Clinical, Neuroimaging, and Genetic Markers Associated with Cognitive and Functional Outcomes After Traumatic Brain Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Criteria
2.2. Demographic and Clinical Variables
2.3. Radiological and Laboratory Examinations
2.4. Statistical Analysis
3. Results
3.1. Demographic Data
3.2. Clinical Presentation and Morphometric Changes
3.3. Cognitive Functions Assessment
3.4. Modeling the Risk of Cognitive Impairments and Functional Impairment in Patients with Encephalopathy After TBI
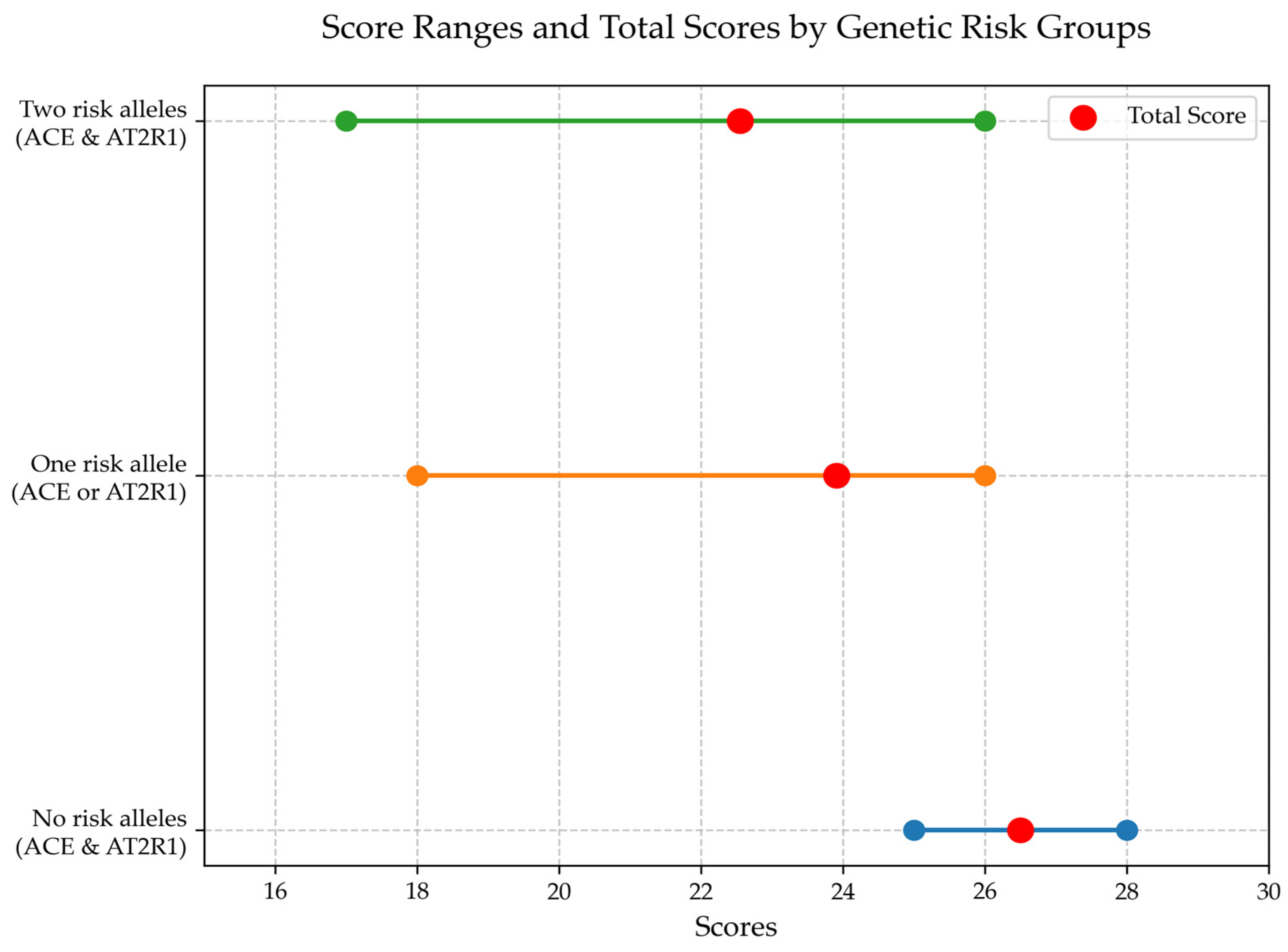
3.5. Analysis of Prognostically Significant Markers for Cognitive Impairment in Patients with Encephalopathy After TBI
- X1: 1—presence of the I allele of the ACE gene, 2—presence of the D allele of the ACE gene;
- X2: 1—presence of the A allele of the AT2R1 gene, 2—presence of the C allele of the AT2R1 gene.
3.6. Analysis of Prognostically Significant Markers for Functional Impairment in Patients with Encephalopathy After TBI
4. Discussion
4.1. Mechanisms Underlying Cognitive and Functional Impairments
4.2. Genetic Contributions to Disease Progression
4.3. Clinical and Functional Implications
5. Conclusions
6. Limitations and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2016 Traumatic Brain Injury and Spinal Cord Injury Collaborators. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 56–87. [Google Scholar] [CrossRef] [PubMed]
- Majdan, M.; Plancikova, D.; Brazinova, A.; Rusnak, M.; Nieboer, D.; Feigin, V.L.; Maas, A. Epidemiology of traumatic brain injuries in Europe: A cross-sectional analysis. Lancet Public Health 2016, 1, e76–e83. [Google Scholar] [CrossRef] [PubMed]
- Maas, A.I.R.; Menon, D.K.; Adelson, P.D.; Andelic, N.; Bell, M.J.; Belli, A.; Bragge, P.; Brazinova, A.; Büki, A.; Chesnut, R.M.; et al. Traumatic brain injury: Integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017, 16, 987–1048. [Google Scholar] [CrossRef]
- Traumatic Brain Injury Center of Excellence. Available online: https://health.mil/Military-Health-Topics/Centers-of-Excellence/Traumatic-Brain-Injury-Center-of-Excellence (accessed on 10 September 2024).
- Bruns, J.J.; Jagoda, A.S. Blast-related mild traumatic brain injury: Mechanisms of injury and impact on clinical care. Mt. Sinai J. Med. A J. Transl. Pers. Med. 2009, 76, 111–118. [Google Scholar] [CrossRef]
- Zhang, J.K.; Botterbush, K.S.; Bagdady, K.; Lei, C.H.; Mercier, P.; Mattei, T.A. Blast-Related Traumatic Brain Injuries Secondary to Thermobaric Explosives: Implications for the War in Ukraine. World Neurosurg. 2022, 167, 176–183.e4. [Google Scholar] [CrossRef]
- Critchley, M. Medical aspects of boxing, particularly from a neurological standpoint. BMJ 1957, 1, 357–362. [Google Scholar] [CrossRef]
- McKee, A.C.; Stein, T.D.; Kiernan, P.T.; Alvarez, V.E. The Neuropathology of Chronic Traumatic Encephalopathy. Brain Pathol. 2015, 25, 350–364. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Montenigro, P.H.; Baugh, C.M.; Daneshvar, D.H.; Mez, J.; E Budson, A.; Au, R.; I Katz, D.; Cantu, R.C.; A Stern, R. Clinical subtypes of chronic traumatic encephalopathy: Literature review and proposed research diagnostic criteria for traumatic encephalopathy syndrome. Alzheimer’s Res. Ther. 2014, 6, 68. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Iverson, G.L.; Gardner, A.J.; Shultz, S.R.; Solomon, G.S.; McCrory, P.; Zafonte, R.; Perry, G.; Hazrati, L.N.; Keene, C.D.; Castellani, R.J. Chronic traumatic encephalopathy neuropathology might not be inexorably progressive or unique to repetitive neurotrauma. Brain 2019, 142, 3672–3693. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schaffert, J.; Didehbani, N.; LoBue, C.; Hart, J.; Rossetti, H.; Lacritz, L.; Cullum, C.M. Frequency and Predictors of Traumatic Encephalopathy Syndrome in a Prospective Cohort of Retired Professional Athletes. Front. Neurol. 2021, 12, 617526. [Google Scholar] [CrossRef]
- The TBI/CTE Group; McKee, A.C.; Cairns, N.J.; Dickson, D.W.; Folkerth, R.D.; Keene, C.D.; Litvan, I.; Perl, D.P.; Stein, T.D.; Vonsattel, J.-P.; et al. The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol. 2016, 131, 75–86. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Katz, D.I.; Bernick, C.; Dodick, D.W.; Mez, J.; Mariani, M.L.; Adler, C.H.; Alosco, M.L.; Balcer, L.J.; Banks, S.J.; Barr, W.B.; et al. National Institute of Neurological Disorders and Stroke Consensus Diagnostic Criteria for Traumatic Encephalopathy Syndrome. Neurology 2021, 96, 848–863. [Google Scholar] [CrossRef] [PubMed]
- Katsumoto, A.; Takeuchi, H.; Tanaka, F. Tau pathology in chronic traumatic encephalopathy and Alzheimer’s disease: Similarities and differences. Front. Neurol. 2019, 10, 980. [Google Scholar] [CrossRef]
- Murray, H.C.; Osterman, C.; Bell, P.; Vinnell, L.; Curtis, M.A. Neuropathology in chronic traumatic encephalopathy: A systematic review of comparative post-mortem histology literature. Acta Neuropathol. Commun. 2022, 10, 108. [Google Scholar] [CrossRef]
- Muzio, L.; Viotti, A.; Martino, G. Microglia in Neuroinflammation and Neurodegeneration: From Understanding to Therapy. Front. Neurosci. 2021, 15, 742065. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peters, M.E.; Lyketsos, C.G. The glymphatic system’s role in traumatic brain injury-related neurodegeneration. Mol. Psychiatry 2023, 28, 2707–2715. [Google Scholar] [CrossRef] [PubMed]
- Bayley, M.T.; Janzen, S.; Harnett, A.; Teasell, R.; Patsakos, E.; Marshall, S.; Bragge, P.; Velikonja, D.; Kua, A.; Douglas, J.; et al. INCOG 2.0 Guidelines for Cognitive Rehabilitation Following Traumatic Brain Injury: Methods, Overview, and Principles. J. Head Trauma Rehabil. 2023, 38, 7–23. [Google Scholar] [CrossRef]
- Hacker, D.; Jones, C.A.; Yasin, E.; Preece, S.; Davies, H.; Hawkins, A.; Belli, A.; Paton, E. Cognitive Outcome After Complicated Mild Traumatic Brain Injury: A Literature Review and Meta-Analysis. J. Neurotrauma 2023, 40, 1995–2014. [Google Scholar] [CrossRef]
- Bryant, A.M.; Rose, N.B.; Temkin, N.R.; Barber, J.K.; Manley, G.T.; McCrea, M.A.; Nelson, L.D.; TRACK-TBI Investigators; Badjatia, N.; Gopinath, S.; et al. Profiles of Cognitive Functioning at 6 Months After Traumatic Brain Injury Among Patients in Level I Trauma Centers: A TRACK-TBI Study. JAMA Netw. Open 2023, 6, e2349118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Griffiths, D.R.; Law, L.M.; Young, C.; Fuentes, A.; Truran, S.; Karamanova, N.; Bell, L.C.; Turner, G.; Emerson, H.; Mastroeni, D.; et al. Chronic Cognitive and Cerebrovascular Function after Mild Traumatic Brain Injury in Rats. J. Neurotrauma 2022, 39, 1429–1441. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stocchetti, N.; Zanier, E.R. Chronic impact of traumatic brain injury on outcome and quality of life: A narrative review. Crit. Care 2016, 20, 148. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mez, J.; Daneshvar, D.H.; Kiernan, P.T.; Abdolmohammadi, B.; Alvarez, V.E.; Huber, B.R.; Alosco, M.L.; Solomon, T.M.; Nowinski, C.J.; McHale, L.; et al. Clinicopathological Evaluation of Chronic Traumatic Encephalopathy in Players of American Football. JAMA 2017, 318, 360–370. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Manley, G.; Gardner, A.J.; Schneider, K.J.; Guskiewicz, K.M.; Bailes, J.; Cantu, R.C.; Castellani, R.J.; Turner, M.; Jordan, B.D.; Randolph, C.; et al. A systematic review of potential long-term effects of sport-related concussion. Br. J. Sports Med. 2017, 51, 969–977. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Namdar, I.; Feldman, R.; Glazer, S.; Meningher, I.; Shlobin, N.A.; Rubovitch, V.; Bikovski, L.; Been, E.; Pick, C.G. Motor Effects of Minimal Traumatic Brain Injury in Mice. J. Mol. Neurosci. 2019, 70, 365–377. [Google Scholar] [CrossRef] [PubMed]
- McCrea, M.A.; Giacino, J.T.; Barber, J.; Temkin, N.R.; Nelson, L.D.; Levin, H.S.; Dikmen, S.; Stein, M.; Bodien, Y.G.; Boase, K.; et al. Functional Outcomes Over the First Year After Moderate to Severe Traumatic Brain Injury in the Prospective, Longitudinal TRACK-TBI Study. JAMA Neurol. 2021, 78, 982–992. [Google Scholar] [CrossRef]
- Huang, Z.; Xu, P.; Hess, D.C.; Zhang, Q. Cellular senescence as a key contributor to secondary neurodegeneration in traumatic brain injury and stroke. Transl. Neurodegener. 2024, 13, 61. [Google Scholar] [CrossRef]
- Hawryluk, G.W.; Bullock, M.R. Past, Present, and Future of Traumatic Brain Injury Research. Neurosurg. Clin. N. Am. 2016, 27, 375–396. [Google Scholar] [CrossRef]
- Yao, L.; Xing, S.; Fu, X.; Song, H.; Wang, Z.; Tang, J.; Zhao, Y. Association between interleukin-10 gene promoter polymorphisms and susceptibility to liver cirrhosis. Int. J. Clin. Exp. Pathol. 2015, 8, 11680–11684. [Google Scholar]
- Gorovenko, N.G.; Kyryachenko, S.P.; Rossokha, Z.I. Study on association of the polymorphic variants of ACE (I/D), AT2R1 (A1166C), TNF-α (G308A), MTHFR (C677T) genes and their combinations with the risk of development of perinatal pathology and gestation reduction. Biopolym. Cell 2011, 27, 206–213. [Google Scholar] [CrossRef]
- Safarinejad, M.R.; Shafiei, N.; Safarinejad, S. The role of endothelial nitric oxide synthase (eNOS) T-786C, G894T, and 4a/b gene polymorphisms in the risk of idiopathic male infertility. Mol. Reprod. Dev. 2010, 77, 720–727. [Google Scholar] [CrossRef]
- McKee, A.C.; Stein, T.D.; Huber, B.R.; Crary, J.F.; Bieniek, K.; Dickson, D.; Alvarez, V.E.; Cherry, J.D.; Farrell, K.; Butler, M.; et al. Chronic traumatic encephalopathy (CTE): Criteria for neuropathological diagnosis and relationship to repetitive head impacts. Acta Neuropathol. 2023, 145, 371–394. [Google Scholar] [CrossRef]
- Verboon, L.N.; Patel, H.C.; Greenhalgh, A.D. The Immune System’s Role in the Consequences of Mild Traumatic Brain Injury (Concussion). Front. Immunol. 2021, 12, 620698. [Google Scholar] [CrossRef]
- Hugon, J.; Hourregue, C.; Cognat, E.; Lilamand, M.; Porte, B.; Mouton-Liger, F.; Dumurgier, J.; Paquet, C. Chronic traumatic encephalopathy. Neurochirurgie 2021, 67, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Mavroudis, I.; Kazis, D.; Chowdhury, R.; Petridis, F.; Costa, V.; Balmus, I.-M.; Ciobica, A.; Luca, A.-C.; Radu, I.; Dobrin, R.P.; et al. Post-Concussion Syndrome and Chronic Traumatic Encephalopathy: Narrative Review on the Neuropathology, Neuroimaging and Fluid Biomarkers. Diagnostics 2022, 12, 740. [Google Scholar] [CrossRef]
- Duve, K.; Shkrobot, S.; Salii, Z. Chronic traumatic encephalopathy: Predictors of the development of cognitive disorders and functional disability. Int. Neurol. J. 2024, 19, 233–240. [Google Scholar] [CrossRef]
- Spoto, G.; Di Rosa, G.; Nicotera, A.G. The Impact of Genetics on Cognition: Insights into Cognitive Disorders and Single Nucleotide Polymorphisms. J. Pers. Med. 2024, 14, 156. [Google Scholar] [CrossRef] [PubMed]
- Kondo, S.; Takada, K.; Kojima, T.; Tanaka, K.; Yakabe, M.; Shibata, E.; Umeda-Kamayama, Y.; Takao, H.; Ogawa, S.; Akishita, M. Marked Cognitive and Activities of Daily Living Improvement by Shunt Embolization in a Very Old Man with Portosystemic Encephalopathy Mimicking Alzheimer Disease: A Case Report. Ann. Geriatr. Med. Res. 2022, 26, 279–283. [Google Scholar] [CrossRef]
- Sit, J.W.; Chair, S.Y.; Choi, K.C.; Chan, C.W.; Lee, D.T.; Chan, A.W.; Cheung, J.L.; Tang, S.W.; Chan, P.S.; E Taylor-Piliae, R. Do empowered stroke patients perform better at self-management and functional recovery after a stroke? A randomized controlled trial. Clin. Interv. Aging 2016, 11, 1441–1450. [Google Scholar] [CrossRef]
- Oliveira-Kumakura, A.R.d.S.; Batista, L.M.O.S.; Spagnol, G.S.; Valler, L. Functionality and quality of life in Brazilian patients 6 months post-stroke. Front. Neurol. 2023, 14, 1020587. [Google Scholar] [CrossRef]

| No. | Gene | International Name of Genetic Polymorphism, rs | Reference to the Source |
|---|---|---|---|
| 1. | IL-1ß | C3953T, g.8967C>T, rs1143634 | [16] |
| 2. | IL-10 | C-592A, g.4433A>C, rs1800872 | [17] |
| 3. | TNFα | G308A, c.-488G>A, rs180062 | [18] |
| 4. | ACE | I/D, c.2306-117_2306-116insAF118569.1:g.14094_14382, rs4340 | [18] |
| 5. | eNOS | 4a/4b, g.150997188 AGGGGTGAGGAAGT CTAGACCTGCTGC [1], rs61722009 | [19] |
| 6. | PON1 | C108T, g.5124C>T, rs705379 | [16] |
| 7. | AT2R1 | A1166C, g.148742201A>C, rs5186 | [18] |
| Severity of TBI | n (%) | Type | n (%) |
|---|---|---|---|
| Mild | 35 (24.14) | Concussion | 20 (57.14) |
| Contusion | 15 (42.86) | ||
| Moderate | 90 (62.07) | Contusion + SAH, epidural or subdural hemorrhage, fractures | 54 (60.00) |
| Contusion + intracerebral hemorrhage, fracture | 7 (7.78) | ||
| Contusion + fracture | 29 (32.22) | ||
| Severe | 20 (13.79) | Severe contusion, diffuse axonal injury, brain compression | 20 (100.00) |
| Clinical Syndromes | Normal Cognitive Functioning | Cognitive Impairment | χ2; p | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | ||||||||
| n | % | n | % | n | % | n | % | |||
| Cephalgia | − | 3 | 60.00 | 2 | 40.00 | 0 | 0 | 0 | 0 | χ2 = 2.63; p = 0.269 |
| + | 38 | 27.14 | 97 | 69.29 | 5 | 3.57 | 0 | 0 | ||
| Emotional-liability syndrome | − | 12 | 31.58 | 23 | 60.53 | 3 | 7.89 | 0 | 0 | χ2 = 3.60; p = 0.165 |
| + | 29 | 27.10 | 76 | 71.03 | 2 | 1.87 | 0 | 0 | ||
| Cerebellar ataxia | − | 29 | 30.53 | 62 | 65.26 | 4 | 4.21 | 0 | 0 | χ2 = 1.32; p = 0.516 |
| + | 12 | 24.00 | 37 | 74.00 | 1 | 2.00 | 0 | 0 | ||
| Pyramidal insufficiency | − | 20 | 25.64 | 54 | 69.23 | 4 | 5.13 | 0 | 0 | χ2 = 1.82; p = 0.403 |
| + | 21 | 31.34 | 45 | 67.16 | 1 | 1.49 | 0 | 0 | ||
| Indicators | Cognitive Impairment | p | ||
|---|---|---|---|---|
| Norm | Mild | Moderate | ||
| RBC (×1012/L) | 5.10 | 4.95 | 4.30 | 0.051 |
| (4.68–5.39) | (4.70–5.28) | (4.20–4.67) | ||
| HGB (g/L) | 152 | 148 | 152 | 0.622 |
| (150–154) | (141–158) | (150–154) | ||
| PLT (×10⁹/L) | 218 | 214 | 231 | 0.944 |
| (196–236) | (189–254) | (190–272) | ||
| WBC (×10⁹/L) | 5.92 | 6.47 | 5.77 | 0.598 |
| (5.25–7.01) | (5.10–7.60) | (5.54–7.07) | ||
| Segmented Neutrophils (%) | 51 | 60 | 47 | <0.001 * |
| (46.8–57.3) | (54.00–65.0) | (41.0–52.0) | ||
| Lymphocytes (%) | 36.0 | 30.00 | 36.0 | 0.004 * |
| (30.0–40.0) | (22.0–35.9) | (32.9–38.0) | ||
| Monocytes (%) | 7.00 | 7.00 | 7.10 | 0.958 |
| (4.00–9.00) | (5.00–8.00) | (2.00–7.50) | ||
| Erythrocyte Sedimentation Rate (mm/hr) | 5.00 | 5.00 | 3.00 | 0.475 |
| (3.00–10.0) | (3.00–11.0) | (2.00–7.00) | ||
| Haematocrit (%) | 46.5 | 45.5 | 46.8 | 0.706 |
| (43.2–48.1) | (42.7–48.0) | (44.8–48.2) | ||
| Factor | Model 1 | Model 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Genotypes | Alleles | |||||||
| β | SE | t | p | β | SE | t | p | |
| Constant | 13.80 | 11.67 | 1.18 | 0.010 * | 2.53 | 2.40 | 1.06 | 0.007 * |
| ACE | −5.14 | 2.21 | −2.32 | 0.032 * | −3.39 | 1.32 | −2.57 | 0.013 * |
| AT2R1 | 1.78 | 1.37 | 1.30 | 0.211 | 2.90 | 1.38 | 2.11 | 0.041 * |
| eNOS | −4.53 | 3.64 | −1.24 | 0.230 | −2.49 | 1.54 | −1.61 | 0.114 |
| IL1β | 1.21 | 1.66 | 0.73 | 0.473 | 0.82 | 1.48 | 0.55 | 0.582 |
| TNFα | 1.63 | 2.42 | 0.67 | 0.509 | 0.29 | 1.55 | 0.19 | 0.853 |
| IL10 | −2.85 | 2.44 | −1.17 | 0.258 | −0.26 | 1.27 | −0.20 | 0.839 |
| PON1 | −1.27 | 1.17 | −1.09 | 0.291 | −0.37 | 0.87 | −0.43 | 0.67 |
| Factor | Model 3 | |||
|---|---|---|---|---|
| β | SE | t | p | |
| Constant | −0.42 | 0.64 | −0.66 | 0.046 * |
| ACE | 0.33 | 0.12 | 2.68 | 0.015 * |
| AT2R1 | −0.16 | 0.13 | −1.19 | 0.250 |
| Sex | 0.17 | 0.38 | 0.46 | 0.654 |
| Age category | 0.12 | 0.21 | 0.55 | 0.587 |
| Somatic comorbidity | 0.25 | 0.28 | 0.89 | 0.385 |
| Disease duration | 0.13 | 0.11 | 1.12 | 0.276 |
| Factor | Model 3 | Model 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| β | SE | t | p | β | SE | t | p | |
| Constant | 1.54 | 0.99 | 1.55 | 0.037 * | 1.56 | 0.37 | 4.24 | 0.001 * |
| ACE | 0.07 | 0.14 | 0.53 | 0.603 | 0.19 | 0.16 | 1.23 | 0.224 |
| AT2R1 | −0.10 | 0.17 | −0.58 | 0.570 | 0.10 | 0.17 | 0.59 | 0.561 |
| eNOS | 0.38 | 0.29 | 1.33 | 0.200 | 0.10 | 0.19 | 0.53 | 0.598 |
| IL1β | 0.05 | 0.23 | 0.22 | 0.828 | −0.00 | 0.23 | −0.02 | 0.983 |
| TNFα | 0.02 | 0.27 | 0.08 | 0.935 | −0.01 | 0.22 | −0.03 | 0.978 |
| IL10 | −0.03 | 0.28 | −0.10 | 0.920 | −0.02 | 0.19 | −0.12 | 0.903 |
| PON1 | −0.40 | 0.14 | −2.84 | 0.011 * | −0.48 | 0.15 | −3.14 | 0.003 * |
| Factor | Model 3 | |||
|---|---|---|---|---|
| β | SE | t | p | |
| Constant | 1.91 | 0.65 | 2.96 | 0.008 * |
| PON1 | −0.42 | 0.13 | −3.39 | 0.003 * |
| Sex | 0.37 | 0.36 | 1.02 | 0.321 |
| Age category | −0.05 | 0.20 | −0.24 | 0.815 |
| Somatic comorbidity | −0.07 | 0.11 | −0.65 | 0.521 |
| Disease duration | 0.34 | 0.28 | 1.21 | 0.241 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duve, K.; Shkrobot, S.; Petakh, P.; Oksenych, V.; Kamyshnyi, O. Clinical, Neuroimaging, and Genetic Markers Associated with Cognitive and Functional Outcomes After Traumatic Brain Injury. J. Clin. Med. 2025, 14, 2796. https://doi.org/10.3390/jcm14082796
Duve K, Shkrobot S, Petakh P, Oksenych V, Kamyshnyi O. Clinical, Neuroimaging, and Genetic Markers Associated with Cognitive and Functional Outcomes After Traumatic Brain Injury. Journal of Clinical Medicine. 2025; 14(8):2796. https://doi.org/10.3390/jcm14082796
Chicago/Turabian StyleDuve, Khrystyna, Svitlana Shkrobot, Pavlo Petakh, Valentyn Oksenych, and Oleksandr Kamyshnyi. 2025. "Clinical, Neuroimaging, and Genetic Markers Associated with Cognitive and Functional Outcomes After Traumatic Brain Injury" Journal of Clinical Medicine 14, no. 8: 2796. https://doi.org/10.3390/jcm14082796
APA StyleDuve, K., Shkrobot, S., Petakh, P., Oksenych, V., & Kamyshnyi, O. (2025). Clinical, Neuroimaging, and Genetic Markers Associated with Cognitive and Functional Outcomes After Traumatic Brain Injury. Journal of Clinical Medicine, 14(8), 2796. https://doi.org/10.3390/jcm14082796









