Update on the Laboratory Diagnosis of Lupus Anticoagulant: Current Challenges and Clinical Involvement
Abstract
:1. Introduction
2. Preanalytical Phase
- (1)
- First of all, to avoid incidental findings that may lead to LAC diagnosis misinterpretation, patients with high APS suspicion must be selected [6]. For this purpose, patients with prolonged unexplained APTT and the rest of the basic hemostasis study within normal parameters (PT time and thrombin time) are candidates for study, especially if thrombosis (arterial or venous) or recurrent miscarriages are associated. It should also be considered in patients < 50 years old who, although they do not meet the clinical criteria, present some autoimmune disorder, such as idiopathic thrombocytopenic purpura, livedo reticularis, or thrombosis, with weak cardiovascular risk factors.
- (2)
- LAC diagnosis should not be performed in the emergency setting unless catastrophic APS (CAPS) is suspected [6]. CAPS is a rare and life-threatening subset of APS characterized by severe thrombotic complications, usually microvascular as well as large vessel, that causes multiorgan failure simultaneously or over a short period of time [7]. In that case, an early diagnosis is required given the severity and mortality associated with this pathology. LAC should not be determined in the acute thrombotic phase or in other pathologies such as infection, surgery, or cancer, in which cytokines are released with the consequent increase in acute phase reactants and factor VIII (FVIII). C-reactive protein has an affinity for the PL present in the reagents, and false positives can occur. Furthermore, an increase in FVIII may shorten the APTT and can induce false negatives. LAC measurement should also be avoided during pregnancy because of the increase in coagulation factors, including FVIII. Anticoagulant treatment can also interfere with the results [6,8].
- (3)
- Regarding the correct handling of the samples, double centrifugation at 2000× g for 15 min at room temperature should be carried out in order to obtain platelet-poor plasma (<10 × 109/L) [6,8]. Ultracentrifugation and the use of filters are not recommended, because microparticles are released. In case of freezing, once double centrifugation is performed, the sample should be frozen only once and within the first 4 h after obtaining the blood sample. The sample can be kept frozen for 14 days at −20° and up to 6 months at −70 °C. Hemolyzed, icteric, insufficient, lipemic, or coagulated samples should not be processed [6,8].
3. LAC Diagnosis
- (1)
- Screening test: this test uses low PL concentrations. PL of screening tests are heavily di luted to enhance the inhibitory effect of possible LAC, prolonging the APTT beyond the high reference limit. An elongated APTT can be observed not only in the presence of LAC but in other situations such as deficiency of intrinsic pathway factors (FXII, FXI, FIX, FVIII) [6,10].
- (2)
- Mixing test: a mixing of the patient’s plasma and a normal plasma pool (NPP), obtained from at least 20–40 healthy subjects, with factor concentrations around 100%, is performed in a 1:1 proportion without incubation for 30 min. The APTT is determined with a reagent with a low PL concentration. The shortening of APTT indicates a factor deficiency, while if the APTT remains prolonged (>4.5 s compared with the NPP), this suggests the presence of an inhibitor, either LAC (PL-dependent inhibitor) or inhibitors against any intrinsic pathway factor (non-PL-dependent inhibitor). In these situations, clinical data are relevant and can help us orientate the diagnosis. LAC is usually associated with thrombosis, while other inhibitors, for example anti-FVIII in acquired or congenital hemophilia A, are associated with bleeding [6,10].
- (3)
- Confirmatory test: this test uses high PL concentrations provided by the manufacturer. To evidence the PL dependence, the confirmatory test must be performed by increasing the concentration of PL used in the screening test.
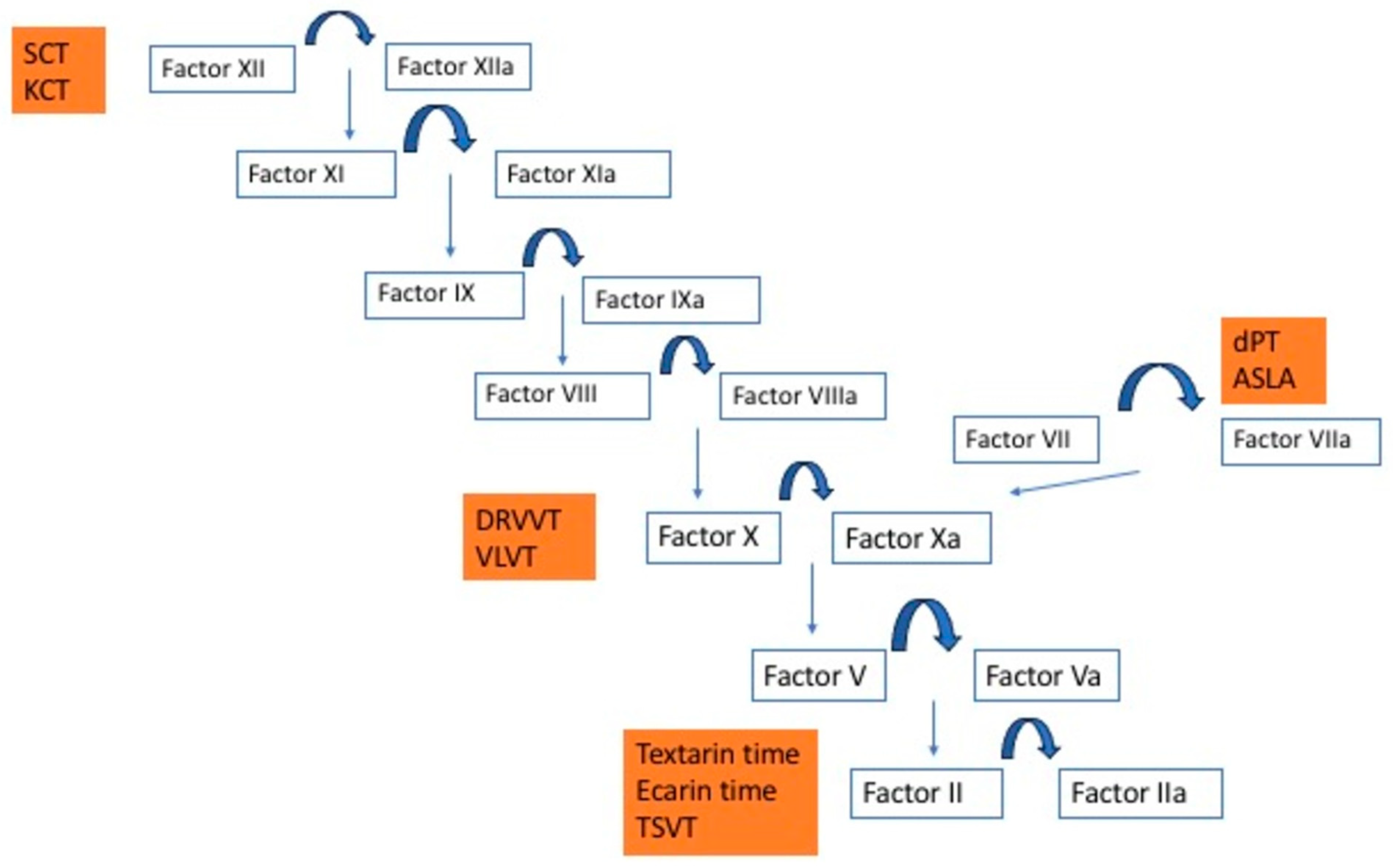
- Dilute prothrombin time (dPT, extrinsic pathway-based test): the high PL concentration in thromboplastin reagents for PT time is such that LAC rarely prolongs routine PT time [6]. Dilution of the thromboplastin to generate a dPT screening test increases LAC sensitivity. A lower thromboplastin dilution, or even undiluted reagent, can be used as the dPT confirmatory test. The dPT assay triggers coagulation via tissue factor and factor VII and contains heparin neutralizer. Although reproducibility is improved because of minimal variation inter- and intralaboratories, it is not available in the majority of laboratories [15,16].
- Kaolin clotting time (KCT, intrinsic pathway-based test): this test uses kaolin as the contact activator. PL are very dilute, making it sensitive to LAC [12,13]. However, KCT is not compatible with some optical analyzers and has poor reproducibility. KCT does not have any commercial confirmatory test, reducing specificity of KCT for LAC [6,15,16].
- Vipera lebetina venom time (VLVT, common pathway-based tests): this assay is similar to DRVVT. Macrovipera lebetina venom activates factor X, and low and high PL screen and confirm reagents are available. Equivalence with DRVVT has not been proven, but it is commercially available. Reagents contain heparin neutralizer [6,15,16].
- Textarin and ecarin time (common pathway-based tests): these assays are provided with snake venom’s factor II activators (Pseudonaja textilis and Echis Carinatus, respectively). They activate the undercarboxylated factor II form produced on VKA, converting it to thrombin or meizothrombin, bypassing factor Xa, and are, therefore, an adequate option in anticoagulated patients. Textarin is a portion of Pseudonaja textilis venom sensitive to PL, factor V, and calcium. Therefore, diluting PL makes textarin sensitive to LAC [6,12,13]. However, the ecarin fraction is cofactor independent, so the lack of PL does not prolong CT if LAC is present. This property makes ecarin time an optimal confirmatory test [6,15,16].
- Taipan snake venom time (TSVT, common pathway-based tests): TSVT is a dilute PL screening test based on factor II activation via Oscutarin C fraction of Oxyuranus scutellatus venom [6]. Pairing TSVT with ecarin time has shown to improve LAC detection in anticoagulated patients. In fact, Moore et at designed an international multicenter study, validating the TSVT and ecarin time pairing for LAC measurement in nonanticoagulated patients and those on VKA and with direct factor Xa inhibitors [17].
4. Interpretation of Results
- (1)
- (2)
- Mixing tests: There are two ways to interpret mixing time results, the use of circulant anticoagulation index (ICA or also called Roxner index) and the mixing-test-specific cutoff point (MTC). The ICA results from the following formula: ([1:1 mix Sample (s) − NPP (s)]/screen Patient (s)) × 100 [1].
- (3)
5. Algorithms for LAC Detection
6. Effect of Anticoagulants on LAC Assays
- (1)
- Heparins.
- (2)
- Vitamin K antagonists (VKA).
- (3)
- Direct oral anticoagulants (DOACs)
- (a)
- Discontinue the DOAC and adjust according to renal function, as established by the DOAC withdrawal guidelines before surgery or a scheduled invasive procedure. The worse the kidney function, the more days of DOAC withdrawal will be necessary to ensure that there is no DOAC activity. The anti-Xa activity of rivaroxaban, apixaban, and edoxaban and the anti-IIa activity of dabigatran could be measured, if available, in the hemostasis laboratory, and LAC study could be carried out if their effect is not detected.
- (b)
- Use neutralizers. There are two commercialized DOAC removing agents: DOAC-stop® (Haematex Research, Hornsby, Australia) and DOAC-remove® (5 Diagnostics, Basel, Switzerland). These neutralizers contain active charcoal compounds able to eliminate DOAC molecules in plasma. Briefly, absorbents and patient plasma are mixed, and then a short incubation and centrifugation of this mixture is performed [1,25]. The supernatant plasma, which is supposed to be DOAC-free, is used for LAC measurement. In addition, filtration techniques can also remove DOAC from the patient plasma [26]. All these neutralizers do not guarantee complete DOAC removal, and an effect on CT may be detected. Therefore, false positives and negatives have been described [1]. Wang et al. studied the impact of DOAC-stop® on thrombin generation assays in NPP before and after adding different anti-Xa DOAC concentrations [27]. In this study, the majority of DOAC levels were <1 ng/mL, except for that of one sample spiked with 502 ng/mL of apixaban, which was reduced to 6 ng/mL. Regarding thrombin generation parameters, a significant increase in the peak height (21.9%) and velocity index (42.6%) were detected following the addition of DOAC-stop®. The decrease in tissue factor pathway inhibitor (TFPI) could explain these findings. Strong correlations were described before and after DOAC-stop® addition for all parameters [27]. They concluded that global assays can be an option for studying the thrombotic state in these patients.
- (c)
- Use of PT activators, not applicable if the patient takes dabigatran.
7. Other Specific Clinical Issues
8. Conclusions
Funding
Conflicts of Interest
Abbreviations
| ADAMTS13 | A disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 |
| aPL | Phospholipid antibodies |
| APS | Antiphospholipid syndrome |
| APTT | Activated partial thromboplastin time |
| ASLA | Activated seven lupus anticoagulant assay |
| BSH | British Society of Hematology |
| CAPS | Catastrophic antiphospholipid syndrome |
| CLSI | Clinical and Laboratory Standards Institute |
| CT | Clotting times |
| DOAC | Direct oral anticoagulants |
| DPT | Dilute prothrombin time |
| DRVVT | Dilute Russell’s viper venom test |
| HELLP | Hemolysis, elevated liver enzyme levels, and low platelet levels |
| ICA | Index of circulating anticoagulant |
| INR | International normalized ratio |
| ISTH | International Society on Thrombosis and Hemostasis |
| KCT | Kaolin clotting time |
| LAC | Lupus anticoagulant |
| MTC | Mixing-test-specific cutoff |
| NPP | Normal plasma pool |
| PL | Phospholipids |
| PT | Prothrombin |
| PS | Phosphatidylserine |
| SCT | Silica clotting time |
| SLE | Systemic lupus eritematosus |
| TFPI | Tissue factor pathway inhibitor |
| TMA | Thrombotic microangiopathy |
| TSVT | Taipan snake venom time |
| TTP | Thrombotic thrombocytopenia purpura |
| VDRL | Venereal Disease Research Laboratory |
| VKA | Vitamin K antagonists |
| VLVT | Vipera lebetina venom time |
References
- Molinari, A.C.; Martini, T.; Banov, L.; Ierardi, A.; Leotta, M.; Strangio, A.; Santoro, R.C. Lupus Anticoagulant Detection under the Magnifying Glass. J. Clin. Med. 2023, 12, 6654. [Google Scholar] [CrossRef] [PubMed]
- Talon, L.; Fourneyron, V.; Senectaire, S.; Tardieu, M.; Tillier, M.; Trapani, A.; Trayaud, A.; Vaissade, A.; Sapin, A.F.; Lebreton, A.; et al. Lupus Anticoagulant Laboratory Diagnosis by applying the 2020 ISTH-SSC Guidelines. Thromb. Res. 2020, 224, 38–45. [Google Scholar] [CrossRef]
- Feinstein, D.I.; Rapaport, S.I. Acquired Inhibitors of Blood Coagulation. Prog. Haemost. Thromb. 1972, 1, 75–95. [Google Scholar]
- Colaco, C.B.; Male, D.K. Anti-phospholipid antibodies in syphilis and a thrombotic subset of SLE: Distinct profile of epitope specificity. Clin. Exp. Immunol. 1985, 59, 449–456. [Google Scholar] [PubMed]
- Barbhaiya, M.; Zuily, S.; Naden, R.; Hendry, A.; Manneville, F.; Amigo, M.C.; Amoura, Z.; Andrade, D.; Andreoli, L.; Artim-Esen, B.; et al. The 2023 ACR/EULAR antiphospholipid syndrome classification criteria. Arth. Reumatol. 2023, 75, 1687–1702. [Google Scholar] [CrossRef] [PubMed]
- Moore, G.W. Testing for Lupus Anticoagulant. Semin. Thromb. Hemost. 2022, 48, 643–660. [Google Scholar] [CrossRef]
- Ambati, A.; Knight, J.S.; Zuo, Y. Antiphospholipid syndrome management: A 2023 update and practical algorithm-based approach. Curr. Opin. Rheumatol. 2023, 35, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Devreese, K.M.; de Groot, P.G.; de Laat, B.; Erkan, D.; Favaloro, E.J.; Mackie, I.; Martinuzzo, M.; Ortel, T.L.; Pengo, V.; Rand, J.H.; et al. Guidance from the Scientific Committee for Lupus Anticoagulant/Antiphospholipid Antibodies of the International Society of Thrombosis and Haemostasis. Update of the Guidelines for Lupus Anticoagulant Detection and Interpretation. J. Thromb. Haemost. 2020, 18, 2828–2839. [Google Scholar] [CrossRef]
- Smith, L.J. Laboratory Diagnosis of the Lupus Anticoagulant. Clin. Lab. Sci. 2017, 30, 7–14. [Google Scholar] [CrossRef]
- Pengo, V.; Tripodi, A.; Reber, G.; Rand, J.H.; Ortel, T.L.; Galli, M.; De Groot, P.G. Update of the Guidelines for Lupus Anticoagulant Detection. Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. J. Thromb. Haemost. 2009, 7, 1737–1740. [Google Scholar] [CrossRef]
- Tripodi, A.; Cohen, H.; Devreese, K.M. Lupus anticoagulant detection in anticoagulated patients. Guidance from the scientific and standardization committee for lupus anticoagulant/antiphospholipid antibodies of the international society on thrombosis and Haemostasis. J. Thromb. Haemost. 2020, 18, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Moore, G.W.; Henley, A.; Greenwood, C.K.; Rangarajan, S. Further evidence of false negatives screening for lupus anticoagulants. Thromb. Res. 2008, 121, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Favaloro, E.J.; Bonar, R.; Marsden, K. Internal quality control and external quality assurance in testing for antiphospholipid antibodies: Part II-Lupus anticoagulant. Semin. Thromb. Hemost. 2012, 38, 404–411. [Google Scholar] [CrossRef]
- Kumano, O.; Ieko, M.; Naito, S.; Yoshida, M.; Takahashi, N. APTT reagent with ellagic acid as activator shows adequate lupus anticoagulant sensitivity in comparison to silica-based reagent. J. Thromb. Hemost. 2012, 10, 2338–2343. [Google Scholar] [CrossRef]
- Moore, G.W. Alternative assays to dRVVT and aPTT for lupus anticoagulant detection. Am. J. Hematol. 2020, 95, 992–998. [Google Scholar] [CrossRef] [PubMed]
- Moore, G.W. Clinical utility of the less commonly employed assays for lupus anticoagulation detection: The evidence. J. Coag Dis. 2010, 2, 69–79. [Google Scholar]
- Moore, G.W.; Jones, P.O.; Platton, S.; Hussain, N.; White, D.; Thomas, W.; Rigano, J.; Pouplard, C.; Gray, E.; Devreese, K.M. International multicenter, multiplatform study to validate Taipan snake venom time as a lupus anticoagulant screening test with ecarin time as the confirmatory test: Communication from the ISTH SSC Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibodies. J. Thromb. Haemost. 2021, 19, 3177–3192. [Google Scholar] [CrossRef]
- Kumano, O.; Moore, G.W. Lupus anticoagulant mixing tests for multiple reagents are more sensitive if interpreted with a mixing test-specific cut-off index of circulating anticoagulant. Res. Pract. Thromb. Haemost. 2018, 2, 105–113. [Google Scholar] [CrossRef]
- Moore, G.W. Reference interval mean clotting times should not be used to calculate lupus anticoagulant mixing tests ratios unless they match the normal pooled plasma clotting time. Thromb. Res. 2017, 159, 16–18. [Google Scholar] [CrossRef]
- Asakrah, S.; Davis, R.; Bhargava, P. Practical considerations and testing nuances for the detection of lupus anticoagulant: Do low phospholipid screen results, assay type, and test ratio matter? Am. J. Clin. Pathol. 2021, 156, 1073–1082. [Google Scholar] [CrossRef]
- Clinical Laboratory Standards Institute (CLSI). Laboratory Testing for the Lupus Anticoagulant: Approved Guideline CLSI Document, H.6.0.-A; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2014. [Google Scholar]
- Keeling, D.; Mackie, I.; Moore, G.W.; Greer, I.A.; Greaves, M. British Committee for Standards in Hematology. Guidelines on the investigation and management of antiphospholipid syndrome. Br. J. Haematol. 2012, 157, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Florin, L.; Desloovere, M.; Devreese, K.M. Evaluation of an automated algorithm for interpretation of lupus anticoagulant testing. Int. J. Lab. Hematol. 2019, 41, 412–417. [Google Scholar] [CrossRef]
- Favaloro, E.J.; Pasalic, L. Lupus anticoagulant testing during anticoagulation, including direct oral anticoagulants. Res. Pract. Thromb. Haemost. 2022, 6, e12676. [Google Scholar] [CrossRef]
- De Kesel, P.M.; Devreese, K.M.J. Direct oral anticoagulant adsorption: Impact on lupus anticoagulant testing. Review of the literature and evaluation on spiked and patient samples. J. Thromb. Haemost. 2020, 18, 2003–2017. [Google Scholar] [CrossRef]
- Linskens, E.A.; De Kesel, P.M.; Devreese, K.M.J. Direct oral anticoagulant removal by a DOAC filter: Impact on lupus anticoagulant testing. Evaluation on spiked and patient samples. Res. Pract. Thromb. Heamost 2022, 6, e12633. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yin, L.H.; Nandurkaar, H.; Ho, P. DOAC-stop can remove direct oral anticoagulants and allow analysis by global coagulation assays. Int. J. Lab. Hematol. 2023, 45, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Pengo, V. Additional laboratory tests to improve on the diagnosis of antiphoshpholipid syndrome. J. Thromb. Haemost. 2020, 18, 3118–3119. [Google Scholar] [CrossRef]
- Obrisca, B.; Vornicu, A.; Procop, A.; Herlea, V.; Terinte-Balcan, G.; Gherghiceanu, M.; Isamail, G. A histology-guided approach to the management of patients with lupus nephritis: Are we there yet? Biomedicines 2022, 10, 1409. [Google Scholar] [CrossRef]
- Asherson, R.A.; Cervera, R. Microvascular and microangiopathic antiphospholipid-associated syndromes (MAPS): Semantic or antisemantic? Autoimmun. Rev. 2008, 7, 164–167. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, J.E.; Han, K.S.; Kim, H.K. Thrombotic risk of reduced ADAMT13 activity in patients with antiphospholipid antibodies. Blood Coag. Fibrinolysis 2016, 27, 907–912. [Google Scholar] [CrossRef]
- Asherson, R.A.; Pierangeli, S.S.; Cervera, R. Is there a microangiopathic antiphospholipid syndrome? Ann. Rheum. Dis. 2007, 66, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Durand, J.M.; Lefevre, P.; Kaplasnki, G.; Soubeyrand, J. Thrombotic microangiopathy and the antiphospholipid antibody syndrome. J. Rheumatol. 1991, 18, 1916–1918. [Google Scholar]
- Díaz-Cremades, J.; Fernández-Fuertes, F.; Ruano, J.A.; Tapia, M.; Soler, S.; Bosch, J.M.; Caballero, M.; González-San Miguel, J.D. Concurrent thrombotic thrombocytopenic purpura and antiphospholipid syndrome: A rare and severe clinical combination. Br. J. Hematol. 2009, 147, 584–585. [Google Scholar] [CrossRef]
- Espinosa, G.; Bucciarelli, S.; Cervera, R.; Lozano, M.; Reverter, J.C.; de la Red, G.; Gil, V.; Ingelmo, M.; Font, J.; Asherson, R.A. Thrombotic microangiopathic haemolytic anemia and antiphospholipid antibodies. Ann. Rheum. Dis. 2004, 63, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Komvilaisak, P.; Wisanuyotin, S.; Jettrisuparb, A.; Wiangnon, S. Lupus anticoagulant-hypoprothombinemia syndrome (LAC-HPS) in children with systemic lupus erythematosus: Report of 3 cases. J. Pediatr. Hematol. Oncol. 2017, 39, e521–e524. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, S.; McCrae, K.R. Diagnosis and management of the antiphospholipid syndrome. Blood Rev. 2017, 31, 406–417. [Google Scholar] [CrossRef]
- Finazzi, G.; Marchioli, R.; Brancaccio, V.; Schinco, P.; Wisloff, F.; Musial, J.; Budo, F.; Berrettini, M.; Testa, S.; Angelo, A.D.; et al. A randomized clinical trial of high-intensity warfarin vs conventional antithrombotic therapy for the prevention of recurrent thrombosis in patients with the antiphospholipid syndrome (WAPS). J. Thromb. Hemost. 2005, 3, 848–853. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marco-Rico, A. Update on the Laboratory Diagnosis of Lupus Anticoagulant: Current Challenges and Clinical Involvement. J. Clin. Med. 2025, 14, 2791. https://doi.org/10.3390/jcm14082791
Marco-Rico A. Update on the Laboratory Diagnosis of Lupus Anticoagulant: Current Challenges and Clinical Involvement. Journal of Clinical Medicine. 2025; 14(8):2791. https://doi.org/10.3390/jcm14082791
Chicago/Turabian StyleMarco-Rico, Ana. 2025. "Update on the Laboratory Diagnosis of Lupus Anticoagulant: Current Challenges and Clinical Involvement" Journal of Clinical Medicine 14, no. 8: 2791. https://doi.org/10.3390/jcm14082791
APA StyleMarco-Rico, A. (2025). Update on the Laboratory Diagnosis of Lupus Anticoagulant: Current Challenges and Clinical Involvement. Journal of Clinical Medicine, 14(8), 2791. https://doi.org/10.3390/jcm14082791




