Cardiovascular–Kidney–Metabolic Syndrome: A New Paradigm in Clinical Medicine or Going Back to Basics?
Abstract
1. Introduction
2. Epidemiological Evidence of CKM Disease Connections
3. Definition and Prevalence of Cardiovascular–Kidney–Metabolic Syndrome
4. Risk Factors for Cardiovascular–Kidney–Metabolic Syndrome
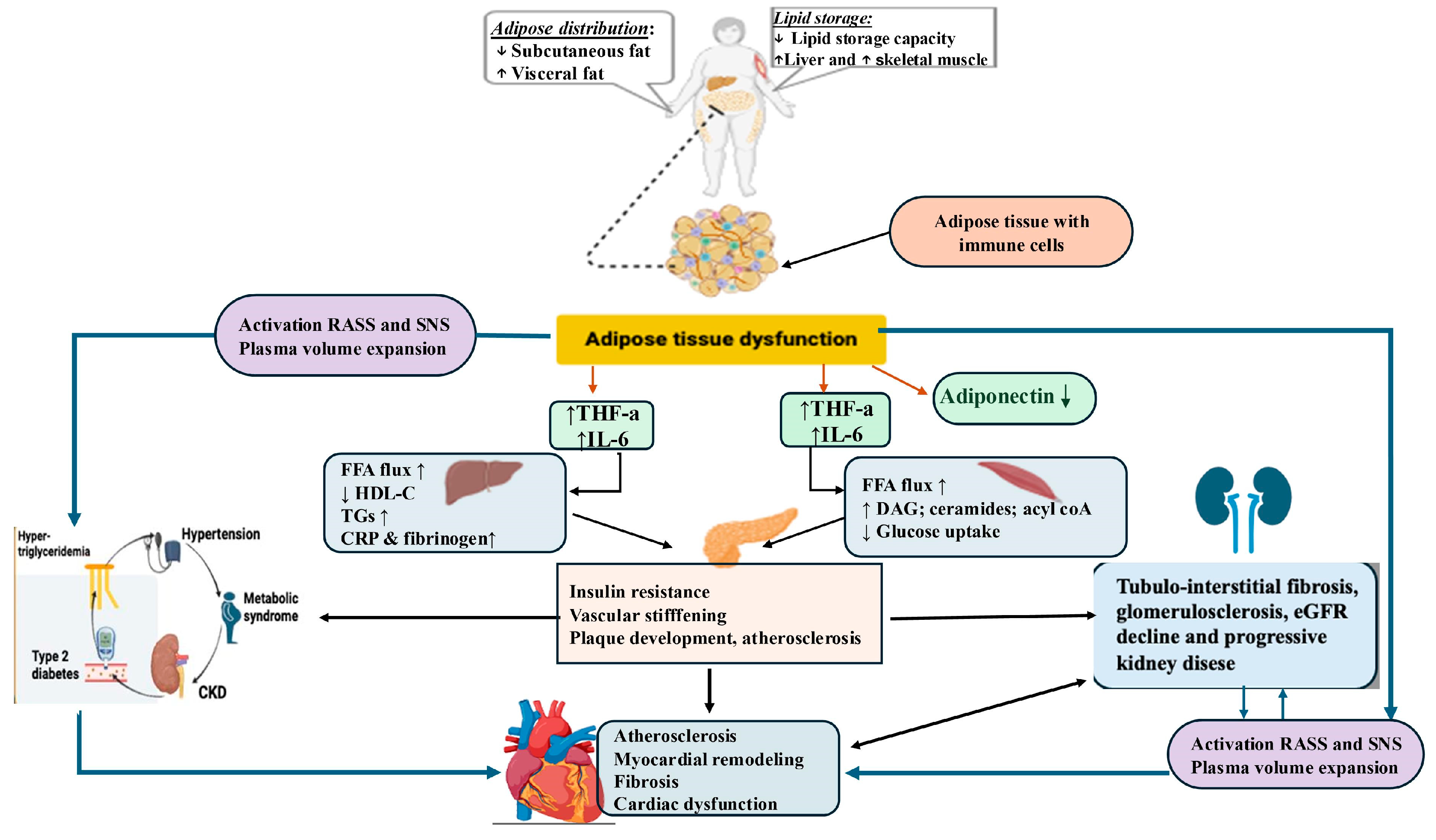
5. Pathophysiological Mechanisms That Underline the Cardiovascular–Kidney–Metabolic Connection
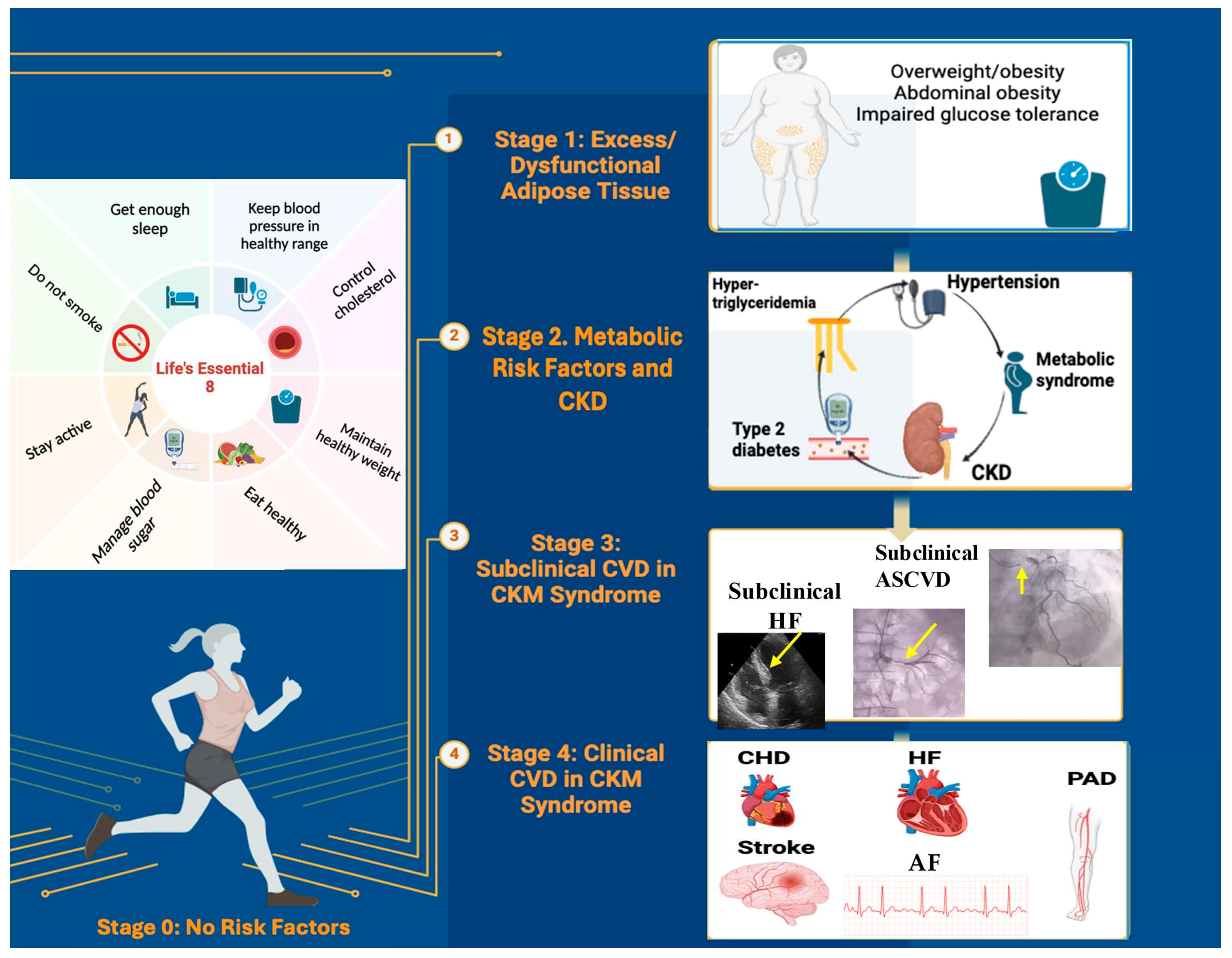
6. Staging of Cardiovascular–Kidney–Metabolic Syndrome
6.1. CKM Syndrome Stage 0: No CKM Risk Factors
6.2. CKM Syndrome Stage 1: Excess and/or Dysfunctional Adiposity
- Stage 1A:
- Excess adiposity manifested by overweight (BMI ≥ 25 kg/m2) or large normal WC (≥80/94 cm for women/men), or obesity (BMI ≥ 30 kg/m2) or abdominal obesity (WC ≥ 88/102 cm for women/men) according to the Centers for Disease Control and Prevention [50]. These intervals are used to assess the likelihood of developing obesity-related diseases [51,52]. However, there is evidence that the BMI predicts MetS differently in racial/ethnic groups. Asians present a higher risk of CVDs and T2D at a lower BMI compared with European populations. In this regard, the WHO has recommended lower anthropometric cutoff points for the Asian population (BMI ≥ 23–27.4 kg/m2 for overweight and ≥27.5 kg/m2 and above for obese, and WC ≥ 80/90 cm for women/men) [52].
- Stage 1B:
- Dysfunctional adiposity manifested by the deposition of visceral and ectopic fat in organs such as the liver, heart, skeletal muscles, pancreas, and kidneys as a marker of higher cardiovascular risk independent of the BMI [53]. This is the consequence of abdominal obesity, which directly contributes to systemic inflammation and oxidative stress, impaired glucose tolerance, and prediabetes (Supplementary Table S1) [54,55]. A new evidence-based definition and diagnostic criteria for obesity were published in 2025. Preclinical obesity is defined as excess fat without evident organ dysfunction or functional limitations, but with a higher risk of progression towards clinical obesity and associated diseases. Clinical obesity is defined as a chronic systemic disease characterized by progressive organ damage and limitations in daily activities caused by excessive fat accumulation [13]. The visceral fat index has become a potentially useful parameter in evaluating the degree of visceral, hormonally active adiposity. The calculation of the visceral fat index is based on BMI, WC, triglycerides, and HDL cholesterol (HDL-C). It characterizes individuals with normal body weight but presents with a cluster of cardiovascular risk factors, including insulin resistance, impaired glucose tolerance, atherogenic lipid profiles, and hypertension, and is independently associated with an increased risk of HF and diastolic dysfunction [29].
6.3. CKM Syndrome Stage 2: Metabolic Risk Factors and CKD
6.4. CKM Syndrome Stage 3: Subclinical CVD
6.5. CKM Syndrome Stage 4: Clinical CVD
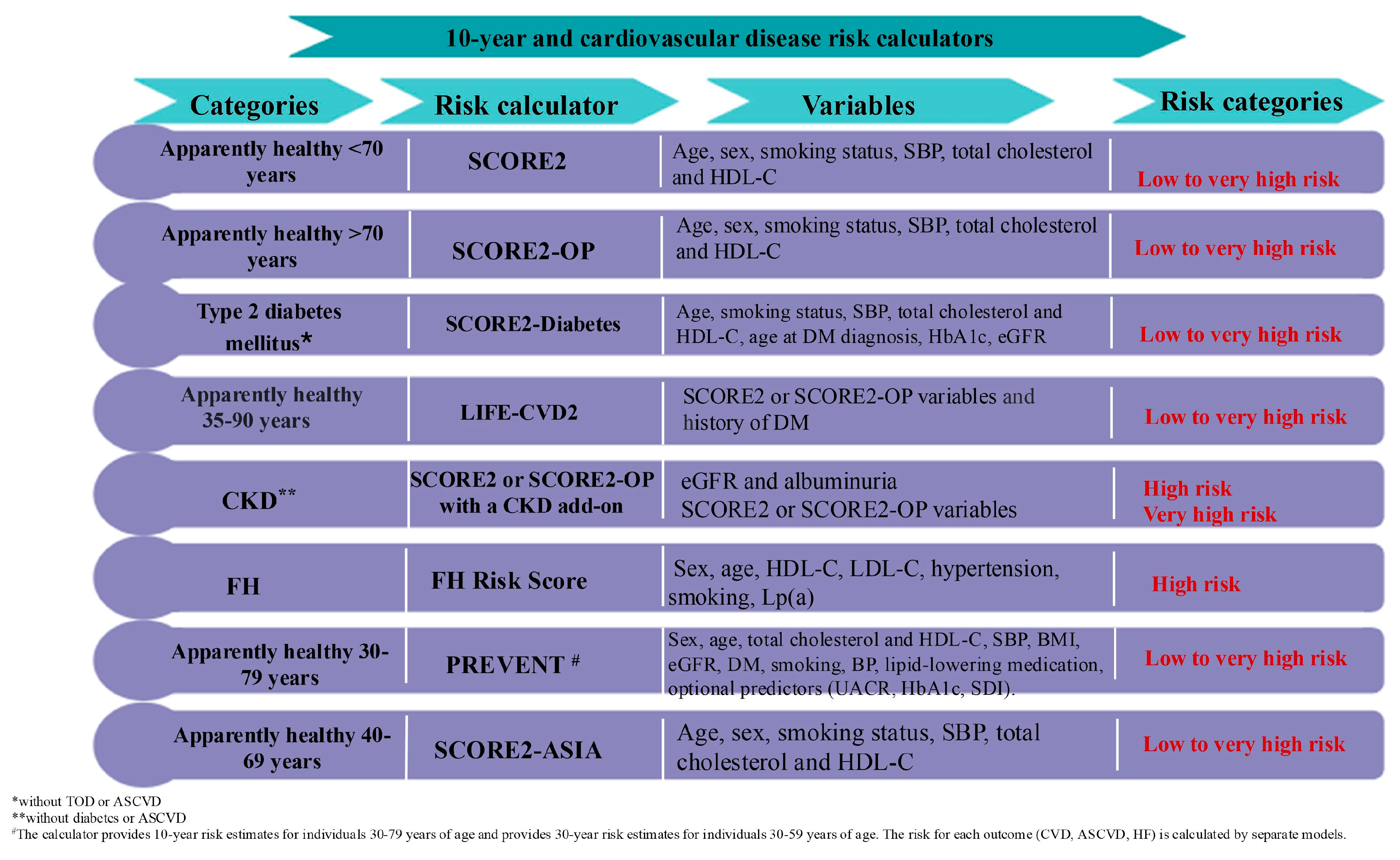
7. Screening for Cardiovascular–Kidney–Metabolic Syndrome
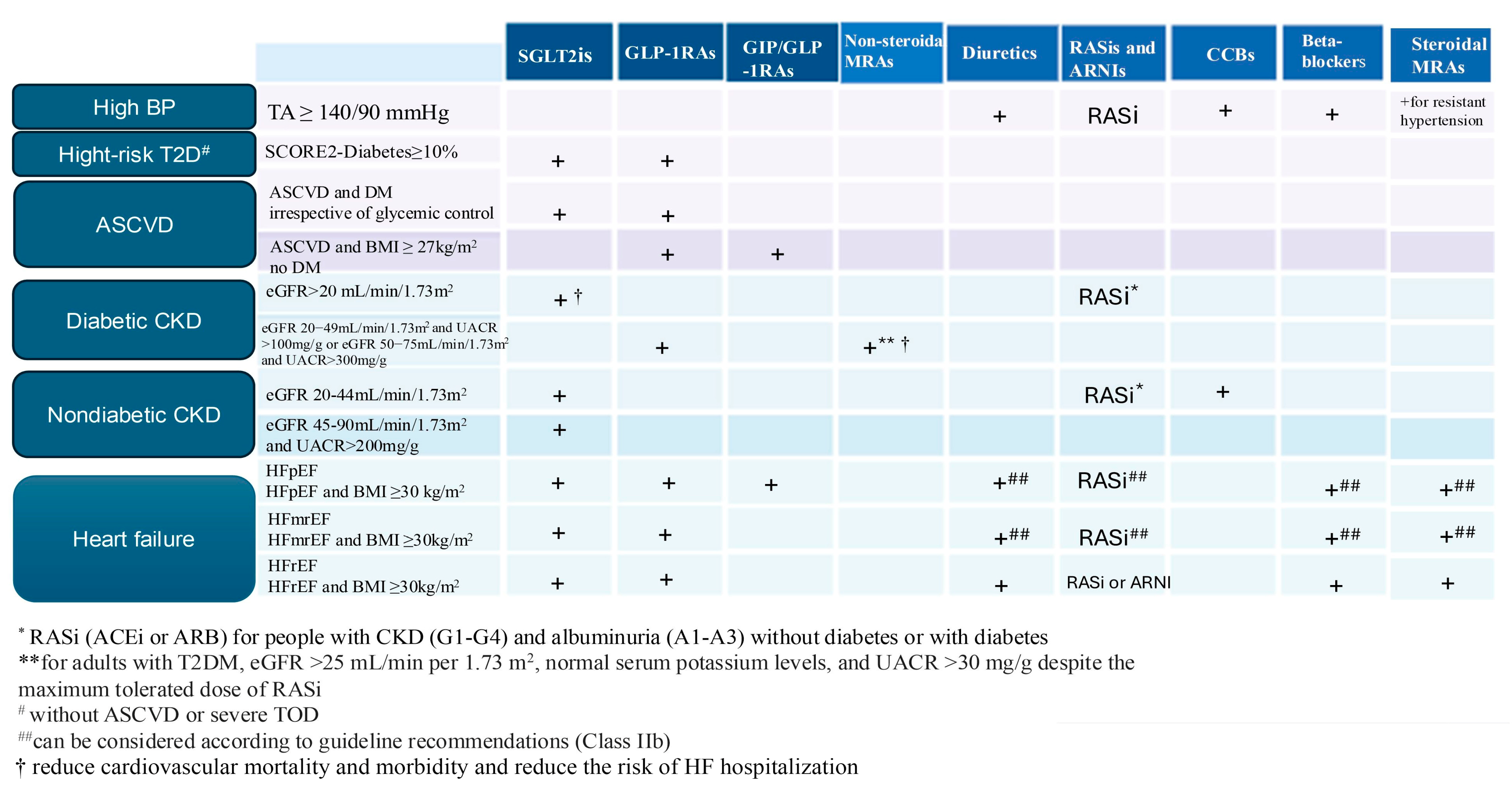
8. Management of Cardiovascular–Kidney–Metabolic Syndrome
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AHA | American Heart Association |
| ACC | American College of Cardiology |
| CKM | Cardiovascular–Kidney–Metabolic |
| CVDs | Cardiovascular Diseases |
| CKD | Chronic Kidney Disease |
| ESKD | End-Stage Kidney Disease |
| MetS | Metabolic Syndrome |
| HF | Heart Failure |
| LVEF | Left Ventricular Ejection Fraction |
| HFpEF | Heart Failure with Preserved Ejection Fraction |
| HFmrEF | Heart Failure with Mildly Reduced Ejection Fraction |
| HFrEF | Heart Failure with Reduced Ejection Fraction |
| WHO | World Health Organization |
| DM | Diabetes Mellitus |
| T1D | Type 1 Diabetes |
| T2D | Type 2 Diabetes |
| CAD | Coronary Artery Disease |
| DKD | Diabetic Kidney Disease |
| eGFR | Glomerular Filtration Rate |
| BMI | Body Mass Index |
| SDOH | Social Determinants of Health |
| FFAs | Free Fatty Acids |
| HDL | High-Density Lipoprotein |
| SNS | Sympathetic Nervous System |
| AGEs | Advanced Glycation End Products |
| KDIGO | Kidney Disease Improving Global Outcomes |
| ASCVD | Atherosclerotic Cardiovascular Disease |
| ADA | American Diabetes Association |
| UACR | Urinary Albumin-to-Creatinine Ratio |
| ESC | European Society of Cardiology |
| Lp(a) | Lipoprotein(a) |
| CAC | Coronary Artery Calcium |
| GLP-1RAs | Glucagon-Like Peptide 1 Receptor Agonists |
| GIP/GLP-1RA | Glucose-Dependent Insulinotropic Polypeptide/Glucagon-Like Peptide-1 Receptor Agonist |
| SGLT2is | Sodium-Glucose Cotransporter-2 Inhibitors |
| MACEs | Major Cardiovascular Events |
| ACEi | Angiotensin-Converting Enzyme Inhibitor |
| ARB | Angiotensin II Receptor Blocker |
| MRAs | Mineralocorticoid Receptor Antagonists |
| SCORE2 | Systematic Coronary Risk Evaluation 2 |
| RASS | Renin–Angiotensin–Aldosterone System |
| RAASi | Renin–Angiotensin–Aldosterone System Inhibitor |
References
- Nikolaou, M.; Theodorakis, N.; Feretzakis, G.; Vamvakou, G.; Hitas, C.; Kalantzi, S.; Spyridaki, A.; Apostolos, A.; Verykios, V.S.; Toutouzas, K. Nationwide Mortality Trends from 2001 to 2020 in Greece: Health Policy Implications under the Scope of Aging Societies. Hell. J. Cardiol. 2024, 28. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Ostrominski, J.; Arnold, S.; Butler, J.; Fonarow, G.C.; Hirsch, J.S.; Palli, S.R.; Donato, B.M.K.; Parrinello, C.M.; O’Connell, T.; Collins, E.B.; et al. Prevalence and overlap of cardiac, renal, and metabolic conditions in US adults, 1999–2020. JAMA Cardiol. 2023, 8, 1050–1060. [Google Scholar] [CrossRef] [PubMed]
- Rangaswami, J.; Bhalla, V.; Blair, J.; Chang, T.; Costa, S.; Lentine, K.L.; Lerma, E.V.; Mezue, K.; Molitch, M.; Mullens, W.; et al. Cardiorenal syndrome: Classification, pathophysiology, diagnosis, and treatment strategies: A scientific statement from the American Heart Association. Circulation 2019, 139, e840–e878. [Google Scholar] [CrossRef]
- Alemany, M. The Metabolic Syndrome, a Human Disease. Int. J. Mol. Sci. 2024, 25, 2251. [Google Scholar] [CrossRef] [PubMed]
- Stefanska, K.; Sattar, N. Incretin-based weight loss therapies and heart failure with preserved ejection fraction: Guideline impactful results, but mechanisms unclear. Cardiovasc. Res. 2025. advance online publication. [Google Scholar] [CrossRef]
- Borlaug, B.A.; Sharma, K.; Shah, S.J.; Ho, J.E. Heart Failure with Preserved Ejection Fraction: JACC Scientific Statement. J. Am. Coll. Cardiol. 2023, 81, 1810–1834. [Google Scholar] [CrossRef]
- Thomas, G.; Sehgal, A.; Kashyap, S.; Srinivas, T.; Kirwan, J.P.; Navaneethan, S.D. Metabolic syndrome and kidney disease: A systematic review and meta-analysis. Clin. J. Am. Soc. Nephrol. 2011, 6, 2364–2373. [Google Scholar] [CrossRef]
- Ndumele, C.; Rangaswami, J.; Chow, S.; Neeland, I.J.; Tuttle, K.R.; Khan, S.S.; Coresh, J.; Mathew, R.O.; Baker-Smith, C.M.; Carnethon, M.R.; et al. Cardiovascular-kidney-metabolic health: A presidential advisory from the American Heart Association. Circulation 2023, 148, 1606–1635. [Google Scholar] [CrossRef]
- Ndumele, C.E.; Neeland, I.J.; Tuttle, K.R.; Chow, S.L.; Mathew, R.O.; Khan, S.S.; Coresh, J.; Baker-Smith, C.M.; Carnethon, M.R.; Després, J.P.; et al. A Synopsis of the Evidence for the Science and Clinical Management of Cardiovascular-Kidney-Metabolic (CKM) Syndrome: A Scientific Statement from the American Heart Association. Circulation 2023, 148, 1636–1664. [Google Scholar] [CrossRef]
- Chertow, G.M. Tackling the Triple Threat: The American Heart Association’s Cardiovascular-Kidney-Metabolic Health Initiative. Presented at the American Society of Nephrology (ASN) Kidney Week 2023, Philadelphia, PA, USA, 3 November 2023. [Google Scholar]
- Braunwald, E. From cardiorenal to cardiovascular-kidney-metabolic syndromes. Eur. Heart J. 2025, 46, 682–684. [Google Scholar] [CrossRef]
- Rubino, F.; Cummings, D.E.; Eckel, R.H.; Cohen, R.V.; Wilding, J.P.H.; Brown, W.A.; Stanford, F.C.; Batterham, R.L.; Farooqi, I.S.; Farpour-Lambert, N.J.; et al. Definition and Diagnostic Criteria of Clinical Obesity. Lancet Diabetes Endocrinol. 2025, 13, 221–262. [Google Scholar] [CrossRef] [PubMed]
- Koskinas, K.C.; Van Craenenbroeck, E.M.; Antoniades, C.; Blüher, M.; Gorter, T.M.; Hanssen, H.; Marx, N.; McDonagh, T.A.; Mingrone, G.; Rosengren, A.; et al. Obesity and cardiovascular disease: An ESC clinical consensus statement. Eur. Heart J. 2024, 45, 4063–4098. [Google Scholar] [CrossRef] [PubMed]
- World Obesity Federation. World Obesity Atlas 2025; World Obesity Federation: London, UK, 2025. [Google Scholar]
- Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; Naghavi, M.; et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar]
- GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 40, 203–234. [Google Scholar]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Claude Mbanya, J.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2023, 204, 110945. [Google Scholar] [CrossRef] [PubMed]
- Marx, N.; Federici, M.; Schütt, K.; Müller-Wieland, D.; Ajjan, R.A.; Antunes, M.J.; Christodorescu, R.M.; Crawford, C.; Di Angelantonio, E.; Eliasson, B.; et al. ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur. Heart J. 2023, 44, 4043–4140. [Google Scholar] [CrossRef]
- The Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet 2010, 375, 2215–2222. [Google Scholar] [CrossRef]
- Mosenzon, O.; Alguwaihes, A.; Leon, J.L.A.; Bayram, F.; Darmon, P.; Davis, T.M.E.; Dieuzeide, G.; Eriksen, K.T.; Hong, T.; Kaltoft, M.S.; et al. CAPTURE: A multinational, cross-sectional study of cardiovascular disease prevalence in adults with type 2 diabetes across 13 countries. Cardiovasc. Diabetol. 2021, 20, 154. [Google Scholar] [CrossRef]
- Thomas, M.C.; Brownlee, M.; Susztak, K.; Sharma, K.; Jandeleit-Dahm, K.A.M.; Zoungas, S.; Rossing, P.; Groop, P.-H.; Cooper, M.E. Diabetic kidney disease. Nat. Rev. Dis. Prim. 2015, 1, 15018. [Google Scholar] [CrossRef]
- Rando, M.M.; Guthoff, M.; Tiwari, V.; Biscetti, F. Editorial: Diagnosis, prevention and treatment in diabetic nephropathy. Front. Endocrinol. 2022, 13, 1011665. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef]
- Ruiz-García, A.; Serrano-Cumplido, A.; Escobar-Cervantes, C.; Arranz-Martínez, E.; Turégano-Yedro, M.; Pallarés-Carratalá, V. Heart Failure Prevalence Rates and Its Association with Other Cardiovascular Diseases and Chronic Kidney Disease: SIMETAP-HF Study. J. Clin. Med. 2023, 12, 4924. [Google Scholar] [CrossRef]
- Szlagor, M.; Dybiec, J.; Młynarska, E.; Rysz, J.; Franczyk, B. Chronic Kidney Disease as a Comorbidity in Heart Failure. Int. J. Mol. Sci. 2023, 24, 2988. [Google Scholar] [CrossRef]
- Trimarco, V.; Izzo, R.; Pacella, D.; Virginia Manzi, M.; Trama, U.; Lembo, M.; Piccinocchi, R.; Gallo, P.; Esposito, G.; Morisco, C.; et al. Increased prevalence of cardiovascular-kidney-metabolic syndrome during COVID-19: A propensity score-matched study. Diabetes Res. Clin. Pract. 2024, 218, 111926. [Google Scholar] [CrossRef]
- Ji, H.; Sabanayagam, C.; Matsushita, K.; Cheng, C.Y.; Rim, T.H.; Sheng, B.; Li, H.; Tham, Y.C.; Cheng, S.; Wong, T.Y. Sex Differences in Cardiovascular-Kidney-Metabolic Syndrome: 30-Year US Trends and Mortality Risks—Brief Report. Arterioscler. Thromb. Vasc. Biol. 2025, 45, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Massy, Z.A.; Drueke, T.B. Combination of Cardiovascular, Kidney, and Metabolic Diseases in a Syndrome Named Cardiovascular-Kidney-Metabolic, With New Risk Prediction Equations. Kidney Int. Rep. 2024, 9, 2608–2618. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Sidell, M.; Arterburn, D.; Daley, M.F.; Desai, J.; Fitzpatrick, S.L.; Horberg, M.A.; Koebnick, C.; McCormick, E.; Oshiro, C.; et al. Racial/ethnic disparities in the prevalence of diabetes and prediabetes by BMI: Patient outcomes research to advance learning (PORTAL) multisite cohort of adults in the U.S. Diabetes Care 2019, 42, 2211–2219. [Google Scholar] [CrossRef]
- Carpenter, C.; Yan, E.; Chen, S.; Hong, K.; Arechiga, A.; Kim, W.S.; Deng, M.; Li, Z.; Heber, D. Body fat and body-mass index among a multiethnic sample of college-age men and women. J. Obes. 2013, 790654. [Google Scholar] [CrossRef]
- Hill-Briggs, F.; Adler, N.; Berkowitz, S.A.; Chin, M.H.; Gary-Webb, T.L.; Navas-Acien, A.; Thornton, P.L.; Haire-Joshu, D. Social determinants of health and diabetes: A scientific review. Diabetes Care 2020, 44, 258–279. [Google Scholar] [CrossRef]
- Powell-Wiley, T.; Baumer, Y.; Baah, F.O.; Baez, A.S.; Farmer, N.; Mahlobo, C.T.; Pita, M.A.; Potharaju, K.A.; Tamura, K.; Wallen, G.R. Social determinants of cardiovascular disease. Circ. Res. 2022, 130, 782–799. [Google Scholar] [CrossRef] [PubMed]
- Larque, E.; Labayen, I.; Flodmark, C.; Lissau, I.; Czernin, S.; Moreno, L.A.; Pietrobelli, A.; Widhalm, K. From conception to infancy: Early risk factors for childhood obesity. Nat. Rev. Endocrinol. 2019, 15, 456–478. [Google Scholar] [CrossRef]
- Arneth, B. Mechanisms of Insulin Resistance in Patients with Obesity. Endocrines 2024, 5, 153–165. [Google Scholar] [CrossRef]
- Klop, B.; Elte, J.W.F.; Cabezas, M.C. Dyslipidemia in Obesity: Mechanisms and Potential Targets. Nutrients 2013, 5, 1218–1240. [Google Scholar] [CrossRef] [PubMed]
- El Meouchy, P.; Wahoud, M.; Allam, S.; Chedid, R.; Karam, W.; Karam, S. Hypertension Related to Obesity: Pathogenesis, Characteristics and Factors for Control. Int. J. Mol. Sci. 2022, 23, 12305. [Google Scholar] [CrossRef] [PubMed]
- Kreiner, F.; Schytz, P.; Heerspink, H.; Idorn, T. Obesity-related kidney disease: Current understanding and future perspectives. Biomedicines 2023, 11, 2498. [Google Scholar] [CrossRef]
- Costantino, V.V.; Gil Lorenzo, A.F.; Bocanegra, V.; Vallés, P.G. Molecular Mechanisms of Hypertensive Nephropathy: Renoprotective Effect of Losartan through Hsp70. Cells 2021, 10, 3146. [Google Scholar] [CrossRef]
- Jha, R.; Lopez-Trevino, S.; Kankanamalage, H.R.; Jha, J.C. Diabetes and Renal Complications: An Overview on Pathophysiology, Biomarkers and Therapeutic Interventions. Biomedicines 2024, 12, 1098. [Google Scholar] [CrossRef]
- Braam, B.; Joles, J.; Danishwar, A.; Gaillard, C. Cardiorenal syndrome—Current understanding and future perspectives. Nat. Rev. Nephrol. 2014, 10, 48–55. [Google Scholar] [CrossRef]
- Buonafine, M.; Bonnard, B.; Jaisser, F. Mineralocorticoid Receptor and Cardiovascular Disease. Am. J. Hypertens. 2018, 31, 1165–1174. [Google Scholar] [CrossRef]
- Kolkhof, P.; Jaisser, F.; Kim, S.Y.; Filippatos, G.; Nowack, C.; Pitt, B. Steroidal and Novel Non-steroidal Mineralocorticoid Receptor Antagonists in Heart Failure and Cardiorenal Diseases: Comparison at Bench and Bedside. Handb. Exp. Pharmacol. 2017, 243, 271–305. [Google Scholar]
- Bauersachs, J.; Jaisser, F.; Toto, R. Mineralocorticoid receptor activation and mineralocorticoid receptor antagonist treatment in cardiac and renal diseases. Hypertension 2015, 65, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Barrera-Chimal, J.; Girerd, S.; Jaisser, F. Mineralocorticoid receptor antagonists and kidney diseases: Pathophysiological basis. Kidney Int. 2019, 96, 302–319. [Google Scholar] [CrossRef] [PubMed]
- Biwer, L.A.; Wallingford, M.C.; Jaffe, I.Z. Vascular mineralocorticoid receptor: Evolutionary mediator of wound healing turned harmful by our modern lifestyle. Am. J. Hypertens. 2019, 32, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Xanthopoulos, A.; Papamichail, A.; Briasoulis, A.; Loritis, K.; Bourazana, A.; Magouliotis, D.E.; Sarafidis, P.; Stefanidis, I.; Skoularigis, J.; Triposkiadis, F. Heart Failure in Patients with Chronic Kidney Disease. J. Clin. Med. 2023, 12, 6105. [Google Scholar] [CrossRef]
- El Chamieh, C.; Liabeuf, S.; Massy, Z. Uremic Toxins and Cardiovascular Risk in Chronic Kidney Disease: What Have We Learned Recently beyond the Past Findings. Toxins 2022, 14, 280. [Google Scholar] [CrossRef]
- Guldan, M.; Unlu, S.; Abdel-Rahman, S.M.; Ozbek, L.; Gaipov, A.; Covic, A.; Soler, M.J.; Covic, A.; Kanbay, M. Understanding the Role of Sex Hormones in Cardiovascular Kidney Metabolic Syndrome: Toward Personalized Therapeutic Approaches. J. Clin. Med. 2024, 13, 4354. [Google Scholar] [CrossRef]
- Wharton, S.; Lau, D.; Vallis, M.; Sharma, A.M.; Biertho, L.; Campbell-Scherer, D.; Adamo, K.; Alberga, A.; Bell, R.; Boulé, N.; et al. Obesity in adults: A clinical practice guideline. CMAJ 2020, 192, E875–E891. [Google Scholar] [CrossRef]
- Koenen, M.; Hill, M.; Cohen, P.; Sowers, J. Obesity, adipose tissue and vascular dysfunction. Circ Res. 2021, 128, 951–968. [Google Scholar] [CrossRef]
- Palaniappan, L.; Wong, E.; Shin, J.; Fortmann, S.P.; Lauderdale, D.S. Asian Americans have greater prevalence of metabolic syndrome despite lower body mass index. Int. J. Obes. 2011, 35, 393–400. [Google Scholar] [CrossRef]
- Despres, J.; Carpentier, A.; Tchernof, A.; Neeland, I.; Poirier, P. Management of obesity in cardiovascular practice: JACC focus seminar. J. Am. Coll. Cardiol. 2021, 78, 513–531. [Google Scholar] [CrossRef] [PubMed]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose tissue dysfunction as determinant of obesity-associated metabolic complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef]
- Powell-Wiley, T.; Poirier, P.; Burke, L.E.; Després, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and cardiovascular disease: A scientific statement from the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef]
- World Health Organization. Classification of Diabetes Mellitus; World Health Organization: Cham, Switzerland, 2019.
- American Diabetes Association Professional Practice Committee. 2. Diagnosis and classification of diabetes: Standards of Care in Diabetes—2025. Diabetes Care 2025, 48 (Suppl. S1), S27–S49. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes CKD Work Group KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2024, 3, S117–S314.
- Wu, X.; Hu, W.; Xu, J.; Shen, J.; Lin, L.; Zhu, J.; Wei, T.; Lv, L. Difference between estimated glomerular filtration rate based on cystatin C versus creatinine and cardiovascular–kidney–metabolic health. Front. Med. 2025, 11, 1477343. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. AHA/ACC/HFSA guideline for the management of heart failure. Circulation 2022, 145, e895–e1032. [Google Scholar] [PubMed]
- Jia, X.; Al Rifai, M.; Ndumele, C.; Virani, S.S.; de Lemos, J.A.; Lee, E.; Shah, A.M.; Echouffo-Tcheugui, J.B.; Bozkurt, B.; Hoogeveen, R.; et al. Reclassification of pre-heart failure stages using cardiac biomarkers: Atherosclerosis Risk in Communities (ARIC) study. JACC Heart Fail. 2023, 11, 449–450. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC) With the Special Contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar]
- Kittelson, K.S.; Gasparotto Junior, A.; Fillmore, N.; da Silva Gomes, R. Cardiovascular-kidney-metabolic syndrome—An integrative review. Prog. Cardiovasc. Dis. 2024, 87, 26–36. [Google Scholar] [CrossRef]
- McEvoy, J.W.; McCarthy, C.P.; Bruno, R.M.; Brouwers, S.; Canavan, M.D.; Ceconi, C.; Christodorescu, R.M.; Daskalopoulou, S.S.; Ferro, C.J.; Gerdts, E.; et al. ESC Guidelines for the management of elevated blood pressure and hypertension. Eur. Heart J. 2024, 45, 3912–4018. [Google Scholar] [CrossRef]
- Claudel, S.E.; Schmidt, I.M.; Gopal, D.M.; Ashish Verma, A. Elevated cardiac biomarkers in relatively healthy U.S. adults. Eur. J. Intern. Med. 2024, 121, 152–154. [Google Scholar] [CrossRef] [PubMed]
- Claudel, S.E.; Ashish, V. Cardiovascular-kidney-metabolic syndrome: A step toward multidisciplinary and inclusive care. Cell Metab. 2023, 35, 2093–2250. [Google Scholar] [CrossRef] [PubMed]
- Lanlan, W.; Zheng, H. Elevated triglyceride glucose index is associated with advanced cardiovascular kidney metabolic syndrome. Sci. Rep. 2024, 14, 31352. [Google Scholar]
- Hong, J.; Zhang, R.; Tang, H.; Wu, S.; Chen, Y.; Tan, X. Comparison of triglyceride glucose index and modified triglyceride glucose indices in predicting cardiovascular diseases incidence among populations with cardiovascular-kidney-metabolic syndrome stages 0–3: A nationwide prospective cohort study. Cardiovasc. Diabetol. 2025, 24, 98. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-W.; Kao, T.-W.; Chang, P.-K.; Chen, W.-L.; Wu, L.-W. Atherogenic index of plasma as predictors for metabolic syndrome, hypertension and diabetes mellitus in Taiwan citizens: A 9-year longitudinal study. Sci. Rep. 2021, 11, 9900. [Google Scholar] [CrossRef]
- Shi, Y.; Wen, M. Sex-specific differences in the effect of the atherogenic index of plasma on prediabetes and diabetes in the NHANES 2011–2018 population. Cardiovasc. Diabetol. 2023, 22, 19. [Google Scholar] [CrossRef]
- Wang, Q.; Zheng, D.; Liu, J.; Fang, L.; Li, Q. Atherogenic index of plasma is a novel predictor of non-alcoholic fatty liver disease in obese participants: A cross-sectional study. Lipids Health Dis. 2018, 17, 284. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, Y.; Lu, Y.; Liu, T.; Yin, P. Atherogenic index of plasma and cardiovascular disease risk in cardiovascular-kidney-metabolic syndrome stage 1 to 3: A longitudinal study. Front. Endocrinol. 2025, 16, 1517658. [Google Scholar] [CrossRef]
- Kronenberg, F.; Mora, S.; Stroes, E.S.G.; Ference, B.A.; Arsenault, B.J.; Berglund, L.; Dweck, M.R.; Koschinsky, M.; Lambert, G.; Mach, F.; et al. Lipoprotein(a) in Atherosclerotic Cardiovascular Disease and Aortic Stenosis: A Consensus Statement of the European Atherosclerosis Society. Eur. Heart J. 2022, 43, 3925–3946. [Google Scholar] [CrossRef]
- Katsiki, N.; Athyros, V. Serum uric acid: A mediator of cardio-reno-metabolic diseases. Expert Rev. Cardiovasc. Ther. 2021, 19, 1127–1128. [Google Scholar] [CrossRef] [PubMed]
- Maloberti, A.; Mengozzi, A.; Russo, E.; Cicero, A.F.G.; Angeli, F.; Agabiti Rosei, E.; Barbagallo, C.M.; Bernardino, B.; Bombelli, M.; Cappelli, F.; et al. The results of the URRAH (Uric Acid Right for Heart Health) project: A focus on hyperuricemia in relation to cardiovascular and kidney disease and its role in metabolic dysregulation. High Blood Press. Cardiovasc. Prevent. 2023, 30, 411–425. [Google Scholar] [CrossRef]
- Gao, C.; Gao, S.; Zhao, R.; Shen, P.; Zhu, X.; Yang, Y.; Duan, C.; Wang, Y.; Ni, H.; Zhou, L.; et al. Association between systemic immune-inflammation index and cardiovascular-kidney-metabolic syndrome. Sci. Rep. 2024, 14, 19151. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, W.; Xie, S.; Xu, Y.; Lin, Z. Joint association of the inflammatory marker and cardiovascular-kidney-metabolic syndrome stages with all-cause and cardiovascular disease mortality: A national prospective study. BMC Public Health 2025, 25, 10. [Google Scholar] [CrossRef]
- Sindhu, D.; Sharma, G.S.M.; Kumbala, D.M. Management of diabetic kidney disease: Where do we stand?: A narrative review. Medicine 2023, 102, e33366. [Google Scholar] [CrossRef] [PubMed]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. Chronic kidney disease and risk management: Standards of care in diabetes. Diabetes Care 2024, 47, S219–S230. [Google Scholar]
- Claudel, S.E.; Verma, A. Albuminuria in Cardiovascular, Kidney, and Metabolic Disorders: A State-of-the-Art Review. Circulation 2025, 151, 716–732. [Google Scholar] [CrossRef]
- Eslam, M.; Newsome, P.; Sarin, S. A new definition for metabolic dysfunction associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- Gaggin, H.K.; Januzzi, J.L., Jr. Update. Cardiac Biomarkers and Heart Failure. Expert analysis. 5 May 2023. Available online: https://www.acc.org/Latest-in-Cardiology/Articles/2015/02/09/13/00/Cardiac-Biomarkers-and-Heart-Failure (accessed on 11 March 2025).
- Kayani, M.; Fatima, N.; Yarra, P.C.; Almansouri, N.E.; Deepshikha, K.; Balasubramanian, A.; Parvathaneni, N.; Mowo-Wale, A.G.; Valdez, J.A.; Nazir, Z. Novel Biomarkers in Early Detection of Heart Failure: A Narrative Review. Cureus 2024, 16, 53445. [Google Scholar] [CrossRef]
- Panattoni, G.; Desimone, P.; Toto, F.; Meringolo, F.; Jacomelli, I.; Rebecchi, M.; Cicogna, F.; Calò, L. Cardiovascular Risk Assessment in Daily Clinical Practice: When and How to Use a Risk Score. Eur. Heart J. Suppl. 2025, 27 (Suppl. S1), i16–i21. [Google Scholar] [CrossRef] [PubMed]
- SCORE2-OP working Group and ESC Cardiovascular Risk Collaboration. SCORE2-OP risk prediction algorithms: Estimating incident cardiovascular event risk in older persons in four geographical risk regions. Eur. Heart J. 2021, 42, 2455–2467. [Google Scholar] [CrossRef]
- SCORE2-Diabetes Working Group and the ESC Cardiovascular Risk Collaboration. SCORE2-Diabetes: 10-year cardiovascular risk estimation in type 2 diabetes in Europe. Eur. Heart J. 2023, 44, 2544–2556. [Google Scholar] [CrossRef]
- Matsushita, K.; Kaptoge, S.; Hageman, S.H.J.; Sang, Y.; Ballew, S.H.; Grams, M.E.; Surapaneni, A.; Sun, L.; Arnlov, J.; Bozic, M.; et al. Including measures of chronic kidney disease to improve cardiovascular risk prediction by SCORE2 and SCORE2-OP. Eur. J. Prev. Cardiol. 2023, 30, 8–16. [Google Scholar] [CrossRef]
- SCORE2 Asia-Pacific Writing Group; Hageman, S.H.J.; Huang, Z.; Lee, H.; Kaptoge, S.; Dorresteijn, J.A.N.; Pennells, L.; Di Angelantonio, E.; Visseren, F.L.J.; Kim, H.C.; et al. Risk prediction of cardiovascular disease in the Asia-Pacific region: The SCORE2 Asia-Pacific model. Eur. Heart J. 2025, 46, 702–715. [Google Scholar] [CrossRef]
- Khan, S.; Coresh, J.; Pencina, M.J.; Ndumele, C.E.; Rangaswami, J.; Chow, S.L.; Palaniappan, L.P.; Sperling, L.S.; Virani, S.S.; Ho, J.E.; et al. Novel prediction equations for absolute risk assessment of total cardiovascular disease incorporating cardiovascular-kidney-metabolic health: A scientific statement from the American Heart Association. Circulation 2023, 48, 1982–2004. [Google Scholar] [CrossRef] [PubMed]
- Hageman, S.H.J.; Kaptoge, S.; de Vries, T.I.; Lu, W.; Kist, J.M.; van Os, H.J.A.; Numans, M.E.; Läll, K.; Bobak, M.; Pikhart, H.; et al. Prediction of individual lifetime cardiovascular risk and potential treatment benefit: Development and recalibration of the LIFE-CVD2 model to four European risk regions. Eur. J. Prev. Cardiol. 2024, 31, 1690–1699. [Google Scholar] [CrossRef] [PubMed]
- Gepner, D.; Young, R.; Delaney, J.; Tattersall, M.C.; Blaha, M.J.; Post, W.S.; Gottesman, R.F.; Kronmal, R.; Budoff, M.J.; Burke, G.L.; et al. Comparison of coronary artery calcium presence, carotid plaque presence, and carotid intima-media thickness for cardiovascular disease prediction in the Multi-Ethnic Study of Atherosclerosis. Circ. Cardiovasc. Imaging 2015, 8, e002262. [Google Scholar] [CrossRef]
- Polonsky, T.; McClelland, R.; Jorgensen, N.W.; Bild, D.E.; Burke, G.L.; Guerci, A.D.; Greenland, P. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA 2010, 303, 1610–1616. [Google Scholar] [CrossRef]
- Emberson, M.A.; Lalande, A.; Wang, D.; McDonough, D.J.; Liu, W.; Gao, Z. Effectiveness of smartphone-based physical activity interventions on individuals’ health outcomes: A systematic review. Biomed. Res. Int. 2021, 6296896. [Google Scholar] [CrossRef]
- Lloyd-Jones, D.M.; Allen, N.B.; Anderson, C.A.M.; Black, T.; Brewer, L.C.; Foraker, R.E.; Grandner, M.A.; Lavretsky, H.; Perak, A.M.; Sharma, G.; et al. Life’s essential 8: Updating and enhancing the American Heart Association’s construct of cardiovascular health: A presidential advisory from the American Heart Association. Circulation 2022, 146, e18–e43. [Google Scholar] [CrossRef] [PubMed]
- Marc-Andre, C. A Review of Current Guidelines for the Treatment of Obesity. Am. J. Manag. Care 2022, 28, 15. [Google Scholar]
- Garvey, W.T.; Mechanick, J.I.; Brett, E.M.; Garber, A.J.; Hurley, D.L.; Jastreboff, A.M.; Nadolsky, K.; Pessah-Pollack, R.; Plodkowski, R.; Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines. American Association of Clinical Endocrinologists and American College of Endocrinology Comprehensive Clinical Practice Guidelines for Medical Care of Patients with Obesity. Endocr. Pract. 2016, 22 (Suppl. S3), 1–203. [Google Scholar]
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J. Am. Coll. Cardiol. 2014, 63 Part B, 2985–3023. [Google Scholar] [CrossRef]
- Lincoff, A.M.; Brown, F.K.; Colhoun, H.M.; Deanfield, J.; Emerson, S.S.; Esbjerg, S.; Hardt-Lindberg, S.; Hovingh, G.K.; Kahn, S.E.; Kushner, R.F.; et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N. Engl. J. Med. 2023, 389, 2221–2232. [Google Scholar] [CrossRef]
- Pi-Sunyer, X.; Astrup, A.; Fujioka, K.; Greenway, F.; Halpern, A.; Krempf, M.; Lau, D.C.; le Roux, C.W.; Violante Ortiz, R.; Jensen, C.B.; et al. A Randomized, Controlled Trial of 3.0 mg of Liraglutide in Weight Management. N. Engl. J. Med. 2015, 373, 11–22. [Google Scholar] [CrossRef]
- Davies, M.J.; Bergenstal, R.; Bode, B.; Kushner, R.F.; Lewin, A.; Skjøth, T.V.; Andreasen, A.H.; Jensen, C.B.; DeFronzo, R.A.; NN8022-1922 Study Group. Efficacy of Liraglutide for Weight Loss Among Patients with Type 2 Diabetes: The SCALE Diabetes Randomized Clinical Trial. JAMA 2015, 314, 687–699. [Google Scholar] [CrossRef]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.; Færch, L.; Jeppesen, O.K.; Pakseresht, A.; Pedersen, S.D.; Perreault, L.; Rosenstock, J.; Shimomura, I.; Viljoen, A.; Wadden, T.A.; et al. Semaglutide 2.4 mg Once a Week in Adults with Overweight or Obesity, and Type 2 Diabetes (STEP 2): A Randomised, Double-Blind, Double-Dummy, Placebo-Controlled, Phase 3 Trial. Lancet 2021, 397, 971–984. [Google Scholar] [CrossRef]
- Kosiborod, M.N.; Abildstrøm, S.Z.; Borlaug, B.A.; Butler, J.; Rasmussen, S.; Davies, M.; Hovingh, G.K.; Kitzman, D.W.; Lindegaard, M.L.; Møller, D.V.; et al. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. N. Engl. J. Med. 2023, 389, 1069–1084. [Google Scholar] [CrossRef]
- Kosiborod, M.N.; Petrie, M.C.; Borlaug, B.A.; Butler, J.; Davies, M.J.; Hovingh, G.K.; Kitzman, D.W.; Møller, D.V.; Treppendahl, M.B.; Verma, S.; et al. Semaglutide in Patients with Obesity-Related Heart Failure and Type 2 Diabetes. N. Engl. J. Med. 2024, 390, 1394–1407. [Google Scholar] [CrossRef] [PubMed]
- Perkovic, V.; Tuttle, K.R.; Rossing, P.; Mahaffey, K.W.; Mann, J.F.E.; Bakris, G.; Baeres, F.M.M.; Idorn, T.; Bosch-Traberg, H.; Lausvig, N.L.; et al. Effects of Semaglutide on Chronic Kidney Disease in Patients with Type 2 Diabetes. N. Engl. J. Med. 2024, 391, 109–121. [Google Scholar] [CrossRef]
- McGuire, D.K.; Marx, N.; Mulvagh, S.L.; Deanfield, J.E.; Inzucchi, S.E.; Pop-Busui, R.; Mann, J.F.E.; Emerson, S.S.; Poulter, N.R.; Engelmann, M.D.M.; et al. Oral Semaglutide and Cardiovascular Outcomes in High-Risk Type 2 Diabetes. N. Engl. J. Med 2025. advance online publication. [Google Scholar] [CrossRef]
- Bonaca, M.P.; Catarig, A.-M.; Houlind, K.; Ludvik, B.; Nordanstig, J.; Ramesh, C.K.; Rasouli, N.; Sourij, H.; Videmark, A.; Verma, S.; et al. Semaglutide and walking capacity in people with symptomatic peripheral artery disease and type 2 diabetes (STRIDE): A phase 3b, double-blind, randomised, placebo-controlled trial. Lancet 2025. advance online publication. [Google Scholar] [CrossRef] [PubMed]
- Jastreboff, A.M.; Aronne, L.J.; Ahmad, N.N.; Wharton, S.; Connery, L.; Alves, B.; Kiyosue, A.; Zhang, S.; Liu, B.; Bunck, M.C.; et al. Tirzepatide Once Weekly for the Treatment of Obesity. N. Engl. J. Med. 2022, 387, 205–216. [Google Scholar] [CrossRef]
- Jastreboff, A.M.; Le Roux, C.W.; Stefanski, A.; Aronne, L.J.; Halpern, B.; Wharton, S.; Wilding, J.P.H.; Perreault, L.; Zhang, S.; Battula, R.; et al. Tirzepatide for Obesity Treatment and Diabetes Prevention. N. Engl. J. Med. 2024, 394, 1445–1463. [Google Scholar] [CrossRef]
- Packer, M.; Zile, M.R.; Kramer, C.M.; Baum, S.J.; Litwin, S.E.; Menon, V.; Ge, J.; Weerakkody, G.J.; Ou, Y.; Bunck, M.C.; et al. Tirzepatide for Heart Failure with Preserved Ejection Fraction and Obesity. N. Engl. J. Med. 2024, 392, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Zile, M.R.; Kramer, C.M.; Murakami, M.; Ou, Y.; Borlaug, B.A. Interplay of Chronic Kidney Disease and the Effects of Tirzepatide in Patients with Heart Failure, Preserved Ejection Fraction, and Obesity The SUMMIT Trial. J. Am. Coll. Cardiol. 2025. advance online publication. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- Solomon, S.D.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; Shah, S.J.; Lindholm, D.; et al. Dapagliflozin in Heart Failure with Preserved and Mildly Reduced Ejection Fraction: Rationale and Design of the DELIVER Trial. Eur. J. Heart Fail. 2021, 23, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner-La Rocca, H.P.; Choi, D.J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- The EMPA-KIDNEY Collaborative Group; Herrington, W.G.; Staplin, N.; Wanner, C.; Green, J.B.; Hauske, S.J.; Emberson, J.R.; Preiss, D.; Judge, P.; Mayne, K.J.; et al. Empagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2023, 388, 117–127. [Google Scholar]
- Agarwal, R.; Filippatos, G.; Pitt, B.; Anker, S.D.; Rossing, P.; Joseph, A.; Kolkhof, P.; Nowack, C.; Gebel, M.; Ruilope, L.M.; et al. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: The FIDELITY pooled analysis. Eur. Heart J. 2022, 43, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Filippatos, G.; Anker, S.D.; Agarwal, R.; Ruilope, L.M.; Rossing, P.; Bakris, G.L.; Tasto, C.; Joseph, A.; Kolkhof, P.; Lage, A.; et al. Finerenone Reduces Risk of Incident Heart Failure in Patients With Chronic Kidney Disease and Type 2 Diabetes: Analyses From the FIGARO-DKD Trial. Circulation 2022, 145, 437–447. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Vaduganathan, M.; Claggett, B.; Jhund, P.S.; Desai, A.S.; Henderson, A.D.; Lam, C.S.P.; Pitt, B.; Senni, M.; et al. FINEARTS-HF Committees and Investigators. Finerenone in heart failure with mildly reduced or preserved ejection fraction. N. Engl. J. Med. 2024, 39, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Sharaiha, R.Z.; Shikora, S.; White, K.P.; Macedo, G.; Toouli, J.; Kow, L. Summarizing Consensus Guidelines on Obesity Management. J. Clin. Gastroenterol. 2023, 57, 967–976. [Google Scholar] [CrossRef]
- Ahwin, P.; Martinez, D. The Relationship Between SGLT2 and Systemic Blood Pressure Regulation. Hypertens. Res. 2024, 47, 2094–2103. [Google Scholar] [CrossRef]
- Krumholz, H.M.; de Lemos, J.A.; Sattar, N.; Linetzky, B.; Sharma, P.; Mast, C.J.; Ahmad, N.N.; Bunck, M.C.; Stefanski, A. Tirzepatide and Blood Pressure Reduction: Stratified Analyses of the SURMOUNT-1 Randomised Controlled Trial. Heart 2024, 110, 1165–1171. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 6. Glycemic goals and hypoglycemia: Standards of Care in Diabetes—2025. Diabetes Care 2025, 48 (Suppl. S1), S128–S145. [Google Scholar] [CrossRef]
- McGuire, D.K.; Shih, W.J.; Cosentino, F.; Charbonnel, B.; Cherney, D.Z.I.; Dagogo-Jack, S.; Pratley, R.; Greenberg, M.; Wang, S.; Huyck, S.; et al. Association of SGLT2 Inhibitors with Cardiovascular and Kidney Outcomes in Patients with Type 2 Diabetes: A Meta-Analysis. JAMA Cardiol. 2021, 6, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Sattar, N.; Lee, M.M.Y.; Kristensen, S.L.; Branch, K.R.H.; Del Prato, S.; Khurmi, N.S.; Lam, C.S.P.; Lopes, R.D.; McMurray, J.J.V.; Pratley, R.E.; et al. Cardiovascular, Mortality, and Kidney Outcomes with GLP-1 Receptor Agonists in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Trials. Lancet Diabetes Endocrinol. 2021, 9, 653–662. [Google Scholar] [CrossRef]
- Handelsman, Y.; Anderson, J.; Bakris, G.L.; Ballantyne, C.M.; Bhatt, D.L.; Bloomgarden, Z.T.; Bozkurt, B.; Budoff, M.J.; Butler, J.; Cherney, D.Z.I.; et al. DCRM 2.0: Multispecialty practice recommendations for the management of diabetes, cardiorenal, and metabolic diseases. Metabolism 2024, 159, 155931. [Google Scholar] [CrossRef] [PubMed]
- Jastreboff, A.M.; Kaplan, L.M.; Frías, J.P.; Wu, Q.; Du, Y.; Gurbuz, S.; Coskun, T.; Haupt, A.; Milicevic, Z.; Hartman, M.L.; et al. Triple-Hormone-Receptor Agonist Retatrutide for Obesity—A Phase 2 Trial. N. Engl. J. Med. 2023, 389, 514–526. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J.; Kaplan, L.M.; Frias, J.P.; Brouwers, B.; Wu, Q.; Thomas, M.K.; Harris, C.; Schloot, N.C.; Du, Y.; Mather, K.J.; et al. Triple hormone receptor agonist retatrutide for metabolic dysfunction-associated steatotic liver disease: A randomized phase 2a trial. Nat. Med. 2024, 30, 2037–2048. [Google Scholar] [CrossRef]
- Kanbay, M.; Guldan, M.; Ozbek, L.; Copur, S.; Covic, A.S.; Covic, A. Exploring the nexus: The place of kidney diseases within the cardiovascular-kidney-metabolic syndrome spectrum. Eur. J. Int. Med. 2024, 127, 1–14. [Google Scholar] [CrossRef]
- Golub, I.; Termeie, O.; Kristo, S.; Schroeder, L.P.; Lakshmanan, S.; Shafter, A.M.; Hussein, L.; Verghese, D.; Aldana-Bitar, J.; Manubolu, V.S.; et al. Major Global Coronary Artery Calcium Guidelines. J. Am. Coll. Cardiol. Imaging 2023, 16, 98–117. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Nuffield Department of Population Health Renal Studies Group; SGLT2 Inhibitor Meta-Analysis Cardio-Renal Trialists’ Consortium. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: Collaborative meta-analysis of large placebo-controlled trials. Lancet 2022, 400, 1788–1801. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Szarek, M.; Steg, P.G.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Voors, A.A.; Metra, M.; et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N. Engl. J. Med. 2021, 384, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Szarek, M.; Pitt, B.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Inzucchi, S.E.; Kosiborod, M.N.; et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N. Engl. J. Med. 2021, 384, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Maddox, T.; Januzzi, J.; Allen, L.A.; Breathett, K.; Brouse, S.; Butler, J.; Davis, L.L.; Fonarow, G.C.; Ibrahim, N.E.; Lindenfeld, J.; et al. 2024 ACC Expert Consensus Decision Pathway for Treatment of Heart Failure with Reduced Ejection Fraction: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2024, 83, 1444–1488. [Google Scholar] [CrossRef]
- Vaduganathan, M.; Docherty, K.; Claggett, B.; Jhund, P.; de Boer, R.A.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. SGLT-2 inhibitors in patients with heart failure: A comprehensive meta-analysis of five randomised controlled trials. Lancet 2022, 400, 757–767. [Google Scholar] [CrossRef] [PubMed]
| Class | Name | Author, Year | Population, Follow-Up | Agent | Main Results with Studied Drug |
|---|---|---|---|---|---|
| GLP1-RA | STEP 1 | Wilding, J.P.H. et al., 2021 [102] | 1961 adults, no T2D, with BMI ≥ 30 kg/m2 or BMI ≥ 27 kg/m2 plus at least one weight-related comorbidity, 68 w | Once weekly s.c. semaglutide 2.4 mg | A mean weight loss of 14.9% |
| STEP 2 | Davies, M. et al., 2021 [103] | 1210 adults with T2D, overweight, or obesity, 68 w. | Once weekly semaglutide, 2.4 mg, semaglutide 1.0 mg plus lifestyle interventions |
Weight loss was 9.6% with semaglutide at a 2.4 mg dose and 7.0% with semaglutide at a 1.0 mg dose Additionally, greater reductions in HbA1c, baseline BMI, or glycemic control status | |
| STEP-HFpEF | Kosiborod, M.N. et al., 2023 [104] | 529 adults with HFpEF (LVEF ≥ 50%) and obesity (BMI ≥ 30 kg/m2), on standard background therapies, 52 w. | Once weekly s.c. semaglutide 2.4 mg |
The mean percentage reduction in body weight was −13.3% Secondary outcomes demonstrated positive changes in the 6 min walk distance test | |
| STEP-HFpEF DM | Kosiborod, M.N et al., 2024 [105] | 616 patients with HFpEF and T2D, 52 w | Once weekly s.c. semaglutide 2.4 mg |
The mean percentage reduction in body weight was −9.8% The change in the 6 min walk distance test was 14.3 m for semaglutide and 1.8 m for placebo | |
| FLOW | Perkovic, V. et al., 2024 [106] | 3533 patients with T2D and CKD, defined by a sustained eGFR decline and albuminuria on standard care, 3.5 y | Once weekly s.c. semaglutide 1 mg |
24% risk reduction of primary events (severe renal decline or CV events), 29% risk reduction of CV mortality, 18% risk reduction of MACEs, and risk reduction of all-cause mortality by 20%; reduction of the risk of new-onset or worsening HF by 27% Slowed the decline in kidney function, with a less steep annual rate of 1.16 mL/min/1.73 m2 | |
| SOUL | McGuire, D.K. et al., 2025 [107] | 9650 participants aged ≥50 years, with T2D and established CAD, cerebrovascular disease, symptomatic PAD, or CKD, 49.5 mo | Oral semaglutide, 14 mg o.d. | 14% relative reduction in MACEs (CV death, non-fatal MI, and non-fatal stroke), primarily driven by a reduction in non-fatal MI | |
| STRIDE | Bonaca M.P. et al., 2025 [108] | 792 patients with T2D and early symptomatic PAD, 52 w | Once weekly s.c. semaglutide 1.0 mg | A significant improvement in maximum walking distance (21%) vs. placebo (8%), pain-free walking distance, quality of life, ankle-brachial index, and disease progression | |
| dual GIP/GLP-1RA | SURMOUNT-1 | Jastreboff, A.M. et al., 2022 [109] | 2539 adults, no T2D, with BMI ≥ 30 kg/m2 or BMI ≥ 27 kg/m2 plus weight-related complications, 72 w. | Once weekly s.c. tirzepatide (5, 10, or 15 mg) plus lifestyle changes | 20.9% mean weight reduction with tirzepatide vs. 3.1% with placebo Improvements in all prespecified cardiometabolic measures were observed with tirzepatide |
| SURMOUNT-2 | Jastreboff, A.M. et al., 2024 [110] | 938 adults with T2D and overweight or obese, 72 w. | Once weekly s.c. tirzepatide (5, 10, or 15 mg) plus lifestyle changes | The least-squares mean reduction in body weight at week 72 was −14.7% with tirzepatide, 15 mg, compared to −3.2% with placebo | |
| SUMMIT | Packer, M. et al., 2024 [111] | 731 patients with HFpEF and obesity, with or without T2D, 60% with CKD, 104 w. | Once weekly s.c. tirzepatide (up to 15 mg) | 38% risk reduction of death from CV causes or a worsening HF event. A significant improvement in KCCQ-CCS at 52 weeks was observed in the tirzepatide group | |
| SUMMIT Substudy | Packer, M. et al., 2025 [112] | 731 participants with HFpEF and BMI ≥ 30 m2/kg, (60% with CKD), 52 w | Once weekly s.c. tirzepatide (up to 15 mg) |
Tirzepatide improved renal function in patients with HFpEF, obesity, and CKD Tirzepatide increased eGFR at 52 weeks, assessed by both creatinine-based and cystatin C–based formulae | |
| SGLT2i | DAPA-HF | McMurray, J.J.V. et al., 2019 [113] | 4744 patients with symptomatic HFrEF and NYHA class II–IV (40% T2D), 18.2 mo | Dapagliflozin 10 mg o.d., in addition to the usual therapy |
26% risk reduction of the primary composite outcome of worsening HF (hospitalization or an urgent visit resulting in intravenous therapy for HF) or death from CV causes HF hospitalizations were reduced by 30%, while CV death was reduced by 18% |
| EMPEROR-Reduced | Packer, M. et al., 2020 [114] | 3730 patients with HFrEF, NYHA class II–IV, median LVEF 27% (50% T2D), 16 mo | Empagliflozin 10 mg o.d., in addition to the usual therapy |
25% risk reduction of the primary composite outcome of death from CV causes or hospitalization for HF The risk for the first HF hospitalization was reduced by 31%, and CV death by 8% | |
| DELIVER | Solomon, S.D. et al., 2021 [115] | 6263 patients with HF and an LVEF of >40% (45% T2D), 2.3 y | Dapagliflozin 10 mg o.d., in addition to the usual therapy | 18% risk reduction of the composite of worsening HF or CV death. Worsening HF was reduced by 21%, and CV death by 12%. Total events and symptom burden were lower in the dapagliflozin group than in the placebo group | |
| EMPEROR-Preserved | Anker, S.D. et al., 2021 [116] | 5988 patients with HF, LVEF > 40% (49% T2D), 26.2 mo | Empagliflozin 10 mg o.d., in addition to the usual therapy |
21% risk reduction of the composite
of CV death or hospitalization for HF 27% risk reduction of HF hospitalizations | |
| DAPA-CKD | Heerspink, H.J.L. et al., 2020 [117] | 4304 pts (67.5% T2D) with CKD (eGFR 25–75 mL/min/1.73 m2, UACR 200–5000 mg/g, 2.4 y | Dapagliflozin 10 mg o.d. |
39% risk reduction for the primary endpoint (eGFR decline ≥ 50%, ESKD or renal/cardiovascular death); 5.3% absolute reduction in primary event rate; 29% reduction in HF hospitalizations; 31% reduction in all-cause mortality Efficacy across all subgroups, including diabetic (36% risk reduction) and non-diabetic (50% risk reduction) patients | |
| EMPA-KIDNEY | Herrington, W. et al., 2023 [118] | 6609 pts (46.2% T2D) with CKD (defined by an eGFR of 20–45 mL/min/1.73 m2 or 45–90 mL/min/1.73 m2, UACR 200–5000 mg/g, 2y. | Empagliflozin 10 mg o.d. | 28% reduction in CKD progression or renal/cardiovascular death; 3.8% absolute reduction in primary event rate; 36% risk reduction in patients with T2D; 18% risk reduction in patients without T2D | |
| ns-MRA | FIDELIO-DKD | Agarwal, R. et al., 2022, [119] | 5734 patients with CKD and T2D, 2.6 y | Finerenone 20 mg (eGFR ≥ 60 mL/min/1.73 m2); 10 mg (eGFR 25–60 mL/min/1.73 m2) | Decreased CKD progression by 18% and CV mortality and morbidity by 14% |
| FIGARO-DKD | Filippatos, G. et al., 2022, [120] | 7437 patients with CKD and T2D, 3.4 y | Finerenone 20 mg (eGFR ≥ 60 mL/min/1.73 m2); 10 mg (eGFR 25–60 mL/min/1.73 m2) | Reduced CV mortality and morbidity by 13% and CKD progression by 13% | |
| FINEARTS-HF | Solomon, SD. et al., 2024, [121] | 6001 patients with symptomatic HF and LVEF ≥ 40%, 32 mo | Finerenone 40 mg daily (20 mg if eGFR < 60 mL/min/1.73 m2). | Reduced HF events by 18% and cardiovascular death by 7%. Kidney disease progression was reduced by 23% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mutruc, V.; Bologa, C.; Șorodoc, V.; Ceasovschih, A.; Morărașu, B.C.; Șorodoc, L.; Catar, O.E.; Lionte, C. Cardiovascular–Kidney–Metabolic Syndrome: A New Paradigm in Clinical Medicine or Going Back to Basics? J. Clin. Med. 2025, 14, 2833. https://doi.org/10.3390/jcm14082833
Mutruc V, Bologa C, Șorodoc V, Ceasovschih A, Morărașu BC, Șorodoc L, Catar OE, Lionte C. Cardiovascular–Kidney–Metabolic Syndrome: A New Paradigm in Clinical Medicine or Going Back to Basics? Journal of Clinical Medicine. 2025; 14(8):2833. https://doi.org/10.3390/jcm14082833
Chicago/Turabian StyleMutruc, Victoria, Cristina Bologa, Victorița Șorodoc, Alexandr Ceasovschih, Bianca Codrina Morărașu, Laurențiu Șorodoc, Oana Elena Catar, and Cătălina Lionte. 2025. "Cardiovascular–Kidney–Metabolic Syndrome: A New Paradigm in Clinical Medicine or Going Back to Basics?" Journal of Clinical Medicine 14, no. 8: 2833. https://doi.org/10.3390/jcm14082833
APA StyleMutruc, V., Bologa, C., Șorodoc, V., Ceasovschih, A., Morărașu, B. C., Șorodoc, L., Catar, O. E., & Lionte, C. (2025). Cardiovascular–Kidney–Metabolic Syndrome: A New Paradigm in Clinical Medicine or Going Back to Basics? Journal of Clinical Medicine, 14(8), 2833. https://doi.org/10.3390/jcm14082833








