Morphometric Blastocyst Assessment: A Retrospective Study Examining the Relationship Between Blastocyst Diameter and Area and Pregnancy Outcomes in Assisted Reproduction Technology Cycles
Abstract
:1. Background
2. Materials and Methods
2.1. Controlled Ovarian Stimulation
2.2. Oocyte Collection, Fertilization and Embryo Assessment
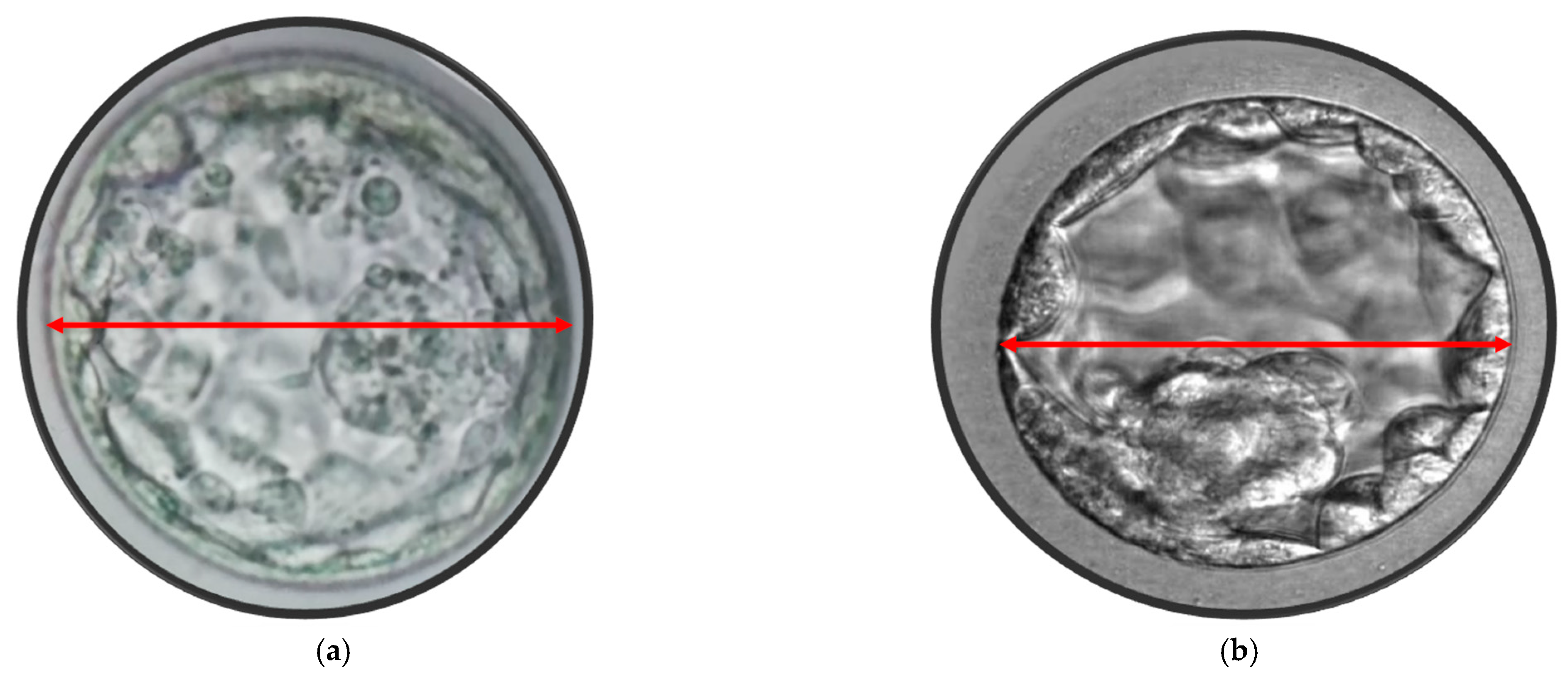
2.3. Blastocyst Morphometric Evaluation
2.4. Statistical Analysis
3. Results
4. Discussion
5. Possible Application of the Research and Novel Technologies
Time-Lapse Technology, AI and Automatic Embryo Selection in ART
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wyns, C.; De Geyter, C.; Calhaz-Jorge, C.; Kupka, M.S.; Motrenko, T.; Smeenk, J.; Bergh, C.; Tandler-Schneider, A.; Rugescu, I.A.; Goossens, V. European IVF Monitoring Consortium (EIM), for the European Society of Human Reproduction and Embryology (ESHRE); ART in Europe, 2018: Results generated from European registries by ESHRE. Hum. Reprod. Open 2022, 2022, hoac022. [Google Scholar] [CrossRef] [PubMed]
- Smeenk, J.; Wyns, C.; De Geyter, C.; Kupka, M.; Bergh, C.; Cuevas Saiz, I.; De Neubourg, D.; Rezabek, K.; Tandler-Schneider, A.; Rugescu, I.A.; et al. European IVF-monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE). ART in Europe, 2019: Results generated from European registries by ESHRE. Hum. Reprod. 2023, 38, 2321–2338. [Google Scholar] [CrossRef] [PubMed]
- Gullo, G.; Cucinella, G.; Stojanovic, V.; Stojkovic, M.; Bruno, C.; Streva, A.V.; Lopez, A.; Perino, A.; Marinelli, S. Ovarian Hyperstimulation Syndrome (OHSS): A Narrative Review and Legal Implications. J. Pers. Med. 2024, 14, 915. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.; Gusmano, M.K.; Patrizio, P. Preterm births, multiples, and fertility treatment: Recommendations for changes to policy and clinical practices. Fertil. Steril. 2014, 102, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Spangmose, A.L.; Ginström Ernstad, E.; Malchau, S.; Forman, J.; Tiitinen, A.; Gissler, M.; Opdahl, S.; Romundstad, L.B.; Bergh, C.; Wennerholm, U.B.; et al. Obstetric and perinatal risks in 4601 singletons and 884 twins conceived after fresh blastocyst transfers: A Nordic study from the CoNARTaS group. Hum. Reprod. 2020, 35, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Cavoretto, P.; Candiani, M.; Giorgione, V.; Inversetti, A.; Abu-Saba, M.M.; Tiberio, F.; Sigismondi, C.; Farina, A. Risk of spontaneous preterm birth in singleton pregnancies conceived after IVF/ICSI treatment: Meta-analysis of cohort studies. Ultrasound Obstet. Gynecol. 2018, 51, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Curchoe, C.L.; Bormann, C.; Hammond, E.; Salter, S.; Timlin, C.; Williams, L.B.; Gilboa, D.; Seidman, D.; Campbell, A.; Morbeck, D. Assuring quality in assisted reproduction laboratories: Assessing the performance of ART Compass—A digital art staff management platform. J. Assist. Reprod. Genet. 2023, 40, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Fordham, D.E.; Rosentraub, D.; Polsky, A.L.; Aviram, T.; Wolf, Y.; Perl, O.; Devir, A.; Rosentraub, S.; Silver, D.H.; Zamir, Y.G.; et al. Embryologist agreement when assessing blastocyst implantation probability: Is data-driven prediction the solution to embryo assessment subjectivity? Hum. Reprod. 2022, 37, 2275–2290. [Google Scholar] [CrossRef]
- Glujovsky, D.; Quinteiro Retamar, A.M.; Alvarez Sedo, C.R.; Ciapponi, A.; Cornelisse, S.; Blake, D. Cleavage-stage versus blastocyst-stage embryo transfer in assisted reproductive technology. Cochrane Database Syst. Rev. 2022, 5, CD002118. [Google Scholar] [CrossRef]
- Homayoon, N.; Arabian, S.; Mangoli, E.; Bayati, F.; Eftekhar, M. Effect of sequential cleavage and blastocyst embryo transfer compared to single cleavage stage embryo transfer on assisted reproductive technology outcome: An RCT. Int. J. Reprod. Biomed. 2024, 22, 433–440. [Google Scholar] [CrossRef]
- Kieslinger, D.C.; Vergouw, C.G.; Ramos, L.; Arends, B.; Curfs, M.H.J.M.; Slappendel, E.; Kostelijk, E.H.; Pieters, M.H.E.C.; Consten, D.; Verhoeven, M.O.; et al. Clinical outcomes of uninterrupted embryo culture with or without time-lapse-based embryo selection versus interrupted standard culture (SelecTIMO): A three-armed, multicentre, double-blind, randomised controlled trial. Lancet 2023, 401, 1438–1446. [Google Scholar] [CrossRef]
- Bhide, P.; Chan, D.Y.L.; Lanz, D.; Alqawasmeh, O.; Barry, E.; Baxter, D.; Carreras, F.G.; Choudhury, Y.; Cheong, Y.; Chung, J.P.W.; et al. Clinical effectiveness and safety of time-lapse imaging systems for embryo incubation and selection in in-vitro fertilisation treatment (TILT): A multicentre, three-parallel-group, double-blind, randomised controlled trial. Lancet 2024, 404, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Chimote, N.M.; Chimote, N.N.; Nath, N.M.; Mehta, B.N. Transfer of spontaneously hatching or hatched blastocyst yields better pregnancy rates than expanded blastocyst transfer. J. Hum. Reprod. Sci. 2013, 6, 183–188. [Google Scholar] [CrossRef]
- Van den Abbeel, E.; Balaban, B.; Ziebe, S.; Lundin, K.; Cuesta, M.J.G.; Klein, B.M.; Helmgaard, L.; Arce, J.C. Association between blastocyst morphology and outcome of single-blastocyst transfer. Reprod. Biomed. Online 2013, 27, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Subira, J.; Craig, J.; Turner, K.; Bevan, A.; Ohuma, E.; McVeigh, E.; Child, T.; Fatum, M. Grade of the inner cell mass, but not trophectoderm, predicts live birth in fresh blastocyst single transfers. Hum. Fertil. 2016, 19, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Zhao, B.; Hu, Z.; Shi, Q. The impact of blastocyst grade on singleton birth weight in fresh IVF-ET cycles in ART: A retrospective study. BMC Pregnancy Childbirth 2024, 24, 588. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.Y.; Wang, E.Y.; Huang, Y.; Guo, X.Y.; Xiong, Y.J.; Yu, Y.P.; Yao, G.D.; Shi, S.L.; Sun, Y.P. Blastocoele expansion degree predicts live birth after single blastocyst transfer for fresh and vitrified/warmed single blastocyst transfer cycles. Fertil. Steril. 2016, 105, 910–919.e1. [Google Scholar] [CrossRef] [PubMed]
- Sciorio, R.; Thong, D.; Thong, K.J.; Pickering, S.J. Clinical pregnancy is significantly associated with the blastocyst width and area: A time-lapse study. J. Assist. Reprod. Genet. 2021, 38, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Zegers-Hochschild, F.; Adamson, G.D.; Dyer, S.; Racowsky, C.; de Mouzon, J.; Sokol, R.; Rienzi, L.; Sunde, A.; Schmidt, L.; Cooke, I.D.; et al. The International Glossary on Infertility and Fertility Care, 2017. Fertil. Steril. 2017, 108, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Ebner, T.; Tritscher, K.; Mayer, R.B.; Oppelt, P.; Duba, H.C.; Maurer, M.; Schappacher-Tilp, G.; Petek, E.; Shebl, O. Quantitative and qualitative trophectoderm grading allows for prediction of live birth and gender. J. Assist. Reprod. Genet. 2016, 33, 49–57. [Google Scholar] [CrossRef]
- Sciorio, R.; Meseguer, M. Focus on time-lapse analysis: Blastocyst collapse and morphometric assessment as new features of embryo viability. Reprod. Biomed. Online 2021, 43, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, B.S.; Daneshmand, S.T.; Garner, F.C.; Aguirre, M.; Thomas, S. Large blastocyst diameter, early blastulation, and low preovulatory serum progesterone are dominant predictors of clinical pregnancy in fresh autologous cycles. Fertil. Steril. 2008, 290, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Sciorio, R.; Thong, J.K.; Pickering, S.J. Comparison of the development of human embryos cultured in either an EmbryoScope or benchtop incubator. J. Assist. Reprod. Genet. 2018, 35, 515–522. [Google Scholar] [CrossRef]
- Sciorio, R.; Rinaudo, P. Culture conditions in the IVF laboratory: State of the ART and possible new directions. J. Assist. Reprod. Genet. 2023, 40, 2591–2607. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yan, Y.; Huang, X.; Sun, L.; Li, Y. Blastocoele expansion: An important parameter for predicting clinical success pregnancy after frozen-warmed blastocysts transfer. Reprod. Biol. Endocrinol. 2019, 17, 15. [Google Scholar] [CrossRef]
- Bourne, H.; Edgar, D.H.; Baker, H.W.G. Sperm Preparation Techniques. In Textbook of Assisted Reproductive Techniques: Laboratory and Clinical Perspectives, 2nd ed.; Gardner, D.K., Weissman, A., Howles, C.M., Shoham, Z., Eds.; Informa Healthcare: New York, NY, USA, 2004; pp. 79–91. [Google Scholar]
- Cutting, R.; Morroll, D.; Roberts, S.A.; Pickering, S.J.; Rutherford, A.; on behalf of the BFS and ACE. Elective single embryo transfer: Guidelines for practice British Fertility Society and Association of Clinical Embryologists. Hum. Fertil. 2008, 11, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Sciorio, R.; Thong, K.J.; Pickering, S.J. Single blastocyst transfer (SET) and pregnancy outcome of day 5 and day 6 human blastocysts vitrified using a closed device. Cryobiology 2018, 84, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Sciorio, R.; Thong, K.J.; Pickering, S.J. Increased pregnancy outcome after day 5 versus day 6 transfers of human vitrified-warmed blastocysts. Zygote 2019, 27, 279–284. [Google Scholar] [CrossRef]
- Sciorio, R.; Tramontano, L.; Campos, G.; Greco, P.F.; Mondrone, G.; Surbone, A.; Greco, E.; Talevi, R.; Pluchino, N.; Fleming, S. Vitrification of human blastocysts for couples undergoing assisted reproduction: An updated review. Front. Cell Dev. Biol. 2024, 12, 1398049. [Google Scholar] [CrossRef] [PubMed]
- Gardner, D.K.; Schoolcraft, W.B. Culture and transfer of human blastocysts. Curr. Opin. Obstet. Gynecol. 1999, 11, 307–311. [Google Scholar] [CrossRef]
- Cruz, M.; Garrido, N.; Herrero, J.; Perez-Cano, I.; Munoz, M.; Meseguer, M. Timing of cell division in human cleavage-stage embryos is linked with blastocyst formation and quality. Reprod. Biomed. Online 2012, 25, 371–381. [Google Scholar] [CrossRef]
- Della Ragione, T.; Verheyen, G.; Papanikolaou, E.G.; Van Landuyt, L.; Devroey, P.; Van Steirteghem, A. Developmental stage on day-5 and fragmentation rate on day-3 can influence the implantation potential of top-quality blastocysts in IVF cycles with single embryo transfer. Reprod. Biol. Endocrinol. 2007, 5, 2. [Google Scholar] [CrossRef]
- Kieslinger, D.C.; De Gheselle, S.; Lambalk, C.B.; De Sutter, P.; Kostelijk, E.H.; Twisk, J.W.; van Rijswijk, J.; Van den Abbeel, E.; Vergouw, C.G. Embryo selection using time-lapse analysis (Early Embryo Viability Assessment) in conjunction with standard morphology: A prospective two-center pilot study. Hum. Reprod. 2016, 31, 2450–2457. [Google Scholar] [CrossRef] [PubMed]
- Coticchio, G.; Lagalla, C.; Sturmey, R.; Pennetta, F.; Borini, A. The enigmatic morula: Mechanisms of development, cell fate determination, self-correction and implications for ART. Hum. Reprod. Updat. 2019, 25, 422–438. [Google Scholar] [CrossRef]
- Lagalla, C.; Coticchio, G.; Sciajno, R.; Tarozzi, N.; Zacà, C.; Borini, A. Alternative patterns of partial embryo compaction: Prevalence, morphokinetic history and possible implications. Reprod. Biomed. Online 2020, 40, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Lagalla, C.; Barberi, M.; Orlando, G.; Sciajno, R.; Bonu, M.A.; Borini, A. A quantitative approach to blastocyst quality evaluation: Morphometric analysis and related IVF outcomes. J. Assist. Reprod. Genet. 2015, 32, 705–712. [Google Scholar] [CrossRef]
- Park, J.K.; Jeon, Y.; Bang, S.; Kim, J.W.; Kwak, I.P.; Lee, W.S. Time-lapse imaging of morula compaction for selecting high-quality blastocysts: A retrospective cohort study. Arch. Gynecol. Obstet. 2024, 309, 2897–2906. [Google Scholar] [CrossRef] [PubMed]
- Marcos, J.; Perez-Albala, S.; Mifsud, A.; Molla, M.; Landeras, J.; Meseguer, M. Collapse of blastocysts is strongly related to lower implantation success: A time-lapse study. Hum. Reprod. 2015, 30, 2501–2508. [Google Scholar] [CrossRef]
- Sciorio, R.; Herrer Saura, R.; Thong, K.J.; Esbert Algam, M.; Pickering, S.J.; Meseguer, M. Blastocyst collapse as an embryo marker of low implantation potential: A time-lapse multicentre study. Zygote 2020, 28, 139–147. [Google Scholar] [CrossRef]
- Sciorio, R.; Thong, K.J.; Pickering, S.J. Spontaneous blastocyst collapse as an embryo marker of low pregnancy outcome: A Time-lapse study. JBRA Assist. Reprod. 2020, 24, 34–40. [Google Scholar] [CrossRef]
- Meseguer, M.; Herrero, J.; Tejera, A.; Hilligsoe, K.M.; Ramsing, N.B.; Remohi, J. The use of morphokinetics as a predictor of embryo implantation. Hum. Reprod. 2011, 26, 2658–2671. [Google Scholar] [CrossRef]
- Utsuno, H.; Ishimaru, T.; Matsumoto, M.; Sasamori, C.; Takahashi, H.; Kimura, H.; Kamijo, S.; Yamada, M.; Tanaka, M.; Hamatani, T. Morphometric assessment of blastocysts: Relationship with the ongoing pregnancy rate. F S Rep. 2022, 4, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Tobias, T.; Sharara, F.I.; Franasiak, J.M.; Heiser, P.W.; Pinckney-Clark, E. Promoting the use of elective single embryo transfer in clinical practice. Fertil. Res. Pract. 2016, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- Reimundo, P.; Gutiérrez Romero, J.M.; Rodríguez Pérez, T.; Veiga, E. Single-embryo transfer: A key strategy to reduce the risk for multiple pregnancy in assisted human reproduction. Adv. Lab. Med. 2021, 2, 179–198. [Google Scholar] [CrossRef]
- Kresowik, J.D.K.; Sparks, A.E.T.; Van Voorhis, B.J. Clinical factors associated with live birth after single embryo transfer. Fertil. Steril. 2012, 98, 1152–1156. [Google Scholar] [CrossRef] [PubMed]
- Sunderam, S.; Boulet, S.L.; Jamieson, D.J.; Kissin, D.M. Effects of patient education on desire for twins and use of elective single embryo transfer procedures during ART treatment: A systematic review. Reprod. Biomed. Soc. Online 2018, 6, 102–119. [Google Scholar] [CrossRef] [PubMed]
- Fouks, Y.; Yogev, Y. Twinning in ART: Single embryo transfer policy. Best Pract. Res. Clin. Obstet. Gynaecol. 2022, 84, 88–95. [Google Scholar] [CrossRef]
- Thompson, S.M.; Onwubalili, N.; Brown, K.; Jindal, S.K.; McGovern, P.G. Blastocyst expansion score and trophectoderm morphology strongly predict successful clinical pregnancy and live birth following elective single embryo blastocyst transfer (eSET): A national study. J. Assist. Reprod. Genet. 2013, 30, 1577–1581. [Google Scholar] [CrossRef]
- Shebl, O.; Haslinger, C.; Kresic, S.; Enengl, S.; Reiter, E.; Oppelt, P.; Ebner, T. The hare and the tortoise: Extreme mitotic rates and how these affect live birth. Reprod. Biomed. Online 2021, 42, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Wirleitner, B.; Schuff, M.; Stecher, A.; Murtinger, M.; Vanderzwalmen, P. Pregnancy and birth outcomes following fresh or vitrified embryo transfer according to blastocyst morphology and expansion stage, and culturing strategy for delayed development. Hum. Reprod. 2016, 31, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Ueno, S.; Yabuuchi, A.; Uchiyama, K.; Okuno, T.; Kobayashi, T.; Segawa, T.; Teramoto, S. Women’s age and embryo developmental speed accurately predict clinical pregnancy after single vitrified-warmed blastocyst transfer. Reprod. Biomed. Online 2014, 29, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, J.; Wu, X.; Cao, S.; Zhou, L.; Wang, Y.; Chen, X.; Lu, J.; Zhao, C.; Chen, M.; et al. Trophectoderm morphology predicts outcomes of pregnancy in vitrified-warmed single blastocyst transfer cycle in a Chinese population. J. Assist. Reprod. Genet. 2014, 31, 1475–1481. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.T.F.; Huang, D.H.; Ahn, H.J.; Arnett, C.; Huang, C.T.F. Early blastocyst expansion in euploid and aneuploid human embryos: Evidence for a non-invasive and quantitative marker for embryo selection. Reprod. Biomed. Online 2019, 39, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.; Fishel, S.; Bowman, N.; Duffy, S.; Sedler, M.; Hickman, C.F. Modelling a risk classification of aneuploidy in human embryos using non-invasive morphokinetics. Reprod. Biomed. Online 2013, 26, 477–485. [Google Scholar] [CrossRef]
- Basile, N.; Nogales del Carmen, M.; Bronet, F.; Florensa, M.; Riqueiros, M.; Rodrigo, L.; Velasco-Garcia, J.; Meseguer, M. Increasing the probability of selecting chromosomally normal embryos by time-lapse morphokinetics analysis. Fertil. Steril. 2014, 101, 699–704. [Google Scholar] [CrossRef]
- Bulletti, F.M.; Sciorio, R.; Conforti, A.; De Luca, R.; Bulletti, C.; Palagiano, A.; Berrettini, M.; Scaravelli, G.; Pierson, R.A. Causes of embryo implantation failure: A systematic review and metaanalysis of procedures to increase embryo implantation potential. Front. Endocrinol. 2025, 15, 1429193. [Google Scholar] [CrossRef]
- Irani, M.; Zaninovic, N.; Rosenwaks, Z.; Xu, K. Does maternal age at retrieval influence the implantation potential of euploid blastocysts? Am. J. Obstet. Gynecol. 2019, 220, 379.e1–379.e7. [Google Scholar] [CrossRef] [PubMed]
- Irani, M.; Reichman, D.; Robles, A.; Melnick, A.; Davis, O.; Zaninovic, N.; Xu, K.; Rosenwaks, Z. Morphologic grading of euploid blastocysts influences implantation and ongoing pregnancy rates. Fertil. Steril. 2017, 107, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Irani, M.; O’Neill, C.; Palermo, G.D.; Xu, K.; Zhang, C.; Qin, X.; Zhan, Q.; Clarke, R.N.; Ye, Z.; Zaninovic, N.; et al. Blastocyst development rate influences implantation and live birth rates of similarly graded euploid blastocysts. Fertil. Steril. 2018, 110, 95–102.e1. [Google Scholar] [CrossRef] [PubMed]
- Ahlstrom, A.; Westin, C.; Reismer, E.; Wikland, M.; Hardarson, T. Trophectoderm morphology: An important parameter for predicting live birth after single blastocyst transfer. Hum. Reprod. 2011, 26, 3289–3296. [Google Scholar] [CrossRef]
- Cimadomo, D.; Capalbo, A.; Levi-Setti, P.E.; Soscia, D.; Orlando, G.; Albani, E.; Parini, V.; Stoppa, M.; Dovere, L.; Tacconi, L.; et al. Associations of blastocyst features, trophectoderm biopsy and other laboratory practice with post-warming behavior and implantation. Hum. Reprod. 2018, 33, 1992–2001. [Google Scholar] [CrossRef] [PubMed]
- Brogliato, C.; Romanini, J.; Berton, C.Z.; Suganuma, C.H.; Vellez, L.T.; Yoshida, I.H.; Barbosa, C.P. Expansion and herniation: Evaluation of the best pregnancy rate predictor after quarter laser assisted hatching in frozen blastocyst transfers. JBRA Assist. Reprod. 2020, 24, 170–172. [Google Scholar] [CrossRef]
- Ezoe, K.; Miki, T.; Akaike, H.; Shimazaki, K.; Takahashi, T.; Tanimura, Y.; Amagai, A.; Sawado, A.; Mogi, M.; Kaneko, S.; et al. Maternal age affects pronuclear and chromatin dynamics, morula compaction and cell polarity, and blastulation of human embryos. Hum. Reprod. 2023, 38, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Coticchio, G.; Ezoe, K.; Lagalla, C.; Shimazaki, K.; Ohata, K.; Ninomiya, M.; Wakabayashi, N.; Okimura, T.; Uchiyama, K.; Kato, K.; et al. Perturbations of morphogenesis at the compaction stage affect blastocyst implantation and live birth rates. Hum. Reprod. 2021, 36, 918–928. [Google Scholar] [CrossRef] [PubMed]
- Coticchio, G.; Mignini Renzini, M.; Novara, P.V.; Lain, M.; De Ponti, E.; Turchi, D.; Fadini, R.; Dal Canto, M. Focused time-lapse analysis reveals novel aspects of human fertilization and suggests new parameters of embryo viability. Hum. Reprod. 2018, 33, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Goto, S.; Kadowaki, T.; Tanaka, S.; Hashimoto, H.; Kokeguchi, S.; Shiotani, M. Prediction of pregnancy rate by blastocyst morphological score and age, based on 1488 single frozen-thawed blastocyst transfer cycles. Fertil. Steril. 2011, 95, 948–952. [Google Scholar] [CrossRef]
- Harper, J.; Magli, M.C.; Lundin, K.; Barratt, C.L.R.; Brison, D. When and how should new technology be introduced into the IVF laboratory? Hum. Reprod. 2011, 27, 303–313. [Google Scholar] [CrossRef]
- Wheeler, M.B.; Walters, E.M.; Beebe, D.J. Toward culture of single gametes: The development of microfluidic platforms for assisted reproduction. Theriogenology 2007, 68 (Suppl. S1), S178–S189. [Google Scholar] [CrossRef]
- Gu, W.; Zhu, X.; Futai, N.; Cho, B.; Takayama, S. Computerized microfluidic cell culture using elastomeric channels and Braille displays. Proc. Natl. Acad. Sci. USA 2004, 101, 15861–15866. [Google Scholar] [CrossRef]
- Volpatti, L.R.; Yetisen, A.K. Commercialization of microfluidic devices. Trends Biotechnol. 2014, 32, 347–350. [Google Scholar] [CrossRef]
- Clark, S.G.; Haubert, K.; Beebe, D.J.; Ferguson, C.E.; Wheeler, M.B. Reduction of polyspermic penetration using biomimetic microfluidic technology during in vitro fertilization. Lab. Chip 2005, 5, 1229–1232. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.K.; Gaudriault, P.; Corbera, A. Lab-on-chip (LoC) application for quality sperm selection: An undelivered promise? Open Res. Eur. 2023, 3, 188. [Google Scholar] [CrossRef] [PubMed]
- Mancini, V.; McKeegan, P.J.; Schrimpe-Rutledge, A.C.; Codreanu, S.G.; Sherrod, S.D.; McLean, J.A.; Picton, H.M.; Pensabene, V. Probing morphological, genetic and metabolomic changes of in vitro embryo development in a microfluidic device. Biotechnol. Prog. 2021, 37, e3194. [Google Scholar] [CrossRef] [PubMed]
- Kushnir, V.A.; Smith, G.D.; Adashi, E.Y. The Future of IVF: The New Normal in Human Reproduction. Reprod. Sci. 2022, 29, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Yanez, L.Z.; Camarillo, D.B. Microfluidic analysis of oocyte and embryo biomechanical properties to improve outcomes in assisted reproductive technologies. Mol. Hum. Reprod. 2017, 23, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Bormann, C.L.; Curchoe, C.L.; Thirumalaraju, P.; Kanakasabapathy, M.K.; Gupta, R.; Pooniwala, R.; Kandula, H.; Souter, I.; Dimitriadis, I.; Shafiee, H. Deep learning early warning system for embryo culture conditions and embryologist performance in the ART laboratory. J. Assist. Reprod. Genet. 2021, 38, 1641–1646. [Google Scholar] [CrossRef]
- Zhan, Q.; Sierra, E.T.; Malmsten, J.; Ye, Z.; Rosenwaks, Z.; Zaninovic, N. The blastocyst score, blastocyst quality ranking tool, is a predictor of blastocyst ploidy and implantation potential. Fertil. Steril. Rep. 2020, 1, 133–141. [Google Scholar] [CrossRef]
- Dirvanauskas, D.; Maskeliunas, R.; Raudonis, V.; Damasevicius, R. Embryo development stage prediction algorithm for automated time lapse incubators. Comput. Methods Programs Biomed. 2019, 177, 161–174. [Google Scholar] [CrossRef]
- Raudonis, V.; Paulauskaite-Taraseviciene, A.; Sutiene, K.; Jonaitis, D. Towards the automation of early-stage human embryo development detection. Biomed. Eng. Online 2019, 18, 120. [Google Scholar] [CrossRef]
- Feyeux, M.; Reignier, A.; Mocaer, M.; Lammers, J.; Meistermann, D.; Barriere, P.; Paul-Gilloteaux, P.; David, L.; Fréour, T. Development of automated annotation software for human embryo morphokinetics. Hum. Reprod. 2020, 35, 557–564. [Google Scholar] [CrossRef]
- Zaninovic, N.; Rosenwaks, Z. Artificial intelligence in human in vitro fertilization and embryology. Fertil. Steril. 2020, 114, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.; Cooke, S.; Illingworth, P.J.; Gardner, D.K. Deep learning as a predictive tool for fetal heart pregnancy following time-lapse incubation and blastocyst transfer. Hum. Reprod. 2019, 34, 1011–1018. [Google Scholar] [CrossRef]
- Chavez-Badiola, A.; Flores-Saiffe, F.A.; Mendizabal-Ruiz, G.; Garcia-Sanchez, R.; Drakeley, A.J.; Garcia-Sandoval, J.P. Predicting pregnancy test results after embryo transfer by image feature extraction and analysis using machine learning. Sci. Rep. 2020, 10, 4394. [Google Scholar] [CrossRef] [PubMed]
- VerMilyea, M.; Hall, J.M.M.; Diakiw, S.M.; Johnston, A.; Nguyen, T.; Perugini, D.; Miller, A.; Picou, A.; Murphy, A.P.; Perugini, M. Development of an artificial intelligence-based assessment model for prediction of embryo viability using static images captured by optical light microscopy during IVF. Hum. Reprod. 2020, 35, 770–784. [Google Scholar] [CrossRef]
- Matos, F.D.; Rocha, J.C.; Nogueira, M.F. A method using artificial neural networks to morphologically assess mouse blastocyst quality. J. Anim. Sci. Technol. 2014, 56, 15. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.C.; Passalia, F.J.; Matos, F.D.; Takahashi, M.B.; Ciniciato, D.S.; Maserati, M.P.; Alves, M.F.; de Almeida, T.G.; Cardoso, B.L.; Basso, A.C.; et al. A method based on artificial intelligence to fully automatize the evaluation of bovine blastocyst images. Sci. Rep. 2017, 7, 7659. [Google Scholar] [CrossRef]
- Saeedi, P.; Yee, D.; Au, J.; Havelock, J. Automatic identification of human blastocyst components via texture. IEEE Trans. Biomed. Eng. 2017, 64, 2968–2978. [Google Scholar]
- Kragh, M.F.; Rimestad, J.; Berntsen, J.; Karstoft, H. Automatic grading of human blastocysts from time-lapse imaging. Comput. Biol. Med. 2019, 115, 103494. [Google Scholar] [CrossRef]
- Chen, T.J.; Zheng, W.L.; Liu, C.H.; Huang, I.; Lai, H.H.; Liu, M. Using deep learning with large dataset of microscope images to develop an automated embryo grading system. Fertil. Reprod. 2019, 1, 51–56. [Google Scholar] [CrossRef]
- Canat, G.; Duval, A.; Gidel-Dissler, N.; Boussommier-Calleja, A. A novel deep learning approach to identify embryo morphokinetics in multiple time lapse systems. Sci. Rep. 2024, 14, 29016. [Google Scholar] [CrossRef]
- Ahlström, A.; Berntsen, J.; Johansen, M.; Bergh, C.; Cimadomo, D.; Hardarson, T.; Lundin, K. Correlations between a deep learning-based algorithm for embryo evaluation with cleavage-stage cell numbers and fragmentation. Reprod. Biomed. Online 2023, 47, 103408. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wei, Q.; Huang, W.; Yin, L.; Ma, T. Can time-lapse culture combined with artificial intelligence improve ongoing pregnancy rates in fresh transfer cycles of single cleavage stage embryos? Front. Endocrinol. 2024, 15, 1449035. [Google Scholar] [CrossRef] [PubMed]
- Jiang, V.S.; Bormann, C.L. Artificial intelligence in the in vitro fertilization laboratory: A review of advancements over the last decade. Fertil. Steril. 2023, 120, 17–23. [Google Scholar] [CrossRef]
- Illingworth, P.J.; Venetis, C.; Gardner, D.K.; Nelson, S.M.; Berntsen, J.; Larman, M.G.; Agresta, F.; Ahitan, S.; Ahlström, A.; Cattrall, F.; et al. Deep learning versus manual morphology-based embryo selection in IVF: A randomized, double-blind noninferiority trial. Nat. Med. 2024, 30, 3114–3120. [Google Scholar] [CrossRef]
- Paternot, G.; Debrock, S.; De Neubourg, D.; D’Hooghe, T.M.; Spiessens, C. Semi-automated morphometric analysis of human embryos can reveal correlations between total embryo volume and clinical pregnancy. Hum. Reprod. 2013, 28, 627–633. [Google Scholar] [CrossRef] [PubMed]

| Group (Diameter ≥ 170 µm) | Group (Diameter < 170 µm) | p-Value | |
|---|---|---|---|
| Maternal age (years) | 33.5 ± 3.48 | 33.7 ± 4.09 | NS |
| Oocyte collected (n) | 11.1 ± 1.48 | 12.1 ± 1.88 | NS |
| Transferred blastocysts (n) | 1 | 1 | NS |
| Basal FSH (mIU/mL) | 7.15 ± 1.51 | 7.64 ± 1.79 | NS |
| Maternal BMI | 28.67 ± 2.16 | 29.17 ± 2.14 | NS |
| Infertility duration (years) | 3.17 ± 1.47 | 3.67 ± 1.75 | NS |
| Endometrial thickness (mm) | 8.83 ± 1.47 | 9.10 ± 1.10 | NS |
| Blastocyst (Area ≥ 25,000 µm2) | Blastocyst (Area < 25,000 µm2) | p-Value | |
|---|---|---|---|
| Maternal age (years) | 33.5 ± 3.48 | 33.6 ± 4.09 | NS |
| Oocyte collected (n) | 12.2 ± 1.34 | 11.0 ± 1.56 | NS |
| Transferred blastocysts (n) | 1 | 1 | NS |
| Basal FSH (mIU/mL) | 8.66 ± 1.51 | 8.04 ± 1.79 | NS |
| Maternal BMI | 29.67 ± 2.16 | 28.45 ± 2.14 | NS |
| Infertility duration (years) | 3.87 ± 1.47 | 3.24 ± 1.75 | NS |
| Endometrial thickness (mm) | 9.20 ± 1.47 | 8.94 ± 1.10 | NS |
| Blastocyst Diameter ≥170 µm | Blastocyst Diameter <170 µm | p-Value | |
|---|---|---|---|
| Beta hCG Positive | 78.8% (339/430) | 48.0% (113/235) | <0.001 |
| Implantation Rate | 68.8% (296/430) | 36.6% (86/235) | <0.001 |
| Clinical Pregnancy Rate (with fetal heartbeat) | 66.3% (285/430) | 35.7% (84/235) | <0.001 |
| Spontaneous abortion | 12.6% (54/430) | 12.3% (29/235) | NS |
| Blastocyst Area ≥25,000 µm2 | Blastocyst Area <25,000 µm2 | p-Value | |
|---|---|---|---|
| Beta hCG Positive | 76.6% (249/325) | 60.3% (205/340) | <0.001 |
| Implantation Rate | 69.8% (227/325) | 47.9% (163/340) | <0.001 |
| Clinical Pregnancy Rate (with fetal heartbeat) | 65.5% (213/325) | 45.9% (156/340) | <0.001 |
| Spontaneous abortion | 11.1% (36/325) | 14.4% (49/340) | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sciorio, R.; Greco, P.F.; Tramontano, L.; Gullo, G.; Greco, E. Morphometric Blastocyst Assessment: A Retrospective Study Examining the Relationship Between Blastocyst Diameter and Area and Pregnancy Outcomes in Assisted Reproduction Technology Cycles. J. Clin. Med. 2025, 14, 2827. https://doi.org/10.3390/jcm14082827
Sciorio R, Greco PF, Tramontano L, Gullo G, Greco E. Morphometric Blastocyst Assessment: A Retrospective Study Examining the Relationship Between Blastocyst Diameter and Area and Pregnancy Outcomes in Assisted Reproduction Technology Cycles. Journal of Clinical Medicine. 2025; 14(8):2827. https://doi.org/10.3390/jcm14082827
Chicago/Turabian StyleSciorio, Romualdo, Pier Francesco Greco, Luca Tramontano, Giuseppe Gullo, and Ermanno Greco. 2025. "Morphometric Blastocyst Assessment: A Retrospective Study Examining the Relationship Between Blastocyst Diameter and Area and Pregnancy Outcomes in Assisted Reproduction Technology Cycles" Journal of Clinical Medicine 14, no. 8: 2827. https://doi.org/10.3390/jcm14082827
APA StyleSciorio, R., Greco, P. F., Tramontano, L., Gullo, G., & Greco, E. (2025). Morphometric Blastocyst Assessment: A Retrospective Study Examining the Relationship Between Blastocyst Diameter and Area and Pregnancy Outcomes in Assisted Reproduction Technology Cycles. Journal of Clinical Medicine, 14(8), 2827. https://doi.org/10.3390/jcm14082827








