of machine learning in thoracic surgery. |
| Herrera et al. [43] | 2023 | Development of a robot-assisted thoracic surgery (RATS) program | Program implementation for robotic thoracic surgery | Lessons learned from implementing RATS in 2500 cases | Provided practical insights into challenges and successes of large-scale RATS program adoption. |
| Wang and Wang [44] | 2023 | Current status and prospect of robot-assisted thoracic surgery: A bibliometric analysis | Bibliometric analysis of robotic thoracic surgery | Statistical analysis of robotic-assisted thoracic surgery trends | Identified trends, research gaps, and areas for future exploration in robotic-assisted thoracic surgery. |
| Cold et al. [45] | 2024 | Artificial Intelligence Improves Novices’ Bronchoscopy Performance | AI in surgical training | AI-assisted bronchoscopy simulation for novices | Demonstrated significant improvements in bronchoscopy performance and learning outcomes for novices. |
| Seastedt et al. [46] | 2022 | A scoping review of artificial intelligence applications in thoracic surgery | Scoping review of AI in thoracic surgery | Comprehensive review of AI applications across thoracic surgery | Identified key areas of AI utility, limitations, and future directions in thoracic surgery. |
| Esteva et al. [47] | 2007 | Neural networks and artificial intelligence in thoracic surgery | Early applications of AI in thoracic surgery | Neural networks for image analysis and decision support | Discussed foundational AI techniques and their potential for improving thoracic surgical outcomes. |
| Gencer and Aydin [48] | 2023 | Can ChatGPT pass the thoracic surgery exam? | AI for educational purposes | Assessment of ChatGPT’s ability to respond to thoracic surgery exam questions | Found ChatGPT to have limitations in specialized thoracic surgery knowledge but potential for educational support. |
| Jones et al. [49] | 2020 | Autonomously Driven: Artificial Intelligence in Cardiothoracic Surgery | AI in cardiothoracic surgery | Overview of autonomous AI systems in surgical decision-making | Explored the development and challenges of autonomous AI in cardiothoracic surgery. |
| AI Modality | Application Area | Example Study | Reported Performance | Clinical Relevance |
|---|---|---|---|---|
| Deep Learning (CNNs) | Pulmonary nodule detection | Yang et al. [57] | Accuracy: 94.2%; AUC: 0.91 | Improves early lung cancer detection and treatment planning |
| NLP + Machine Learning | Respiratory disease diagnosis (EHR) | Liang et al. [22] | F1 Score: 0.927 (Top 3 diagnoses) | Supports rapid and scalable diagnosis via EHR analysis |
| Random Forest | Post-transplant survival prediction | Tian et al. [29] | Stratified survival: 14.8 vs. 52.9 months | Assists in identifying high-risk transplant patients |
| AI-Enhanced 3D Reconstruction | Surgical planning and intraoperative guidance | Li et al. [107] | Dice score: 89.2%; time savings: 24 min | Optimizes anatomical accuracy and reduces operative time |
| Deep Learning | Gene mutation prediction (EGFR/KRAS) | Zhao et al. [26] | High accuracy for EGFR/KRAS prediction | Supports targeted therapy decisions in surgical oncology |
| Hybrid AI (CNN + analytical) | Lung ultrasound imaging | Števík et al. [20] | Sensitivity: 92.8%; Specificity: 83.4% | Improves diagnostic accuracy in intraoperative imaging |
| Ensemble Deep Learning | Immunotherapy response prediction | Saad et al. [93] | C-index for OS: 0.75; outperformed clinical risk factors | Enhances immunotherapy stratification for surgical candidates |
| Machine Learning | Preoperative risk assessment | Poullis [75] | Enhanced prediction vs. traditional models | Enables personalized surgical risk modeling |
| AI-Based Radiomics | Prognosis and staging via imaging | Zheng et al. [72] | AUROC: 0.83 (detection); 0.74 (nodal metastasis) | Improves staging accuracy and treatment selection |
| Artificial Neural Networks | Radiotherapy toxicity and outcome prediction | Chan et al. [103] | Accuracy: 64.7%; predictive for survival outcomes | Informs cardiac-sparing strategies during radiotherapy |
| Computer Vision + ML | Intraoperative skill assessment and guidance | Ostberg et al. [42] | Real-time feedback and skill tracking; qualitative validation | Improves surgical precision and supports team-based performance optimization |
| AI-Powered Monitoring Systems | Postoperative complication detection | Hu and He [34] | Reduced complication rates and enhanced vital sign stability | Enables early detection of postoperative issues and supports enhanced recovery protocols |
| Predictive ML Models | Postoperative survival and readmission risk | She et al. [79] | Outperformed TNM staging in survival prediction | Supports personalized follow-up strategies and resource allocation post-surgery |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leivaditis, V.; Maniatopoulos, A.A.; Lausberg, H.; Mulita, F.; Papatriantafyllou, A.; Liolis, E.; Beltsios, E.; Adamou, A.; Kontodimopoulos, N.; Dahm, M. Artificial Intelligence in Thoracic Surgery: A Review Bridging Innovation and Clinical Practice for the Next Generation of Surgical Care. J. Clin. Med. 2025, 14, 2729. https://doi.org/10.3390/jcm14082729
Leivaditis V, Maniatopoulos AA, Lausberg H, Mulita F, Papatriantafyllou A, Liolis E, Beltsios E, Adamou A, Kontodimopoulos N, Dahm M. Artificial Intelligence in Thoracic Surgery: A Review Bridging Innovation and Clinical Practice for the Next Generation of Surgical Care. Journal of Clinical Medicine. 2025; 14(8):2729. https://doi.org/10.3390/jcm14082729
Chicago/Turabian StyleLeivaditis, Vasileios, Andreas Antonios Maniatopoulos, Henning Lausberg, Francesk Mulita, Athanasios Papatriantafyllou, Elias Liolis, Eleftherios Beltsios, Antonis Adamou, Nikolaos Kontodimopoulos, and Manfred Dahm. 2025. "Artificial Intelligence in Thoracic Surgery: A Review Bridging Innovation and Clinical Practice for the Next Generation of Surgical Care" Journal of Clinical Medicine 14, no. 8: 2729. https://doi.org/10.3390/jcm14082729
APA StyleLeivaditis, V., Maniatopoulos, A. A., Lausberg, H., Mulita, F., Papatriantafyllou, A., Liolis, E., Beltsios, E., Adamou, A., Kontodimopoulos, N., & Dahm, M. (2025). Artificial Intelligence in Thoracic Surgery: A Review Bridging Innovation and Clinical Practice for the Next Generation of Surgical Care. Journal of Clinical Medicine, 14(8), 2729. https://doi.org/10.3390/jcm14082729







