Redo-Transcatheter Aortic Valve Replacement Procedural Optimization and Patient Selection: From Bench to Clinical Practice
Abstract
:1. Introduction
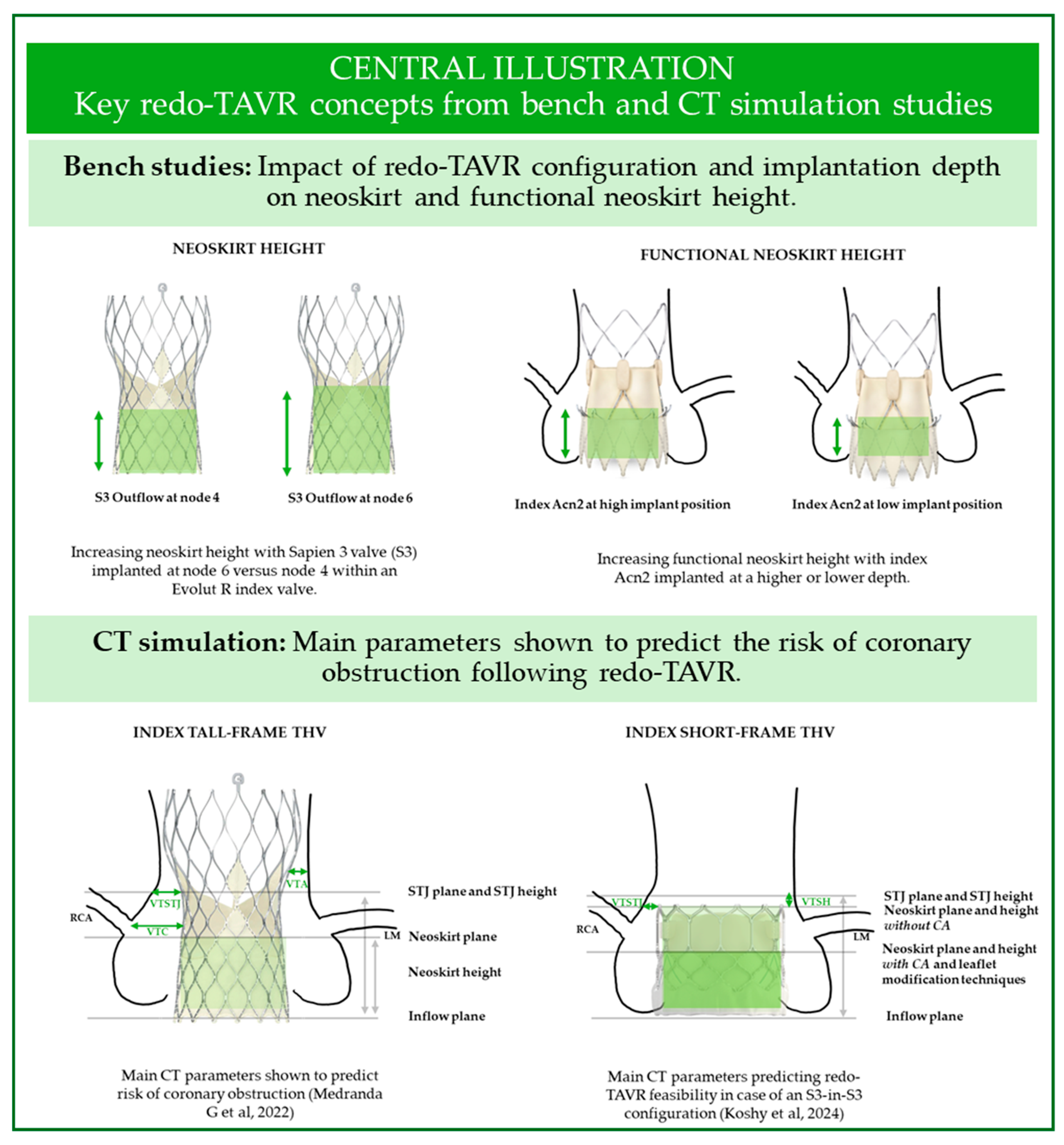
2. Insights from Bench Studies
2.1. Leaflet Function
2.2. Hydrodynamic Performance
2.3. Coronary Access: The Impact of Neoskirt Height
2.4. Coronary Access: The Importance of Commissural Alignment
3. Insights from CT Studies
3.1. CT Pre-Procedural Planning: Predicting Coronary Obstruction
3.1.1. Choice of the Redo-TAVR Valve
3.1.2. Index Valve Is a SEV
3.1.3. Index Valve Is a BEV
3.2. CT Studies Evaluating Feasibility of Coronary Access in Redo-TAVR Patients
3.2.1. Coronary Artery Origin and the Neoskirt Plane
3.2.2. Commissural Alignment
3.2.3. Commissural Alignment of Self-Expanding Valves
3.2.4. Commissural Alignment of Balloon-Expanding Valves
4. Insights from Clinical Studies
4.1. Real-World Indications for Redo-TAVR
4.2. Clinical Experience with Redo-TAVR: Success and Failure
4.3. Safety and Clinical Outcomes of Redo-TAVR Procedures
5. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BEV | Balloon-expandable valve |
| CA | Commissural alignment |
| CMA | Commissural misalignment |
| CT | Computed tomography |
| HALT | Hypo-attenuated leaflet thickening |
| TAVR | Transcatheter aortic valve replacement |
| THV | Transcatheter heart valve |
| SAVR | Surgical aortic valve replacement |
| SEV | Self-expandable valve |
| VTA | Valve-to-aorta distance |
| VTC | Valve-to-coronary distance |
References
- Aziz, M.; Simonato, M.; Webb, J.G.; Abdel-Wahab, M.; McElhinney, D.; Duncan, A.; Tchetche, D.; Barbanti, M.; Petronio, A.S.; Maisano, F.; et al. Mortality Prediction after Transcatheter Treatment of Failed Bioprosthetic Aortic Valves Utilizing Various International Scoring Systems: Insights from the Valve-in-Valve International Data (VIVID). Catheter Cardiovasc. Interv. 2018, 92, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Heen, A.F.; Lytvyn, L.; Shapiro, M.; Guyatt, G.H.; Siemieniuk, R.A.C.; Zhang, Y.; Manja, V.; Vandvik, P.O.; Agoritsas, T. Patient Values and Preferences on Valve Replacement for Aortic Stenosis: A Systematic Review. Heart 2021, 107, 1289–1295. [Google Scholar] [CrossRef]
- Landes, U.; Richter, I.; Danenberg, H.; Kornowski, R.; Sathananthan, J.; De Backer, O.; Søndergaard, L.; Abdel-Wahab, M.; Yoon, S.H.; Makkar, R.R.; et al. Outcomes of Redo Transcatheter Aortic Valve Replacement According to the Initial and Subsequent Valve Type. JACC Cardiovasc. Interv. 2022, 15, 1543–1554. [Google Scholar] [CrossRef] [PubMed]
- Akodad, M.; Sellers, S.; Landes, U.; Meier, D.; Tang, G.H.L.; Gada, H.; Rogers, T.; Caskey, M.; Rutkin, B.; Puri, R.; et al. Balloon-Expandable Valve for Treatment of Evolut Valve Failure. JACC Cardiovasc. Interv. 2022, 15, 368–377. [Google Scholar] [CrossRef]
- Akodad, M.; Meier, D.; Sellers, S.; de Backer, O.; Mylotte, D.; Landes, U.; Frawley, C.; Lynch, L.; Tang, G.H.L.; Sondergaard, L.; et al. A Bench Study of Balloon-Expandable Valves for the Treatment of Self-Expanding Valve Failure. EuroIntervention 2023, 19, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Akodad, M.; Kütting, M.; Sellers, S.; Kirsten, A.; Marx, P.; Kim, I.; Cheung, A.; Leipsic, J.; Søndergaard, L.; Toggweiler, S.; et al. Redo Transcatheter Aortic Valve Implantation with the ALLEGRA Transcatheter Heart Valve: Insights from Bench Testing. Cardiovasc. Eng. Technol. 2022, 13, 930–938. [Google Scholar] [CrossRef]
- Sathananthan, J.; Fraser, R.; Landes, U.; Rich, C.; Sellers, S.; Leipsic, J.; Blanke, P.; Lutter, G.; Frank, D.; Puehler, T.; et al. Repeat Transcatheter Aortic Valve Implantation and Implications for Transcatheter Heart Valve Performance: Insights from Bench Testing. EuroIntervention 2021, 17, 856–864. [Google Scholar] [CrossRef]
- Meier, D.; Landes, U.; Sondergaard, L.; De Backer, O.; Lutter, G.; Puehler, T.; Akodad, M.; Tzimas, G.; Blanke, P.; Payne, G.W.; et al. Redo-TAVI with SAPIEN 3 in SAPIEN XT or SAPIEN 3 - Impact of Pre- and Post-Dilatation on Final THV Expansion. EuroIntervention 2023, 19, 757. [Google Scholar] [CrossRef]
- Meier, D.; Akodad, M.; Landes, U.; Barlow, A.M.; Chatfield, A.G.; Lai, A.; Tzimas, G.; Tang, G.H.L.; Puehler, T.; Lutter, G.; et al. Coronary Access Following Redo TAVR: Impact of THV design, implant technique, and cell misalignment. Cardiovasc. Interv. 2022, 15, 1519–1531. [Google Scholar] [CrossRef]
- Meier, D.; Grant, D.; Frawley, C.; Akodad, M.; Landes, U.; Khokhar, A.A.; Dudek, D.; George, I.; Rinaldi, M.J.; Kim, W.-K.; et al. Redo-TAVI with the ACURATE Neo2 and Prime XL for Balloon-Expandable Transcatheter Heart Valve Failure. EuroIntervention 2024, 20, e376–e388. [Google Scholar] [CrossRef]
- ISO 5840-3:2021; Cardiovascular Implants—Cardiac Valve Prostheses—Part 3: Heart Valve Substitutes Implanted by Transcatheter Techniques. ISO: Geneva, Switzerland, 2021.
- Hatoum, H.; Yousefi, A.; Lilly, S.; Maureira, P.; Crestanello, J.; Dasi, L.P. An in Vitro Evaluation of Turbulence after Transcatheter Aortic Valve Implantation. J. Thorac. Cardiovasc. Surg. 2018, 156, 1837–1848. [Google Scholar] [CrossRef] [PubMed]
- Sathananthan, J.; Sellers, S.; Fraser, R.; Dvir, D.; Hensey, M.; Murdoch, D.J.; Blanke, P.; Pibarot, P.; Toggweiler, S.; Wood, D.; et al. Impact of Implant Depth on Hydrodynamic Function with the ACURATE Neo Transcatheter Heart Valve Following Valve-in-Valve Transcatheter Aortic Valve Replacement in Mitroflow Bioprosthetic Valves: An Ex Vivo Bench Study. EuroIntervention 2019, 15, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Midha, P.A.; Raghav, V.; Condado, J.F.; Okafor, I.U.; Lerakis, S.; Thourani, V.H.; Babaliaros, V.; Yoganathan, A.P. Valve Type, Size, and Deployment Location Affect Hemodynamics in an In Vitro Valve-in-Valve Model. JACC Cardiovasc. Interv. 2016, 9, 1618–1628. [Google Scholar] [CrossRef]
- Martin, C.; Sun, W. Transcatheter Valve Underexpansion Limits Leaflet Durability: Implications for Valve-in-Valve Procedures. Ann. Biomed. Eng. 2017, 45, 394–404. [Google Scholar] [CrossRef]
- Gunning, P.S.; Saikrishnan, N.; Yoganathan, A.P.; McNamara, L.M. Total Ellipse of the Heart Valve: The Impact of Eccentric Stent Distortion on the Regional Dynamic Deformation of Pericardial Tissue Leaflets of a Transcatheter Aortic Valve Replacement. J. R. Soc. Interface 2015, 12, 20150737. [Google Scholar] [CrossRef]
- Sathananthan, J.; Nigade, A.; Meier, D.; Navarro, D.; Spencer, J.; Lai, A.; Gill, H.; Pirelli, L.; Webb, J.G.; Wood, D.A.; et al. Hydrodynamic Assessment of Explanted Degenerated Transcatheter Aortic Valves: Novel Insights Into Noncalcific and Calcific Mechanisms. JACC Cardiovasc. Interv. 2024, 17, 1340–1351. [Google Scholar] [CrossRef]
- De Backer, O.; Landes, U.; Fuchs, A.; Yoon, S.H.; Mathiassen, O.N.; Sedaghat, A.; Kim, W.K.; Pilgrim, T.; Buzzatti, N.; Ruile, P.; et al. Coronary Access After TAVR-in-TAVR as Evaluated by Multidetector Computed Tomography. JACC Cardiovasc. Interv. 2020, 13, 2528–2538. [Google Scholar] [CrossRef]
- Ochiai, T.; Oakley, L.; Sekhon, N.; Komatsu, I.; Flint, N.; Kaewkes, D.; Yoon, S.H.; Raschpichler, M.; Patel, V.; Tiwana, R.; et al. Risk of Coronary Obstruction Due to Sinus Sequestration in Redo Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2020, 13, 2617–2627. [Google Scholar] [CrossRef]
- Rogers, T.; Greenspun, B.C.; Weissman, G.; Torguson, R.; Craig, P.; Shults, C.; Gordon, P.; Ehsan, A.; Wilson, S.R.; Goncalves, J.; et al. Feasibility of Coronary Access and Aortic Valve Reintervention in Low-Risk TAVR Patients. JACC Cardiovasc. Interv. 2020, 13, 726–735. [Google Scholar] [CrossRef]
- Yudi, M.B.; Sharma, S.K.; Tang, G.H.L.; Kini, A. Coronary Angiography and Percutaneous Coronary Intervention After Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2018, 71, 1360–1378. [Google Scholar] [CrossRef]
- Tang, G.H.L.; Zaid, S.; Ahmad, H.; Undemir, C.; Lansman, S.L. Transcatheter Valve Neo-Commissural Overlap with Coronary Orifices after Transcatheter Aortic Valve Replacement: Implication on Coronary Reaccess. Circ. Cardiovasc. Interv. 2018, 11, e007263. [Google Scholar] [CrossRef] [PubMed]
- Gunning, P.S.; Vaughan, T.J.; McNamara, L.M. Simulation of Self Expanding Transcatheter Aortic Valve in a Realistic Aortic Root: Implications of Deployment Geometry on Leaflet Deformation. Ann. Biomed. Eng. 2014, 42, 1989–2001. [Google Scholar] [CrossRef]
- Bailey, J.; Curzen, N.; Bressloff, N.W. The Impact of Imperfect Frame Deployment and Rotational Orientation on Stress within the Prosthetic Leaflets during Transcatheter Aortic Valve Implantation. J. Biomech. 2017, 53, 22–28. [Google Scholar] [CrossRef]
- Barbanti, M.; Costa, G. The Value of Bench Studies to Anticipate Long-Term Caveats of Transcatheter Aortic Valve Replacement. Cardiovasc. Interv. 2022, 15, 1540–1542. [Google Scholar] [CrossRef]
- Tchétché, D.; Chevalier, B.; Holzhey, D.; Harnath, A.; Schäfer, U.; Teiger, E.; Manigold, T.; Modine, T.; Souteyrand, G.; Champagnac, D.; et al. TAVR for Failed Surgical Aortic Bioprostheses Using a Self-Expanding Device: 1-Year Results From the Prospective VIVA Postmarket Study. JACC Cardiovasc. Interv. 2019, 12, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Dvir, D.; Webb, J.; Brecker, S.; Bleiziffer, S.; Hildick-Smith, D.; Colombo, A.; Descoutures, F.; Hengstenberg, C.; Moat, N.E.; Bekeredjian, R.; et al. Transcatheter Aortic Valve Replacement for Degenerative Bioprosthetic Surgical Valves: Results from the Global Valve-in-Valve Registry. Circulation 2012, 126, 2335–2344. [Google Scholar] [CrossRef]
- Ribeiro, H.B.; Rodés-Cabau, J.; Blanke, P.; Leipsic, J.; Kwan Park, J.; Bapat, V.; Makkar, R.; Simonato, M.; Barbanti, M.; Schofer, J.; et al. Incidence, Predictors, and Clinical Outcomes of Coronary Obstruction Following Transcatheter Aortic Valve Replacement for Degenerative Bioprosthetic Surgical Valves: Insights from the VIVID Registry. Eur. Heart J. 2018, 39, 687–695. [Google Scholar] [CrossRef]
- Medranda, G.A.; Soria Jimenez, C.E.; Torguson, R.; Case, B.C.; Forrestal, B.J.; Ali, S.W.; Shea, C.; Zhang, C.; Wang, J.C.; Gordon, P.; et al. Lifetime Management of Patients with Symptomatic Severe Aortic Stenosis: A Computed Tomography Simulation Study. EuroIntervention 2022, 18, e407. [Google Scholar] [CrossRef]
- Grubb, K.J.; Shekiladze, N.; Spencer, J.; Perdoncin, E.; Tang, G.H.L.; Xie, J.; Lisko, J.; Sanchez, J.Z.; Lucas, L.M.; Sathananthan, J.; et al. Feasibility of Redo-TAVI in Self-Expanding Evolut Valves: A CT Analysis from the Evolut Low Risk Trial Substudy. EuroIntervention 2023, 19, e330. [Google Scholar] [CrossRef]
- Tang, G.H.L.; Spencer, J.; Rogers, T.; Grubb, K.J.; Gleason, P.; Gada, H.; Mahoney, P.; Dauerman, H.L.; Forrest, J.K.; Reardon, M.J.; et al. Feasibility of Coronary Access Following Redo-TAVR for Evolut Failure: A Computed Tomography Simulation Study. Circ. Cardiovasc. Interv. 2023, 16, e013238. [Google Scholar] [CrossRef]
- Koshy, A.N.; Tang, G.H.L.; Khera, S.; Vinayak, M.; Berdan, M.; Gudibendi, S.; Hooda, A.; Safi, L.; Lerakis, S.; Dangas, G.D.; et al. Redo-TAVR Feasibility After SAPIEN 3 Stratified by Implant Depth and Commissural Alignment: A CT Simulation Study. Circ. Cardiovasc. Interv. 2024, 17, e013766. [Google Scholar] [CrossRef] [PubMed]
- Arslani, K.; Tirado-Conte, G.; Van Mieghem, N.M.; Mylotte, D.; Tang, G.H.L.; Bapat, V.N.; Leroux, L.; Tchétché, D.; Grubb, K.J.; De Backer, O. Redo-TAVI Feasibility and Coronary Accessibility Following Index TAVI with the Evolut Valve in Patients with Bicuspid Aortic Valve Stenosis. EuroIntervention 2024, 20, 1416–1418. [Google Scholar] [CrossRef] [PubMed]
- Bieliauskas, G.; Kobari, Y.; Khokhar, A.A.; Abdel-Wahab, M.; Abdelhafez, A.; Fukui, M.; Kofoed, K.F.; Dudek, D.; Fuchs, A.; Cavalcante, J.; et al. Feasibility of Redo-TAVI in the Self-Expanding ACURATE Neo2 Valve: A Computed Tomography Study. EuroIntervention 2024, 20, 1405–1415. [Google Scholar] [CrossRef]
- Fuchs, A.; Kofoed, K.F.; Yoon, S.H.; Schaffner, Y.; Bieliauskas, G.; Thyregod, H.G.; Makkar, R.; Søndergaard, L.; De Backer, O.; Bapat, V. Commissural Alignment of Bioprosthetic Aortic Valve and Native Aortic Valve Following Surgical and Transcatheter Aortic Valve Replacement and Its Impact on Valvular Function and Coronary Filling. JACC Cardiovasc. Interv. 2018, 11, 1733–1743. [Google Scholar] [CrossRef]
- Raschpichler, M.; Flint, N.; Yoon, S.H.; Kaewkes, D.; Patel, C.; Singh, C.; Patel, V.; Kashif, M.; Borger, M.A.; Chakravarty, T.; et al. Commissural Alignment After Balloon-Expandable Transcatheter Aortic Valve Replacement Is Associated with Improved Hemodynamic Outcomes. JACC Cardiovasc. Interv. 2022, 15, 1126–1136. [Google Scholar] [CrossRef]
- Lancellotti, P.; Pibarot, P.; Chambers, J.; Edvardsen, T.; Delgado, V.; Dulgheru, R.; Pepi, M.; Cosyns, B.; Dweck, M.R.; Garbi, M.; et al. Recommendations for the Imaging Assessment of Prosthetic Heart Valves: A Report from the European Association of Cardiovascular Imaging Endorsed by the Chinese Society of Echocardiography, the Inter-American Society of Echocardiography, and the Brazilian Department of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 589–590. [Google Scholar] [CrossRef]
- Søndergaard, L.; Ihlemann, N.; Capodanno, D.; Jørgensen, T.H.; Nissen, H.; Kjeldsen, B.J.; Chang, Y.; Steinbrüchel, D.A.; Olsen, P.S.; Petronio, A.S.; et al. Durability of Transcatheter and Surgical Bioprosthetic Aortic Valves in Patients at Lower Surgical Risk. J. Am. Coll. Cardiol. 2019, 73, 546–553. [Google Scholar] [CrossRef]
- Bieliauskas, G.; Wong, I.; Bajoras, V.; Wang, X.; Kofoed, K.F.; De Backer, O.; Søndergaard, L. Patient-Specific Implantation Technique to Obtain Neo-Commissural Alignment with Self-Expanding Transcatheter Aortic Valves. Cardiovasc. Interv. 2021, 14, 2097–2108. [Google Scholar] [CrossRef]
- Kitamura, M.; Wilde, J.; Dumpies, O.; Gutberlet, M.; Gohmann, R.; Shibata, M.; Noack, T.; Thiele, H.; Holzhey, D.; Abdel-Wahab, M. Patient-Specific Neocommissural Alignment of the Evolut Valve: A Pilot Study in Transcatheter Aortic Valve-in-Valve Replacement. JACC Cardiovasc. Interv. 2021, 14, 934–936. [Google Scholar] [CrossRef]
- Kitamura, M.; Wilde, J.; Gohmann, R.; Majunke, N.; Gutberlet, M.; Shibata, M.; Kiefer, P.; Desch, S.; Thiele, H.; Holzhey, D.; et al. Commissural Alignment of the ACURATE Neo Valve in Transcatheter Aortic Valve Replacement. Cardiovasc. Interv. 2021, 14, 1740–1742. [Google Scholar] [CrossRef]
- Quagliana, A.; Montarello, N.J.; Willemen, Y.; Bække, P.S.; Jørgensen, T.H.; De Backer, O.; Sondergaard, L. Commissural Alignment and Coronary Access after Transcatheter Aortic Valve Replacement. J. Clin. Med. 2023, 12, 2136. [Google Scholar] [CrossRef] [PubMed]
- Spilias, N.; Sabbak, N.; Harb, S.C.; Yun, J.J.; Vargo, P.R.; Unai, S.; Puri, R.; Reed, G.W.; Krishnaswamy, A.; Kapadia, S.R. A Novel Method of Assessing Commissural Alignment for the SAPIEN 3 Transcatheter Aortic Valve. Cardiovasc. Interv. 2021, 14, 1269–1272. [Google Scholar] [CrossRef] [PubMed]
- Akodad, M.; Tzimas, G.; Meier, D.; Haugan, D.; Gibson, H.; Ringhofer, J.; Everett, R.J.; Sathananthan, J.; Wood, D.A.; Webb, J.G.; et al. Quantification of Commissural Alignment of Balloon-Expandable THV on Fluoroscopy: A Comparison Study with Post-TAVR CT. Cardiovasc. Interv. 2022, 15, 2374–2383. [Google Scholar] [CrossRef]
- Akodad, M.; Meier, D.; Tzimas, G.; Leipsic, J.; Blanke, P.; Wood, D.A.; Webb, J.G.; Sathananthan, J. A Simplified Fluoroscopic Method for Commissural Alignment Assessment with a Balloon-Expandable Transcatheter Heart Valve. JACC Case Rep. 2023, 13. [Google Scholar] [CrossRef]
- Landes, U.; Webb, J.G.; De Backer, O.; Sondergaard, L.; Abdel-Wahab, M.; Crusius, L.; Kim, W.K.; Hamm, C.; Buzzatti, N.; Montorfano, M.; et al. Repeat Transcatheter Aortic Valve Replacement for Transcatheter Prosthesis Dysfunction. J. Am. Coll. Cardiol. 2020, 75, 1882–1893. [Google Scholar] [CrossRef]
- Tang, G.H.L.; Zaid, S.; Kleiman, N.S.; Goel, S.S.; Fukuhara, S.; Marin-Cuartas, M.; Kiefer, P.; Abdel-Wahab, M.; De Backer, O.; Søndergaard, L.; et al. Explant vs Redo-TAVR After Transcatheter Valve Failure: Mid-Term Outcomes From the EXPLANTORREDO-TAVR International Registry. JACC Cardiovasc. Interv. 2023, 16, 927–941. [Google Scholar] [CrossRef] [PubMed]
- Makkar, R.R.; Kapadia, S.; Chakravarty, T.; Cubeddu, R.J.; Kaneko, T.; Mahoney, P.; Patel, D.; Gupta, A.; Cheng, W.; Kodali, S.; et al. Outcomes of Repeat Transcatheter Aortic Valve Replacement with Balloon-Expandable Valves: A Registry Study. Lancet 2023, 302, 1529–1540. [Google Scholar] [CrossRef]
- Carroll, J.D.; Mack, M.J.; Vemulapalli, S.; Herrmann, H.C.; Gleason, T.G.; Hanzel, G.; Deeb, G.M.; Thourani, V.H.; Cohen, D.J.; Desai, N.; et al. STS-ACC TVT Registry of Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2020, 76, 2492–2516. [Google Scholar] [CrossRef]
- Parma, R.; Joner, M.; Saia, F.; Cuisset, T.; Delgado, V.; Rodes-Cabau, J.; Modine, T.; Van Belle, E.; Fovino, L.N.; Landes, U.; et al. Procedural and Clinical Outcomes of Patients Undergoing a TAVI in TAVI Procedure: Rationale and Design of the Multicentre, Prospective, Observational ReTAVI Registry. Eur. J. Clin. Invest. 2024, 54, e14241. [Google Scholar] [CrossRef]
- Bapat, V. Valve-in-Valve Apps: Why and How They Were Developed and How to Use Them. EuroIntervention 2014, 10, U44–U51. [Google Scholar] [CrossRef]
| Study | Redo-TAVR Valve Types | Specific Valves | Design | Main Outcomes | Results |
|---|---|---|---|---|---|
| Akodad M. et al. [4] | BEV in SEV | Sapien 3 (S3, 20–29 mm) in Evolut (23–34 mm) | S3 outflow implanted at Evolut nodes 4, 5 and 6 | 1. Neoskirt height | Shortest at node 4, highest at node 6 |
| 2. Leaflet overhang | Greatest when low implantation depth | ||||
| 3. Hydrodynamic performance * | Acceptable RF < 20% for all except 29 mm S3 at node 4 of 29 mm Evolut | ||||
| 4. Embolization risk | Low at all implant depths | ||||
| Akodad M. et al. [5] | BEV in SEV | Sapien 3 Ultra (S3U) in ACURATE neo2 (ACn2) | Low implant (S3U outflow at ACn2 upper crown) vs. high implant (S3U outflow at base of ACn2 commissural post) | 1. Neoskirt height | Shorter when S3U at low implant depth |
| 2. Leaflet overhang | Moderate< 50% for all except 26 mm S3U implanted low in L ACn2 | ||||
| 3. Leaflet deflection | >2 mm gap between neoskirt–outer border of THV frame | ||||
| 4. Valve expansion | S3U under-expanded, 78–92% of expected nominal area | ||||
| 5. Hydrodynamic performance * | Favorable in all configurations | ||||
| Akodad M. et al. [6] | SEV (ALLEGRA 27 mm) in various SEV and BEV | ALLEGRA in:
| Each configuration at −4, 0, and 4 mm implantation depth (distance from lower border of redo valve to lower border of index valve) | 1. Hydrodynamic performance | All configurations were compatible, except ALLEGRA in EvP at −4 mm (outflow constrained by Evolut Pro waist) |
| 2. Transvalvular gradients | <20 mmHg in all compatible configurations | ||||
| 3. Pinwheeling | In EvP: more important irrespective of implantation depth In the other THVs: worse at high implantation depth | ||||
| 4. Neoskirt height | Higher for tall frame THVs | ||||
| Sathananthan J. et al. [7] | SEV/BEV in SEV/BEV | S3, Evolut Pro, Acn, ALLEGRA, Portico in Sapien XT (SXT) and Evolut R | Sapien XT (23-29 mm) Evolut R (23-29 mm) | 1. Anchoring | Most stable S3 in an Evolut R requires adequate sizing to prevent embolization; ALLEGRA and Portico embolized from 29 mm SXT |
| 2. Hydrodynamic performance | Acceptable for all valves implanted within the SXT; Improved for Evolut R, Acn implanted high; High RF if S3 implanted low in Evolut R | ||||
| 3. Transvalvular gradients | Acceptable | ||||
| Meier D. et al. [8] | BEV in BEV | S3 (23 mm) in SX (23 mm) or S3 (23 mm) | Evaluation of index and redo valve expansion and hydrodynamic performance without and with pre and/or post-dilation (23 mm non-compliant balloon) | 1. Expansion without pre/post-dilation: | S3 under-expanded |
| 2. Pre and post-dilation | Redo S3 remained under-expanded when implanted in an SXT | ||||
| Redo S3 achieved nominal diameter when implanted in an S3 | |||||
| Index valve was overexpanded (12%) | |||||
| 3. Hydrodynamic performance | Acceptable | ||||
| Increased pinwheeling in under-expanded valves | |||||
| Meier D. et al. [9] | SEV/BEV in BEV | S3, Evolut Pro, Acn, Portico, in SXT, and Evolut R | Micro-computed tomography determination of neoskirt height and size lowest accessible cell for coronary access | 1. Shorter neoskirts | Most cases with index SXT Shortest: Portico in SXT |
| 2. Higher neoskirts | Various configurations within EvolutR Highest: high implant of 26 mm Evolut Pro in 25 mm Evolut R | ||||
| 3. Largest accessible cell | Acn in SXT | ||||
| 4. Smallest accessible cell | Evolut Pro in Evolut R; Misalignment in this configuration reduced cell area by 30–50% | ||||
| Meier D. et al. [10] | SEV in BEV | Acn2 or Ac XL in S3 vs. S3 in S3 | BEV implanted nominally low or nominally high | 1. Hydrodynamic performance | More favorable for Acn2/Ac XL in S3 Implantation height had minimal impact |
| 2. Pinwheeling | Less for Acn2/Ac XL in S3 | ||||
| 3. Neoskirt length | Slightly taller for Acn2/Ac XL | ||||
| Author | Study Design | Main Outcomes | Results |
|---|---|---|---|
| Medranda GA et al. [29] | CT redo-TAVR simulation using paired, pre-, and post-TAVR CT studies N = 213 | Patients classified into Low risk of coronary obstruction:
| Patients predicted to be at low risk: 25.4% |
| Patients predicted to be at high risk and likely requiring leaflet modification: 27.7% | |||
| Redo-TAVR feasible only if first valve a BEV and likely requiring leaflet modification: 46.9% | |||
| Grubb KJ et al. [30] | Post-TAVR CT scans N = 204 | Evaluation of five redo-TAVR implant depths: S3-in-Evolut inflow-to-inflow, S3 outflow at Evolut nodes 4, 5, 6, Evolut-in-Evolut inflow-to-inflow | Lowest risk of coronary obstruction: S3 outflow at Evolut node 4 |
Highest risk of coronary obstruction:
| |||
| Tang GHL [31] | CT simulation of index (Evolut) and redo-TAVR (S3) valves N = 219 | Impact of implantation depth of index and redo-TAVR on coronary access | Highest rate of coronary access (97%):
|
Lowest rate of coronary access (31%):
| |||
| Koshy AN [32] | CT simulation of index (S3) and redo-TAVR (S3) valves N = 1900 | Impact of implantation depth of index THV in an S3-in-S3 simulation study | Reduced redo-TAVR unfeasibility in case of higher index implant position
|
| CA associated with redo-TAVR feasibility in all patients |
| Registry | Study Design | Main Outcomes | Results |
|---|---|---|---|
| Redo-TAVR International Registry [46] | Investigator-initiated, international, 37 centers Core lab assessment for baseline echocardiography and CT N = 212 | 30-day mortality:
| 5.4% 1.3% |
| THV failure mode if: | |||
| Valvular regurgitation: 73% Valve stenosis: 16% Mixed: 11% | ||
| Valvular regurgitation: 30% Valve stenosis: 37% Mixed: 33% | ||
| Retrospective analysis of Redo-TAVR International Registry [3] | Propensity score matching according to index or redo SEV or BEV N = 221 | Index valve type | If index valve is SEV |
| Earlier failure: 3.7 ± 2.3 years vs. 4.9 ± 2.1 years; p < 0.001 | ||
| More frequently with AR: 47.3% vs. 16.2%; p < 0.001 | ||
| Redo-TAVR success | |||
| Similar | ||
| Higher in case of redo-SEV: 77.2% vs. 64.3%; p = 0.045 Lower gradients with redo-SEV: 10.3 mmHg vs. 15.2 mmHg; p < 0.001 | ||
| EXPLANT-OR-REDO-TAVR international registry [47] | International registry, 29 centers Patients with failed TAVR treated by surgical explant vs. redo-TAVR N = 396 | Treatment based on index valve failure mode: | |
| More frequently by TAVR explant: 17.1% vs. 0.5%; p < 0.001 | ||
| More frequently by redo-TAVR: 63.7% vs. 51.9%; p = 0.023 | ||
| Mortality | |||
| Lower for redo-TAVR 3.4% vs. 13.6%, p < 0.001 | ||
| Lower for redo-TAVR 15.4% vs. 32.4%, p = 0.001 | ||
| TVT registry [48] | Multicenter registry based in the USA Propensity score matching of 1320 BEV redo-TAVR patients with 1320 BEV TAVR patients | Stroke at 30 days | Similar 2% vs. 1.9%, p = 0.84 |
| Stroke at 1 year | Similar 3.2% vs. 3.5%, p = 0.80 | ||
| Mortality at 30 days | Similar 4.7% vs. 4.0%, p = 0.36 | ||
| Mortality at 1 year | Similar 17.5% vs. 19.0%, p = 0.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sava, R.I.; Garot, P.; Benamer, H.; Gall, E.; Pezel, T.; Djebbar, M.; Sayah, N.; Meier, D.; Tzimas, G.; Garot, J.; et al. Redo-Transcatheter Aortic Valve Replacement Procedural Optimization and Patient Selection: From Bench to Clinical Practice. J. Clin. Med. 2025, 14, 2770. https://doi.org/10.3390/jcm14082770
Sava RI, Garot P, Benamer H, Gall E, Pezel T, Djebbar M, Sayah N, Meier D, Tzimas G, Garot J, et al. Redo-Transcatheter Aortic Valve Replacement Procedural Optimization and Patient Selection: From Bench to Clinical Practice. Journal of Clinical Medicine. 2025; 14(8):2770. https://doi.org/10.3390/jcm14082770
Chicago/Turabian StyleSava, Ruxandra I., Philippe Garot, Hakim Benamer, Emmanuel Gall, Théo Pezel, Morad Djebbar, Neila Sayah, David Meier, Georgios Tzimas, Jérôme Garot, and et al. 2025. "Redo-Transcatheter Aortic Valve Replacement Procedural Optimization and Patient Selection: From Bench to Clinical Practice" Journal of Clinical Medicine 14, no. 8: 2770. https://doi.org/10.3390/jcm14082770
APA StyleSava, R. I., Garot, P., Benamer, H., Gall, E., Pezel, T., Djebbar, M., Sayah, N., Meier, D., Tzimas, G., Garot, J., Leclercq, F., & Akodad, M. (2025). Redo-Transcatheter Aortic Valve Replacement Procedural Optimization and Patient Selection: From Bench to Clinical Practice. Journal of Clinical Medicine, 14(8), 2770. https://doi.org/10.3390/jcm14082770





