Early Driving Pressure Is Associated with Major Adverse Kidney Events at 30 Days in ARDS Patients with SARS-CoV-2
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Population
2.2. Definition of MAKEs and Persistent AKI
2.3. Determinations of Urinary Renal Stress Biomarkers
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Study Participants
3.2. Characteristics of Patients with MAKEs at 30 Days
3.3. Characteristics of Patients with Persistent Acute Kidney Injury (pAKI)
3.4. Risk Factors for MAKEs: Multivariable and Sensitivity Analysis
3.5. Risk Factor for Persistent Acute Kidney Injury (pAKI)
4. Discussion
Limitations, Strengths, and Weakness
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chan, L.; Chaudhary, K.; Saha, A.; Chauhan, K.; Vaid, A.; Zhao, S.; Paranjpe, I.; Somani, S.; Richter, F.; Miotto, R.; et al. AKI in Hospitalized Patients with COVID-19. J. Am. Soc. Nephrol. JASN 2021, 32, 151–160. [Google Scholar] [CrossRef]
- Maeda, A.; Inokuchi, R.; Bellomo, R.; Doi, K. Heterogeneity in the definition of major adverse kidney events: A scoping review. Intensive Care Med. 2024, 50, 1049–1063. [Google Scholar] [CrossRef]
- Fisher, M.; Neugarten, J.; Bellin, E.; Yunes, M.; Stahl, L.; Johns, T.S.; Abramowitz, M.K.; Levy, R.; Kumar, N.; Mokrzycki, M.H.; et al. AKI in Hospitalized Patients with and without COVID-19: A Comparison Study. J. Am. Soc. Nephrol. JASN 2020, 31, 2145–2157. [Google Scholar] [CrossRef]
- Darmon, M.; Clec’h, C.; Adrie, C.; Argaud, L.; Allaouchiche, B.; Azoulay, E.; Bouadma, L.; Garrouste-Orgeas, M.; Haouache, H.; Schwebel, C.; et al. Acute respiratory distress syndrome and risk of AKI among critically ill patients. Clin. J. Am. Soc. Nephrol. CJASN 2014, 9, 1347–1353. [Google Scholar] [CrossRef]
- Hirsch, J.S.; Ng, J.H.; Ross, D.W.; Sharma, P.; Shah, H.H.; Barnett, R.L.; Hazzan, A.D.; Fishbane, S.; Jhaveri, K.D.; Northwell COVID-19 Research Consortium; et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020, 98, 209–218. [Google Scholar] [CrossRef]
- Chang, R.; Elhusseiny, K.M.; Yeh, Y.C.; Sun, W.Z. COVID-19 ICU and mechanical ventilation patient characteristics and outcomes-A systematic review and meta-analysis. PLoS ONE 2021, 16, e0246318. [Google Scholar] [CrossRef]
- Singbartl, K. Renal-pulmonary crosstalk. Contrib. Nephrol. 2011, 174, 65–70. [Google Scholar] [CrossRef]
- Leite, T.T.; Gomes, C.A.M.; Valdivia, J.M.C.; Libório, A.B. Respiratory parameters and acute kidney injury in acute respiratory distress syndrome: A causal inference study. Ann. Transl. Med. 2019, 7, 742. [Google Scholar] [CrossRef]
- Gattarello, S.; Lombardo, F.; Romitti, F.; D’albo, R.; Velati, M.; Fratti, I.; Pozzi, T.; Nicolardi, R.; Fioccola, A.; Busana, M.; et al. Determinants of acute kidney injury during high-power mechanical ventilation: Secondary analysis from experimental data. Intensive Care Med. Exp. 2024, 12, 31. [Google Scholar] [CrossRef]
- Upadhyaya, V.D.; Shariff, M.Z.; Mathew, R.O.; Hossain, M.A.; Asif, A.; Vachharajani, T.J. Management of Acute Kidney Injury in the Setting of Acute Respiratory Distress Syndrome: Review Focusing on Ventilation and Fluid Management Strategies. J. Clin. Med. Res. 2020, 12, 1–5. [Google Scholar] [CrossRef]
- Seubert, M.E.; Goeijenbier, M. Controlled Mechanical Ventilation in Critically Ill Patients and the Potential Role of Venous Bagging in Acute Kidney Injury. J. Clin. Med. 2024, 13, 1504. [Google Scholar] [CrossRef] [PubMed]
- Mekontso Dessap, A.; Ware, L.B.; Bagshaw, S.M. How could biomarkers of ARDS and AKI drive clinical strategies? Intensive Care Med. 2016, 42, 800–802. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Shang, N.; Levitman, A.; Corker, A.; Kudose, S.; Yaeh, A.; Neupane, U.; Stevens, J.; Sampogna, R.; Mills, A.M.; et al. Elevated Neutrophil Gelatinase-Associated Lipocalin Is Associated With the Severity of Kidney Injury and Poor Prognosis of Patients With COVID-19. Kidney Int. Rep. 2021, 6, 2979–2992. [Google Scholar] [CrossRef]
- Casas-Aparicio, G.; la Barrera, C.A.-D.; Escamilla-Illescas, D.; León-Rodríguez, I.; Del Río-Estrada, P.M.; Calderón-Dávila, N.; González-Navarro, M.; Olmedo-Ocampo, R.; Castillejos-López, M.; Figueroa-Hernández, L.; et al. Role of Urinary Kidney Stress Biomarkers for Early Recognition of Subclinical Acute Kidney Injury in Critically Ill COVID-19 Patients. Biomolecules 2022, 12, 275. [Google Scholar] [CrossRef]
- Menez, S.; Moledina, D.G.; Thiessen-Philbrook, H.; Wilson, F.P.; Obeid, W.; Simonov, M.; Yamamoto, Y.; Corona-Villalobos, C.P.; Chang, C.; Garibaldi, B.T.; et al. Prognostic Significance of Urinary Biomarkers in Patients Hospitalized With COVID-19. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2022, 79, 257–267.e1. [Google Scholar] [CrossRef] [PubMed]
- ARDS Definition of Task Force; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef]
- Yüce, M.; Filiztekin, E.; Özkaya, K.G. COVID-19 diagnosis -A review of current methods. Biosens. Bioelectron. 2021, 172, 112752. [Google Scholar] [CrossRef]
- Suter, P.M.; Fairley, B.; Isenberg, M.D. Optimum end-expiratory airway pressure in patients with acute pulmonary failure. N. Engl. J. Med. 1975, 292, 284–289. [Google Scholar] [CrossRef]
- Chawla, L.S.; Bellomo, R.; Bihorac, A.; Goldstein, S.L.; Siew, E.D.; Bagshaw, S.M.; Bittleman, D.; Cruz, D.; Endre, Z.; Fitzgerald, R.L.; et al. Acute kidney disease and renal recovery: Consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat. Rev. Nephrol. 2017, 13, 241–257. [Google Scholar] [CrossRef]
- Bellani, G.; Laffey, J.G.; Pham, T.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; Van Haren, F.; Larsson, A.; McAuley, D.F.; et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016, 315, 788–800. [Google Scholar] [CrossRef]
- Antonucci, E.; Garcia, B.; Chen, D.; Matthay, M.A.; Liu, K.D.; Legrand, M. Incidence of acute kidney injury and attributive mortality in acute respiratory distress syndrome randomized trials. Intensive Care Med. 2024, 50, 1240–1250. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.B.P.; Meade, M.O.; Slutsky, A.S.; Brochard, L.; Costa, E.L.V.; Schoenfeld, D.A.; Stewart, T.E.; Briel, M.; Talmor, D.S.; Mercat, A.; et al. Driving pressure and survival in the acute respiratory distress syndrome. N. Engl. J. Med. 2015, 372, 747–755. [Google Scholar] [CrossRef]
- Kunadu, A.Q.; Nalamalapu, S.R.; Hafiz, M.; Sahebazamani, M. Driving Pressure and Mortality. Am. J. Respir. Crit. Care Med. 2022, 206, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, T.; Vasques, F.; Rapetti, F.; Maiolo, G.; Collino, F.; Romitti, F.; Camporota, L.; Cressoni, M.; Cadringher, P.; Quintel, M.; et al. Driving pressure and mechanical power: New targets for VILI prevention. Ann. Transl. Med. 2017, 5, 286. [Google Scholar] [CrossRef] [PubMed]
- Kummer, R.L.; Marini, J.J. The Respiratory Mechanics of COVID-19 Acute Respiratory Distress Syndrome-Lessons Learned? J. Clin. Med. 2024, 13, 1833. [Google Scholar] [CrossRef]
- Silva, P.L.; Ball, L.; Rocco, P.R.M.; Pelosi, P. Physiological and Pathophysiological Consequences of Mechanical Ventilation. Semin. Respir. Crit. Care Med. 2022, 43, 321–334. [Google Scholar] [CrossRef]
- Kaur, S.; Bansal, R.; Kollimuttathuillam, S.; Gowda, A.M.; Singh, B.; Mehta, D.; Maroules, M. The looming storm: Blood and cytokines in COVID-19. Blood Rev. 2021, 46, 100743. [Google Scholar] [CrossRef] [PubMed]
- Hepokoski, M.; Englert, J.A.; Baron, R.M.; Crotty-Alexander, L.E.; Fuster, M.M.; Beitler, J.R.; Malhotra, A.; Singh, P. Ventilator-induced lung injury increases expression of endothelial inflammatory mediators in the kidney. Am. J. Physiol. Ren. Physiol. 2017, 312, F654–F660. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Acute Respiratory Distress Syndrome Network; Brower, R.G.; Matthay, M.A.; Morris, A.; Schoenfeld, D.; Thompson, B.T.; Wheeler, A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1301–1308. [Google Scholar] [CrossRef]
- Grieco, D.L.; Maggiore, S.M.; Bellani, G.; Spadaro, S.; Spinelli, E.; Tonetti, T.; Menga, L.S.; Pozzi, M.; Battaglini, D.; Di Mussi, R.; et al. Individualized positive end-expiratory pressure guided by end-expiratory lung volume in early acute respiratory distress syndrome: Study protocol for the multicenter, randomized IPERPEEP trial. Trials 2022, 23, 63. [Google Scholar] [CrossRef]
- van den Akker, J.P.; Egal, M.; Groeneveld, A.B. Invasive mechanical ventilation as a risk factor for acute kidney injury in the critically ill: A systematic review and meta-analysis. Crit. Care 2013, 17, R98. [Google Scholar] [CrossRef]
- Ottolina, D.; Zazzeron, L.; Trevisi, L.; Agarossi, A.; Colombo, R.; Fossali, T.; Passeri, M.; Borghi, B.; Ballone, E.; Rech, R.; et al. Acute kidney injury (AKI) in patients with COVID-19 infection is associated with ventilatory management with elevated positive end-expiratory pressure (PEEP). J. Nephrol. 2022, 35, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Basse, P.; Morisson, L.; Barthélémy, R.; Julian, N.; Kindermans, M.; Collet, M.; Huot, B.; Gayat, E.; Mebazaa, A.; Chousterman, B.G. Relationship between positive end-expiratory pressure levels, central venous pressure, systemic inflammation and acute renal failure in critically ill ventilated COVID-19 patients: A monocenter retrospective study in France. Acute Crit. Care 2023, 38, 172–181. [Google Scholar] [CrossRef]
- Benites, M.H.; Suarez-Sipmann, F.; Kattan, E.; Cruces, P.; Retamal, J. Ventilation-induced acute kidney injury in acute respiratory failure: Do PEEP levels matter? Crit. Care 2025, 29, 130. [Google Scholar] [CrossRef] [PubMed]
- Borlino, S.C.; Hagry, J.; Lai, C.; Rocca, E.; Fouqué, G.; Rosalba, D.; Fasan, M.; Shi, R.; Recanatini, A.; Cisterna, I.; et al. The Effect of Positive End-Expiratory Pressure on Pulmonary Vascular Resistance Depends on Lung Recruitability in Patients with Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2024, 210, 900–907. [Google Scholar] [CrossRef]
- Lumlertgul, N.; Amprai, M.; Tachaboon, S.; Dinhuzen, J.; Peerapornratana, S.; Kerr, S.J.; Srisawat, N. Urine Neutrophil Gelatinase-associated Lipocalin (NGAL) for Prediction of Persistent AKI and Major Adverse Kidney Events. Sci. Rep. 2020, 10, 8718. [Google Scholar] [CrossRef] [PubMed]
- Titeca-Beauport, D.; Daubin, D.; Van Vong, L.; Belliard, G.; Bruel, C.; Alaya, S.; Chaoui, K.; Andrieu, M.; Rouquette-Vincenti, I.; Godde, F.; et al. Urine cell cycle arrest biomarkers distinguish poorly between transient and persistent AKI in early septic shock: A prospective, multicenter study. Crit. Care 2020, 24, 280. [Google Scholar] [CrossRef]
- Hjortrup, P.B.; Haase, N.; Wetterslev, M.; Perner, A. Clinical review: Predictive value of neutrophil gelatinase-associated lipocalin for acute kidney injury in intensive care patients. Crit. Care 2013, 17, 211. [Google Scholar] [CrossRef]
- Endre, Z.H. Assessing Renal Recovery after Acute Kidney Injury: Can Biomarkers Help? Nephron 2018, 140, 86–89. [Google Scholar] [CrossRef]
- Seeley, E.J. Updates in the management of acute lung injury: A focus on the overlap between AKI and ARDS. Adv. Chronic Kidney Dis. 2013, 20, 14–20. [Google Scholar] [CrossRef]
| Characteristics | Overall n = 45 | MAKE–30 Days n = 31 | No-MAKE–30 Days n = 14 | p-Value |
|---|---|---|---|---|
| Age, years γ | 57.73 (18.64) | 63.94 (15.98) | 44 (17.09) | <0.01 |
| Men [n] ø | 30 (66.67) | 18 (58.06) | 12 (85.71) | 0.094 |
| Weight, kg γ | 77.22 (16.25) | 76.42 (14.76) | 79 (19.66) | 0.627 |
| Height, cm γ | 163.7 (11.39) | 161.55 (10.33) | 168.57 (12.51) | 0.055 |
| BMI, kg/m2 γ | 28.69 (5.42) | 29.2 (5.77) | 27.58 (4.56) | 0.358 |
| Comorbidities | ||||
| Hypertension [n] ø | 19 (42.22) | 13 (41.94) | 6 (42.86) | 1.000 |
| Diabetes [n] ø | 10 (24.44) | 10 (32.26) | 1 (7.14) | 0.132 |
| Heart disease ø | 3 (6.67) | 3 (9.68) | 0 (0) | 0.541 |
| HIV ø | 3 (6.67) | 1 (3.23) | 2 (14.29) | 0.224 |
| Other comorbidities ø | 2 (4.44) | 1 (3.23) | 1 (7.14) | 0.530 |
| Laboratories | ||||
| Leucocytes, 103/mm3 γ | 13.9 (9.65–16.6) | 15.7 (10.3–17.4) | 11.49 (8.86–14.15) | 0.148 |
| Neutrophils, 103/mm3 γ | 12.5 (8–15.5) | 13.1 (8–16) | 10.65 (8–13) | 0.239 |
| Lymphocytes, 103/mm3 γ | 0.7 (0.5–1) | 0.7 (0.4–1) | 0.8 (0.5–1.2) | 0.438 |
| Hemoglobin, g/dL γ | 14.37 (1.68) | 14.33 (1.77) | 14.5 (1.51) | 0.754 |
| Hematocrit, % γ | 43.07 (5.31) | 42.93 (5.8) | 43.4 (4.22) | 0.785 |
| Platelets, 103/mm3 γ | 273.1 (98.35) | 283.51 (105.84) | 250.26 (77.92) | 0.299 |
| Sodium, mmol/L γ | 137.2 (5.71) | 137.03 (6.3) | 137.57 (4.33) | 0.773 |
| Potassium, mmol/L γ | 4.31 (0.66) | 4.35 (0.75) | 4.24 (0.41) | 0.614 |
| Chloride, mmol/L γ | 102 (99–105) | 102 (97–106) | 102.5 (100–105) | 0.768 |
| Calcium, mg/dL γ | 8.06 (0.57) | 8.04 (0.61) | 8.11 (0.51) | 0.715 |
| Magnesium, mg/dL γ | 2.2 (1.9–2.4) | 2.2 (1.9–2.6) | 2.2 (1.9–2.3) | 0.459 |
| Phosphate, mg/dL γ | 3.8 (3.1–4.5) | 4 (2.9–5.2) | 3.7 (3.2–3.9) | 0.244 |
| Glucose, mg/dL γ | 138 (112–188) | 149 (127–206) | 108 (85–138) | 0.006 |
| HbA1C, % γ | 5.855 (5.5–7.3) | 6.16 (5.77–7.62) | 5.57 (5.17–5.59) | 0.066 |
| BUN mg/dL γ | 23 (18–31) | 28 (20–35) | 20 (16–26) | 0.020 |
| Creatinine at admission mg/dL γ | 0.95 (0.695–1.285) | 0.95 (0.75–1.36) | 0.73 (0.62–1.01) | 0.041 |
| Baseline creatinine, mg/dL ρ | 0.65 (0.43–0.79) | 0.69 (0.44–0.87) | 0.53 (0.36–0.7) | 0.050 |
| LDH, U/L γ | 635.5 (279.6) | 661.3 (267.82) | 578.37 (306.85) | 0.363 |
| CPK, U/L γ | 85 (38–191) | 99 (38–191) | 71.5 (38–205) | 0.893 |
| ESR, mm/hr ρ | 33 (20–50) | 34 (18–50) | 31.5 (27–44) | 0.773 |
| Ferritin, ng/mL ρ | 1135.11 (453–1914.19) | 1158.6 (433.52–1855) | 1122.56 (701.67–2923) | 0.694 |
| D-Dimer, µg/mL ρ | 2.31 (0.475–6.065) | 2.62 (0.59–6) | 1.08 (0.44–6.13) | 0.495 |
| Procalcitonin ng/mL γ | 0.21 (0.12–0.4) | 0.21 (0.11–0.73) | 0.24 (0.14–0.38) | 0.893 |
| Procalcitonin > 0.5 ng/mL ø | 10 (22.22) | 8 (25.8) | 2 (14.29) | 0.469 |
| C-Reactive protein, mg/dL γ | 17.05 (7.94) | 17.49 (7.31) | 16.04 (9.5) | 0.587 |
| Troponin-I, pg/mL ρ | 16.3 (4.8–64.6) | 44.5 (9.3–72.5) | 2.9 (2.2–6.85) | <0.01 |
| BNP, pg/mLρ | 62.9 (30.2–197.2) | 102 (40.8–252.8) | 36.5 (16.5–63.1) | 0.009 |
| Fibrinogen, mg/dL γ | 691.5 (608–768.5) | 693 (605–768) | 690 (613–785) | 0.976 |
| Critical Care Variables | ||||
| pH γ | 7.29 (0.11) | 7.27 (0.12) | 7.36 (0.08) | 0.009 |
| pO2, mmHg γ | 66.8 (58.2–80) | 64.5 (57–85) | 70 (63–78.5) | 0.229 |
| pCO2, mmHg γ | 49 (39–58) | 50 (39–68) | 44.4 (37–55) | 0.117 |
| PaO2/FiO2, mmHg γ | 132.0 (58.07) | 122.12 (62.82) | 154.14 (39.37) | 0.045 |
| SpO2 | 92 (88.5–94.8) | 91 (84–94.8) | 92.5 (92–97) | 0.042 |
| HCO3-, mmol/L ρ | 22.2 (20.3–24.8) | 21.9 (19.8–23.3) | 23 (22–25) | 0.364 |
| Fluid balance, mL γ | 1051 (512–1685) | 1230 (540–1826) | 720.5 (380.2–1237.6) | 0.159 |
| SOFAρ | 8 (8–9) | 8 (8–9) | 8 (8–9) | 0.967 |
| MAP, mmHg γ | 74 (71–78) | 75 (71.5–78) | 72 (69–80) | 0.524 |
| HR, bpm ρ | 80 (70–100) | 81 (72–101) | 77 (67–87) | 0.569 |
| Vasoactive drugs ø | 18 (40) | 13 (41.94) | 5 (35.71) | 0.753 |
| Prone-position ventilation ø | 32 (71.11) | 25 (80.65) | 7 (50) | 0.072 |
| Urinary Kidney Biomarkers | ||||
| IGFBP7, ng/mL γ | 11.79 (6.63–30.77) | 14.32 (6.71–45.03) | 7.79 (5.69–12.86) | 0.047 |
| TIMP-2, ng/mL γ | 4.79 (1.79–10.53) | 4.43 (1.95–10.35) | 5.39 (1.05–13.48) | 0.980 |
| IL-6, pg/mL ρ | 0.72 (0.31–1.91) | 1.59 (0.39–2.38) | 0.36 (0.18–0.86) | 0.024 |
| [(TIMP-2)(IGFBP-7)]/1000 ρ | 0.05 (0.01–0.3) | 0.1 (0.02–0.33) | 0.04 (0.01–0.16) | 0.226 |
| N-Gal ρ | 24.7 (8.8–82.2) | 50.2 (9.9–110.7) | 10.95 (7.4–24.7) | 0.042 |
| Ventilatory parameters after compliance-guided PEEP titration | ||||
| PEEP, cmH2O ρ | 8 (8–12) | 8 (7–12) | 10 (8–12) | 0.447 |
| Tidal Volume, mL γ | 394.5 (64.97) | 383.93 (63.46) | 418.23 (64.39) | 0.115 |
| Pmax, cmH2O γ | 27.41 (6.04) | 29.07 (5.56) | 24 (5.71) | 0.008 |
| Pplat, cmH2O γ | 24.6 (4.91) | 25.94 (4.73) | 21.64 (4.09) | 0.005 |
| ΔP, cmH2O γ | 13 (11–18) | 15 (12–20) | 12 (10–13) | 0.006 |
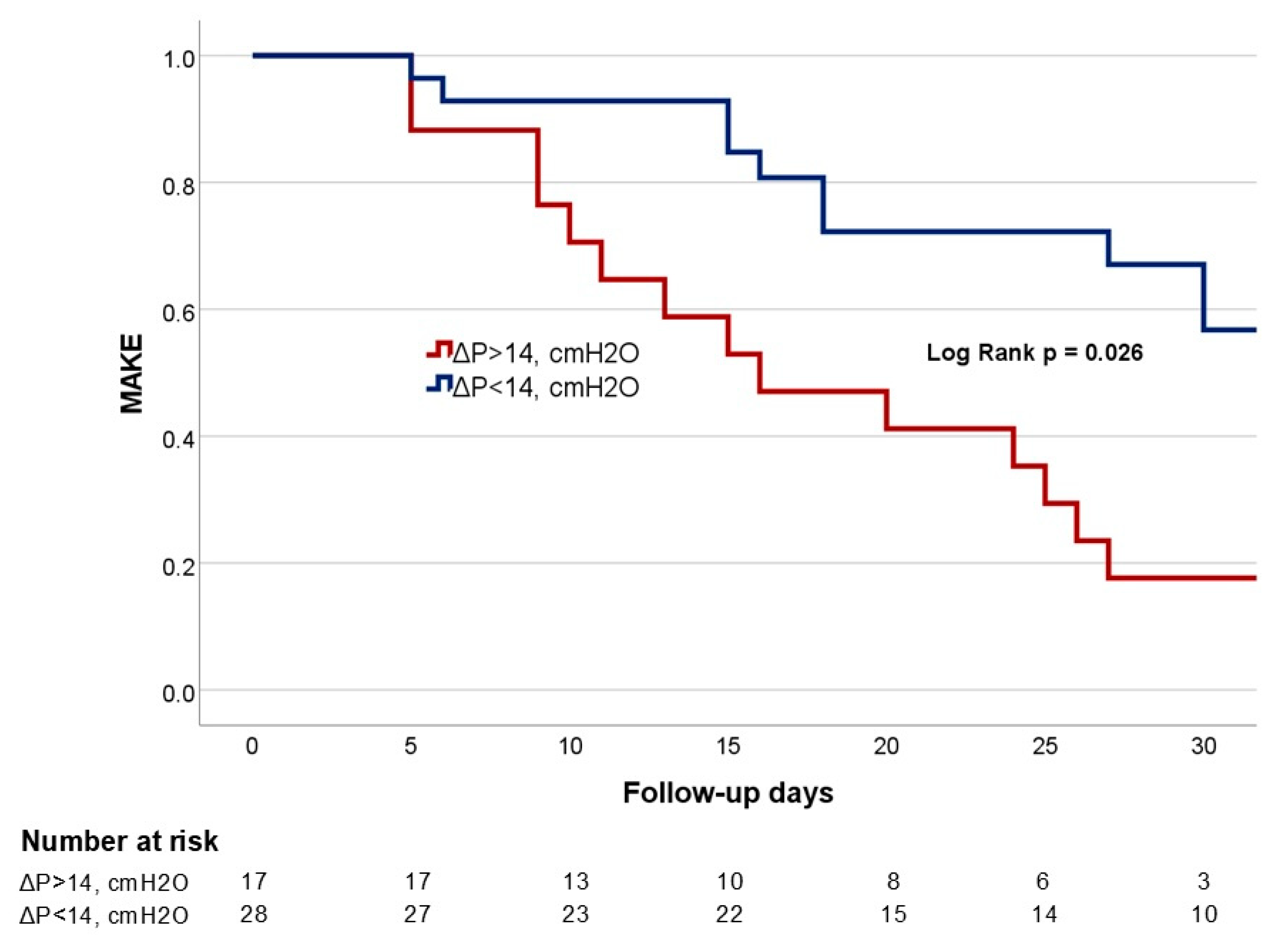
| ΔP > 14, cmH2O ø | 17 (37.78) | 16 (51.61) | 1 (7.14) | 0.007 |
| Cstat, ml/cmH2O γ | 29.02 (9.68) | 25.37 (7.9) | 37.14 (8.42) | < 0.001 |
| Outcomes | ||||
| Extubation ø | 23 (51.11) | 10 (32.26) | 13 (92.86) | <0.01 |
| Days on IMV ø | 17 (9–48) | 22 (10–54) | 16 (9–42) | 0.343 |
| Death ø | 22 (48.89) | 21 (67.74) | 1 (7.14) | <0.01 |
| Variables | Unadjusted ORs (95% CI) | p-Value | Adjusted ORs (95% CI) | p-Value |
|---|---|---|---|---|
| Age, years | 1.07 (1.02–1.12) | 0.003 | 1.23 (1.00–1.22) | 0.038 |
| Male | 0.23 (0.04–1.21) | 0.083 | 0.15 (0.03–5.78) | 0.432 |
| Diabetes | 6.19 (0.70–54.15) | 0.099 | 0.62 (0.02–16.77) | 0.619 |
| ΔP, cmH2O | 1.36 (1.06–1.74) | 0.013 | 1.62 (1.01–2.60) | 0.043 |
| Urinary N-Gal | 1.01 (0.99–1.04 | 0.089 | 1.01 (0.98–1.05) | 0.323 |
| Urinary IL-6, pg/ml | 2.13 (0.95–4.75) | 0.064 | 1.81 (0.62–5.33) | 0.281 |
| Variables | Unadjusted ORs (95% CI) | p-Value | Adjusted ORs (95% CI) | p-Value |
|---|---|---|---|---|
| Age, years | 1.06 (1.02–1.10) | 0.005 | 1.05 (1.00–1.10) | 0.038 |
| Men | 0.58 (1.66–2.55) | 0.401 | 0.66 (0.12–3.58) | 0.639 |
| Procalcitonin, ng/ml | 4.08 (0.70–23.7) | 0.117 | 2.61 (0.40–16.87) | 0.311 |
| Urinary N-Gal > 40 ng/mL | 8.43 (2.11–33.61) | 0.002 | 8.54 (1.75–41.65) | 0.008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casas-Aparicio, G.; Caballero-Islas, A.E.; León-Ortiz, A.; Escamilla-Illescas, D.; Rueda-Escobedo, Y.; Ascención-López, C.; Hernández-Quino, D.; Flores-Vargas, A.; Sosa-Chombo, J.; Tolentino-de La Mora, A.; et al. Early Driving Pressure Is Associated with Major Adverse Kidney Events at 30 Days in ARDS Patients with SARS-CoV-2. J. Clin. Med. 2025, 14, 2783. https://doi.org/10.3390/jcm14082783
Casas-Aparicio G, Caballero-Islas AE, León-Ortiz A, Escamilla-Illescas D, Rueda-Escobedo Y, Ascención-López C, Hernández-Quino D, Flores-Vargas A, Sosa-Chombo J, Tolentino-de La Mora A, et al. Early Driving Pressure Is Associated with Major Adverse Kidney Events at 30 Days in ARDS Patients with SARS-CoV-2. Journal of Clinical Medicine. 2025; 14(8):2783. https://doi.org/10.3390/jcm14082783
Chicago/Turabian StyleCasas-Aparicio, Gustavo, Adrián E. Caballero-Islas, Antonio León-Ortiz, David Escamilla-Illescas, Yovanna Rueda-Escobedo, Carlos Ascención-López, Diana Hernández-Quino, Aimee Flores-Vargas, Jesús Sosa-Chombo, Abraham Tolentino-de La Mora, and et al. 2025. "Early Driving Pressure Is Associated with Major Adverse Kidney Events at 30 Days in ARDS Patients with SARS-CoV-2" Journal of Clinical Medicine 14, no. 8: 2783. https://doi.org/10.3390/jcm14082783
APA StyleCasas-Aparicio, G., Caballero-Islas, A. E., León-Ortiz, A., Escamilla-Illescas, D., Rueda-Escobedo, Y., Ascención-López, C., Hernández-Quino, D., Flores-Vargas, A., Sosa-Chombo, J., Tolentino-de La Mora, A., Saucedo-Pruneda, A., & Piten-Isidro, E. (2025). Early Driving Pressure Is Associated with Major Adverse Kidney Events at 30 Days in ARDS Patients with SARS-CoV-2. Journal of Clinical Medicine, 14(8), 2783. https://doi.org/10.3390/jcm14082783






