Prognostic Value of the Red Cell Distribution Width-to-eGFR Ratio (RGR) Across Chronic Heart Failure Phenotypes: A Retrospective Observational Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Red Cell Distribution Width-to-Estimated Glomerular Filtration Rate Ratio (RGR)
2.2. Statistical Analysis
3. Results
3.1. Study Population, Demographic and Clinical Profile
3.2. Length of Hospital Stay
3.3. Laboratory Data
3.4. Predictors of Prolonged Hospitalization
3.5. Predictors of In-Hospital Mortality
3.6. Cox Proportional Hazards Regression for In-Hospital Mortality
3.7. Predictors of 6-Month All-Cause Mortality
4. Discussions
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AF | Atrial fibrillation |
| AUC | Area under curve |
| BMI | Body mass index |
| CAD | Coronary artery disease |
| CHF | Chronic heart failure |
| CI | Confidence interval |
| CKD | Chronic kidney disease |
| COPD | Chronic obstructive pulmonary disease |
| DBP | Diastolic blood pressure |
| eGFR | Estimated glomerular filtration rate |
| ELOS | Extended length of hospital stay |
| ESC | European Society of Cardiology |
| HF | Heart failure |
| HFA | Heart Failure Association |
| HFmrEF | Heart failure with mildly reduced ejection fraction |
| HFpEF | Heart failure with preserved ejection fraction |
| HFrEF | Heart failure with reduced ejection fraction |
| HR | Heart rate |
| IQR | Interquartile range |
| LOS | Length of hospital stay |
| LVEF | Left ventricle ejection fraction |
| NT-proBNP | N-terminal prohormone of brain natriuretic peptide |
| NYHA-FC | New York Heart Association functional class |
| RDW | Red cell distribution width |
| RDW-SD | Red cell distribution width standard deviation |
| RGR | Red cell distribution width-to-estimated glomerular filtration rate ratio |
| ROC | Receiver operating characteristic |
| SBP | Systolic blood pressure |
| T2DM | Type 2 diabetes mellitus |
| USA | United States |
References
- Seferović, P.M.; Vardas, P.; Jankowska, E.A.; Maggioni, A.P.; Timmis, A.; Milinković, I.; Polovina, M.; Gale, C.P.; Lund, L.H.; Lopatin, Y.; et al. The Heart Failure Association Atlas: Heart failure epidemiology and management statistics 2019. Eur. J. Heart Fail. 2021, 23, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Ide, T.; Kaku, H.; Matsushima, S.; Tohyama, T.; Enzan, N.; Funakoshi, K.; Sumita, Y.; Nakai, M.; Nishimura, K.; Miyamoto, Y.; et al. Clinical characteristics and outcomes of hospitalized patients with heart failure from the large-scale Japanese Registry of Acute Decompensated Heart Failure (JROADHF). Circ. J. 2021, 25, 1438–1450. [Google Scholar] [CrossRef] [PubMed]
- Osenenko, K.M.; Kuti, E.; Deighton, A.M.; Pimple, P.; Szabo, S.M. Burden of hospitalization for heart failure in the United States: A systematic literature review. J. Manag. Care Spec. Pharm. 2022, 28, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, V.; Nagai, T.; Chiang, C.E.; Reddy, Y.N.V.; Chao, T.F.; Zakeri, R.; Bloom, C.; Nakai, M.; Nishimura, K.; Hung, C.L.; et al. Hospitalization for heart failure in the United States, UK, Taiwan, and Japan: An international comparison of administrative health records on 413,385 individual patients. J. Card. Fail. 2022, 28, 353–366. [Google Scholar] [CrossRef]
- Bates, B.A.; Enzan, N.; Tohyama, T.; Gandhi, P.; Matsushima, S.; Tsutsui, H.; Setoguchi, S.; Ide, T. Management and outcomes of heart failure hospitalization among older adults in the United States and Japan. ESC Heart Fail. 2024, 11, 3395–3405. [Google Scholar] [CrossRef]
- Smith, A.B.; Jung, M.; Pressler, S.J. Pain and Heart Failure During Transport by Emergency Medical Services and Its Associated Outcomes: Hospitalization, Mortality, and Length of Stay. West. J. Nurs. Res. 2024, 46, 172–182. [Google Scholar] [CrossRef]
- Zheng, J.; Tisdale, R.L.; Heidenreich, P.A.; Sandhu, A.T. Disparities in Hospital Length of Stay Across Race and Ethnicity Among Patients with Heart Failure. Circ. Heart Fail. 2022, 15, e009362. [Google Scholar] [CrossRef]
- Salvagno, G.L.; Sanchis-Gomar, F.; Picanza, A.; Lippi, G. Red Blood Cell Distribution Width: A Simple Parameter with Multiple Clinical Applications. Crit. Rev. Clin. Lab. Sci. 2015, 52, 86–105. [Google Scholar] [CrossRef]
- Xanthopoulos, A.; Giamouzis, G.; Dimos, A.; Skoularigki, E.; Starling, R.C.; Skoularigis, J.; Triposkiadis, F. Red Blood Cell Distribution Width in Heart Failure: Pathophysiology, Prognostic Role, Controversies and Dilemmas. J. Clin. Med. 2022, 11, 1951. [Google Scholar] [CrossRef]
- Felker, G.M.; Allen, L.A.; Pocock, S.J.; Shaw, L.K.; McMurray, J.J.; Pfeffer, M.A.; Swedberg, K.; Wang, D.; Yusuf, S.; Michelson, E.L.; et al. Red Cell Distribution Width as a Novel Prognostic Marker in Heart Failure: Data from the CHARM Program and the Duke Databank. J. Am. Coll. Cardiol. 2007, 50, 40–47. [Google Scholar] [CrossRef]
- Senthong, V.; Hudec, T.; Neale, S.; Wu, Y.; Hazen, S.L.; Tang, W.H. Relation of Red Cell Distribution Width to Left Ventricular End-Diastolic Pressure and Mortality in Patients with and Without Heart Failure. Am. J. Cardiol. 2017, 119, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Wołowiec, Ł.; Rogowicz, D.; Banach, J.; Buszko, K.; Surowiec, A.; Błażejewski, J.; Bujak, R.; Sinkiewicz, W. Prognostic Significance of Red Cell Distribution Width and Other Red Cell Parameters in Patients with Chronic Heart Failure During Two Years of Follow-Up. Kardiol. Pol. 2016, 74, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Zhang, Z.; Wang, Y.; Jiang, F.; Yang, K.; He, F.; Zhang, C. Predictive Value of Left Ventricular Myocardial Strain by Four-Dimensional Speckle Tracking Echocardiography Combined with Red Cell Distribution Width in Heart Failure with Preserved Ejection Fraction. Echocardi-Ography 2019, 36, 1074–1083. [Google Scholar] [CrossRef] [PubMed]
- Vizzardi, E.; Sciatti, E.; Bonadei, I.; Pezzali, N.L.; Lombardi, C.M.; Metra, M. Red Cell Distribution Width and Chronic Heart Failure: Prognostic Role Beyond Echocardiographic Parameters. Monaldi Arch. Chest Dis. 2016, 84, 59. [Google Scholar] [CrossRef]
- Bonaque, J.C.; Pascual-Figal, D.A.; Manzano-Fernández, S.; González-Cánovas, C.; Vidal, A.; Muñoz-Esparza, C.; Garrido, I.P.; Pas-tor-Pérez, F.; Valdés, M. Red Blood Cell Distribution Width Adds Prognostic Value for Outpatients with Chronic Heart Failure. Rev. Esp. Cardiol. (Engl. Ed.) 2012, 65, 606–612. [Google Scholar] [CrossRef]
- Al-Najjar, Y.; Goode, K.M.; Zhang, J.; Cleland, J.G.; Clark, A.L. Red Cell Distribution Width: An Inexpensive and Powerful Prognostic Marker in Heart Failure. Eur. J. Heart Fail. 2009, 11, 1155–1162. [Google Scholar] [CrossRef]
- Rahamim, E.; Zwas, D.R.; Keren, A.; Elbaz-Greener, G.; Ibrahimli, M.; Amir, O.; Gotsman, I. The Ratio of Hemoglobin to Red Cell Distribution Width: A Strong Predictor of Clinical Outcome in Patients with Heart Failure. J. Clin. Med. 2022, 11, 886. [Google Scholar] [CrossRef]
- Hillege, H.L.; Nitsch, D.; Pfeffer, M.A.; Swedberg, K.; McMurray, J.J.; Yusuf, S.; Granger, C.B.; Michelson, E.L.; Ostergren, J.; Cornel, J.H.; et al. Renal Function as a Predictor of Outcome in a Broad Spectrum of Patients with Heart Failure. Circulation 2006, 113, 671–678. [Google Scholar] [CrossRef]
- Dries, D.L.; Exner, D.V.; Domanski, M.J.; Greenberg, B.; Stevenson, L.W. The Prognostic Implications of Renal Insufficiency in Asymptomatic and Symptomatic Patients with Left Ventricular Systolic Dysfunction. J. Am. Coll. Cardiol. 2000, 35, 681–689. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef]
- Gardner, R.S.; Ozalp, F.; Murday, A.J.; Robb, S.D.; McDonagh, T.A. N-Terminal Pro-Brain Natriuretic Peptide: A New Gold Standard in Predicting Mortality in Patients with Advanced Heart Failure. Eur. Heart J. 2003, 24, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
- Mueller, C.; McDonald, K.; de Boer, R.A.; Maisel, A.; Cleland, J.G.F.; Kozhuharov, N.; Coats, A.J.S.; Metra, M.; Mebazaa, A.; Ruschitzka, F.; et al. Heart Failure Association of the European Society of Cardiology Practical Guidance on the Use of Natriuretic Peptide Concentrations. Eur. J. Heart Fail. 2019, 21, 715–731. [Google Scholar] [CrossRef] [PubMed]
- Madamanchi, C.; Alhosaini, H.; Sumida, A.; Runge, M.S. Obesity and Natriuretic Peptides, BNP and NT-ProBNP: Mechanisms and Di-agnostic Implications for Heart Failure. Int. J. Cardiol. 2014, 176, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Cappellini, M.D.; Motta, I. Anemia in clinical practice—Definition and classification: Does hemoglobin change with aging? Semin. Hematol. 2015, 52, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.iso.org/obp/ui/#iso:std:iso:15189:ed-4:v1:en (accessed on 18 April 2025).
- Analysis Checklist: Outliers. Available online: https://www.graphpad.com/guides/prism/latest/statistics/stat_checklist_identifying_outliers.htm (accessed on 12 March 2025).
- Marusteri, M.; Bacarea, V. Comparing Groups for Statistical Differences: How to Choose the Right Statistical Test? Biochem. Med. 2010, 20, 15–32. [Google Scholar] [CrossRef]
- Interpreting Results: Kruskal-Wallis Test. Available online: https://www.graphpad.com/guides/prism/latest/statistics/how_the_kruskal-wallis_test_works.htm (accessed on 12 March 2025).
- Šimundić, A.M. Measures of Diagnostic Accuracy: Basic Definitions. EJIFCC 2009, 19, 203–211. [Google Scholar]
- Ruopp, M.D.; Perkins, N.J.; Whitcomb, B.W.; Schisterman, E.F. Youden Index and Optimal Cut-Point Estimated from Observations Affected by a Lower Limit of Detection. Biom. J. 2008, 50, 419–430. [Google Scholar] [CrossRef]
- Muhlestein, J.B.; Lappe, D.L.; Anderson, J.L.; Muhlestein, J.B.; Budge, D.; May, H.T.; Bennett, S.T.; Bair, T.L.; Horne, B.D. Both Initial Red Cell Distribution Width (RDW) and Change in RDW During Heart Failure Hospitalization Are Associated with Length of Hospital Stay and 30-Day Outcomes. Int. J. Lab. Hematol. 2016, 38, 328–337. [Google Scholar] [CrossRef]
- Melchio, R.; Rinaldi, G.; Testa, E.; Giraudo, A.; Serraino, C.; Bracco, C.; Spadafora, L.; Falcetta, A.; Leccardi, S.; Silvestri, A.; et al. Red Cell Distribution Width Predicts Mid-Term Prognosis in Patients Hospitalized with Acute Heart Failure: The RDW in Acute Heart Failure (RE-AHF) Study. Intern. Emerg. Med. 2019, 14, 239–247. [Google Scholar] [CrossRef]
- Huang, Y.L.; Hu, Z.D.; Liu, S.J.; Sun, Y.; Qin, Q.; Qin, B.D.; Zhang, W.W.; Zhang, J.R.; Zhong, R.Q.; Deng, A.M. Prognostic Value of Red Blood Cell Distribution Width for Patients with Heart Failure: A Systematic Review and Meta-Analysis of Cohort Studies. PLoS ONE 2014, 9, e104861. [Google Scholar] [CrossRef]
- Kim, M.; Lee, C.J.; Kang, H.J.; Son, N.H.; Bae, S.; Seo, J.; Oh, J.; Rim, S.J.; Jung, I.H.; Choi, E.Y.; et al. Red Cell Distribution Width as a Prognosticator in Patients with Heart Failure. ESC Heart Fail. 2023, 10, 834–845. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.R.; Roalfe, A.K.; Adoki, I.; Hobbs, F.D.R.; Taylor, C.J. Survival of Patients with Chronic Heart Failure in the Community: A Sys-tematic Review and Meta-Analysis. Eur J Heart Fail. 2019, 21, 1306–1325. [Google Scholar] [CrossRef] [PubMed]
- Oliva, F.; Sormani, P.; Contri, R.; Campana, C.; Carubelli, V.; Cirò, A.; Morandi, F.; Di Tano, G.; Mortara, A.; Senni, M.; et al. Heart Rate as a Prognostic Marker and Therapeutic Target in Acute and Chronic Heart Failure. Int J Cardiol. 2018, 253, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Spoletini, I.; Coats, A.J.S.; Piepoli, M.F.; Rosano, G.M.C. Heart Rate and Blood Pressure Monitoring in Heart Failure. Eur. Heart J. Suppl. 2019, 21, M13–M16. [Google Scholar] [CrossRef]
- Raphael, C.E.; Whinnett, Z.I.; Davies, J.E.; Fontana, M.; Ferenczi, E.A.; Manisty, C.H.; Mayet, J.; Francis, D.P. Quantifying the Paradoxical Effect of Higher Systolic Blood Pressure on Mortality in Chronic Heart Failure. Heart 2009, 95, 56–62. [Google Scholar] [CrossRef]
- van der Wal, H.H.; van Deursen, V.M.; van der Meer, P.; Voors, A.A. Comorbidities in Heart Failure. Handb. Exp. Pharmacol. 2017, 243, 35–66. [Google Scholar] [CrossRef]
- Widmer, F. Herzinsuffizienz und Komorbiditäten [Comorbidity in Heart Failure]. Ther. Umsch. 2011, 68, 103–106. [Google Scholar] [CrossRef]
- Stamerra, C.A.; D’Elia, E.; Gori, M.; Roncali, F.; Cereda, A.; Gavazzi, A.; Ferri, C.; Senni, M. Red cell distribution width (RDW) is correlated to time of oxygen desaturation < 90% and length of sleep apneas in patients with sleep disorder breathing (SDB) and acute heart failure with preserved ejection fraction (HFpEF). Front. Cardiovasc. Med. 2023, 10, 1045702. [Google Scholar] [CrossRef]
- Lippi, G.; Targher, G.; Montagnana, M.; Salvagno, G.L.; Zoppini, G.; Guidi, G.C. Relationship between red blood cell distribution width and kidney function tests in a large cohort of unselected outpatients. Scand. J. Clin. Lab. Investig. 2008, 68, 745–748. [Google Scholar] [CrossRef]
- Oh, H.J.; Park, J.T.; Kim, J.K.; Yoo, D.E.; Kim, S.J.; Han, S.H.; Kang, S.W.; Choi, K.H.; Yoo, T.H. Red blood cell distribution width is an independent predictor of mortality in acute kidney injury patients treated with continuous renal replacement therapy. Nephrol. Dial. Transplant. 2012, 27, 589–594. [Google Scholar] [CrossRef]
- Förhécz, Z.; Gombos, T.; Borgulya, G.; Pozsonyi, Z.; Prohászka, Z.; Jánoskuti, L. Red cell distribution width in heart failure: Prediction of clinical events and relationship with markers of ineffective erythropoiesis, inflammation, renal function, and nutritional state. Am. Heart J. 2009, 158, 659–666. [Google Scholar] [CrossRef]


| Parameter | Entire Cohort n = 627 | HFrEF n = 275 | HFmrEF n = 170 | HFpEF n = 182 | p Value |
|---|---|---|---|---|---|
| Age (years, median) | 71 (62–77) | 70 (59–77) | 71 (63–77.75) | 73 (66.25–78.75) | 0.004 * |
| Male (n, %) | 345 (55.02) | 175 (63.63) | 95 (55.88) | 75 (41.20) | <0.001 ** |
| Urban areas (n, %) | 394 (62.83) | 174 (63.27) | 114 (67.06) | 106 (58.24) | 0.369 ** |
| Excess body weight (kg/m2, median) | 28.41 (24.84–33) | 27.68 (24.75–32.08) | 29.3 (25.64–32.83) | 29.33 (24.57–34.1) | 0.234 * |
| Clinical characteristics | |||||
| HR (bpm, median, IQR) | 77 (67–92) | 82 (70–100) | 72 (63.5–87.75) | 74 (65–85) | <0.001 * |
| SBP (mmHg, median, IQR) | 130 (120–140) | 125 (110–140) | 130 (120–140) | 135 (125–140) | <0.001 * |
| DBP (mmHg, median, IQR) | 80 (70–85) | 80 (70–89.5) | 75 (70–85) | 80 (70–88.75) | 0.319 * |
| NYHA - FC | <0.001 ** | ||||
| II | 203 (32.38) | 58 (21.09) | 60 (35.29) | 85 (46.7) | - |
| III | 355 (56.62) | 164 (59.64) | 105 (61.76) | 86 (47.25) | - |
| IV | 69 (11) | 53 (19.27) | 5 (2.94) | 11 (6.04) | - |
| Comorbidities | |||||
| Total number (median) | 5 (4–6) | 5 (4–6) | 5 (4–6) | 5 (4–6) | 0.337 * |
| Excess body weight (n, %) | 460 (73.37) | 198 (43.04) | 132 (28.7) | 130 (28.26) | 0.332 ** |
| CAD (n, %) | 303 (48.33) | 136 (49.45) | 92 (54.12) | 75 (41.21) | 0.047 ** |
| Prior documented MI (n, %) | 153 (24.4) | 90 (32.73) | 39 (22.94) | 24 (13.19) | <0.001 ** |
| Hypertension (n, %) | 517 (82.46) | 207 (75.27) | 150 (88.24) | 160 (87.91) | <0.001 ** |
| Valvular heart disease ≥ moderate (n, %) | 512 (81.66) | 236 (85.82) | 135 (79.41) | 141 (77.47) | 0.053 ** |
| Prior valvular surgery (n, %) | 33 (5.26) | 9 (3.27) | 8 (4.71) | 16 (8.79) | 0.033 ** |
| AF (n, %) | 285 (45.45) | 129 (46.91) | 68 (40) | 88 (48.35) | 0.236 ** |
| History of myocarditis (n, %) | 11 (1.75) | 7 (2.55) | 4 (2.35) | 0 (0) | 0.100 ** |
| T2DM (n, %) | 221 (35.25) | 95 (34.55) | 58 (34.12) | 68 (37.36) | 0.774 ** |
| COPD (n, %) | 95 (15.15) | 48 (17.45) | 33 (19.41) | 14 (7.69) | 0.003 ** |
| Anemia (n, %) | 170 (27.11) | 71 (25.82) | 49 (28.82) | 50 (27.47) | 0.780 ** |
| Prior documented CKD (n, %) | 185 (29.51) | 80 (29.09) | 54 (31.76) | 51 (28.02) | 0.729 ** |
| Dysthyroidism (n, %) | 113 (18.02) | 42 (15.27) | 27 (15.88) | 44 (24.18) | 0.037 ** |
| Length of hospital stay | |||||
| LOS (days, median) | 7 (5–10) | 7 (5–10.5) | 7 (5–9) | 7 (5–9) | 0.111 * |
| ELOS (n, %) | 272 (43.38) | 137 (49.82) | 69 (40.59) | 66 (36.26) | 0.011 ** |
| ELOS (days, median) | 11 (9–14) | 11 (9–13) | 10 (8–13) | 12 (9–14) | 0.945 * |
| Mortality | |||||
| Overall mortality | 95 (15.15) | 49 (17.45) | 25 (14.70) | 23 (12.08) | 0.288 ** |
| In-hospital mortality (n, %) | 32 (5.1) | 13 (4.73) | 11 (6.47) | 8 (4.40) | 0.630 ** |
| 6-months mortality (n, %) | 63 (10.04) | 35 (12.72) | 14 (8.23) | 14 (7.69) | 0.141 ** |
| Parameter | Entire Cohort n = 627 | HFrEF n = 275 | HFmrEF n = 170 | HFpEF n = 182 | p Value |
|---|---|---|---|---|---|
| RDW (fl) | 46.3 (43.1–50.4) | 46.8 (43.9–51.75) | 46.05 (43–49.43) | 45.7 (42.03–50.23) | 0.112 * |
| Creatinine (mg/dL) | 1.01 (0.8–1.29) | 1.05 (0.84–1.33) | 1.02 (0.82–1.29) | 0.94 (0.71–1.2) | 0.004 * |
| eGFR (mL/min/1.73 m2) | 73.68 (54.75–97.09) | 71.56 (54.66–92.25) | 71.63 (52.87–95.87) | 78.24 (58.19–102.59) | 0.056 * |
| RGR | 0.64 (0.47–0.88) | 0.66 (0.5–0.9) | 0.64 (0.47–0.89) | 0.58 (0.43–0.85) | 0.042 * |
| NT-proBNP (pg/mL) | 3.46 (2.82–3.81) | 3.73 (3.39–4.02) | 3.36 (2.66–3.76) | 2.98 (2.59–3.51) | <0.001 * |
| Parameter | Dependent Expected Value (ELOS) | ||
|---|---|---|---|
| Entire Cohort (n = 627) | AUC (95% CI) | p Value | Cut-Off Point |
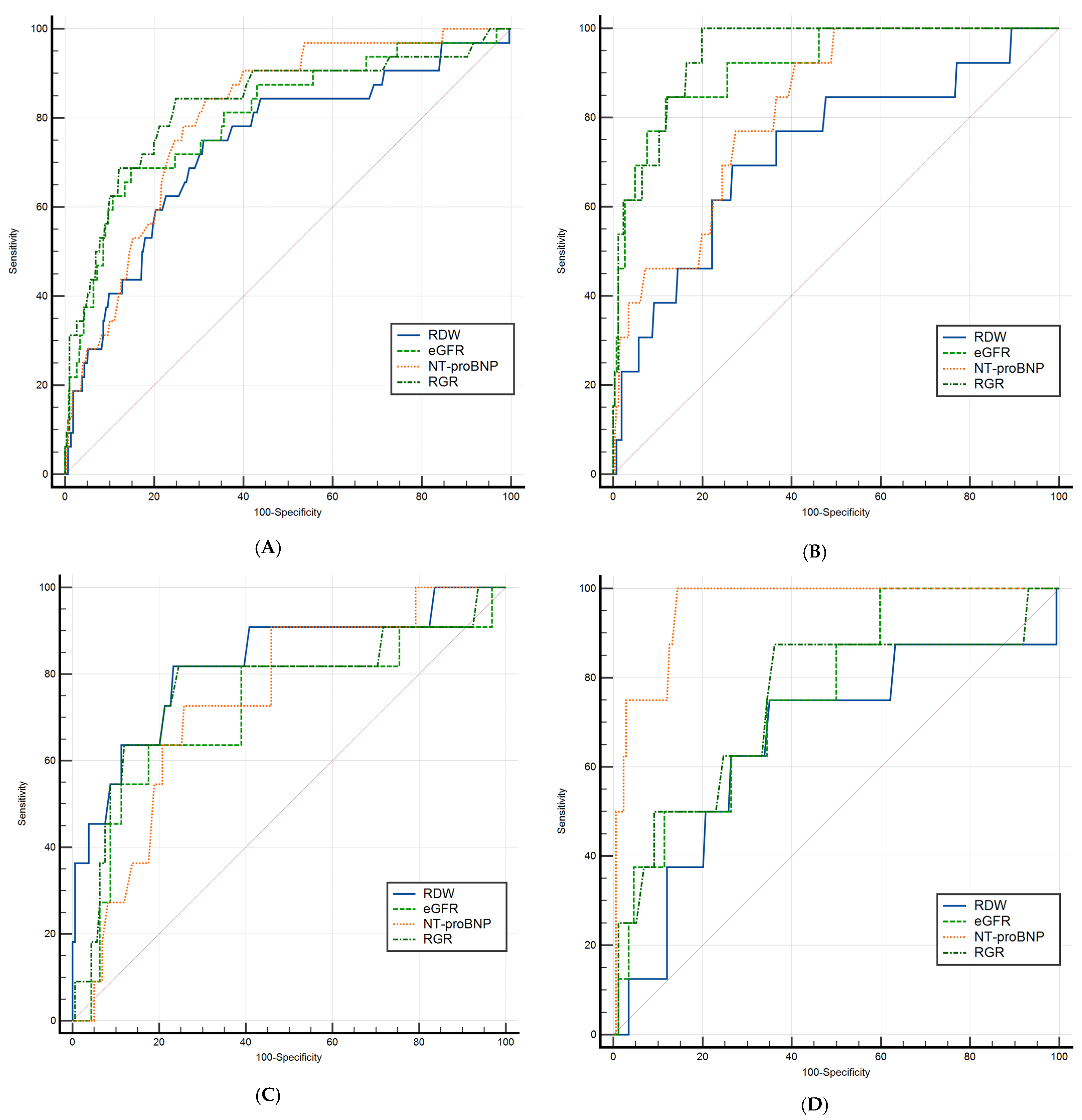
| RDW | 0.603 (0.563–0.641) | <0.001 | >46.6 |
| eGFR | 0.539 (0.499–0.579) | 0.093 | ≤57.5 |
| RGR | 0.570 (0.530–0.609) | 0.002 | >0.92 |
| NT-proBNP | 0.647 (0.608–0.685) | <0.001 | >3.2 |
| HFrEF (n = 275) | |||
| RDW | 0.620 (0.560–0.678) | <0.001 | >45.8 |
| eGFR | 0.503 (0.443–0.564) | 0.927 | ≤81.89 |
| RGR | 0.553 (0.450–0.653) | 0.364 | >0.55 |
| NT-proBNP | 0.623 (0.563–0.680) | <0.001 | >3.79 |
| HFmrEF (n = 170) | |||
| RDW | 0.548 (0.470–0.624) | 0.291 | >47.8 |
| eGFR | 0.525 (0.447–0.602) | 0.586 | ≤50.41 |
| NT-proBNP | 0.624 (0.547–0.697) | 0.004 | >3.32 |
| RGR | 0.537 (0.459–0.614) | 0.422 | >1.01 |
| HFpEF (n = 182) | |||
| RDW | 0.606 (0.531–0.678) | 0.167 | >46.8 |
| eGFR | 0.581 (0.506–0.653) | 0.087 | ≤61.95 |
| RGR | 0.619 (0.545–0.690) | 0.008 | >0.65 |
| NT-proBNP | 0.628 (0.553–0.698) | 0.002 | >2.99 |
| Parameter | Dependent Expected Value (In-Hospital Mortality) | ||
|---|---|---|---|
| Entire Cohort (n = 627) | AUC (95% CI) | p Value | Cut-Off Point |
| RDW | 0.741 (0.705–0.775) | <0.001 | >48.7 |
| eGFR | 0.806 (0.773–0.836) | <0.001 | ≤46.84 |
| RGR | 0.830 (0.798–0.859) | <0.001 | >0.84 |
| NT-proBNP | 0.821 (0.771–0.865) | <0.001 | >3.79 |
| HFrEF (n = 275) | |||
| RDW | 0.727 (0.671–0.779) | 0.004 | >50.4 |
| eGFR | 0.919 (0.881–0.949) | <0.001 | ≤45 |
| RGR | 0.945 (0.910–0.968) | <0.001 | >0.91 |
| NT-proBNP | 0.822 (0.772–0.866) | <0.001 | >3.79 |
| HFmrEF (n = 170) | |||
| RDW | 0.826 (0.760–0.880) | <0.001 | >48.8 |
| eGFR | 0.715 (0.641–0.781) | 0.026 | ≤46.84 |
| RGR | 0.769 (0.699–0.830) | 0.003 | >0.85 |
| NT-proBNP | 0.740 (0.667–0.804) | <0.001 | >3.72 |
| HFpEF (n = 182) | |||
| RDW | 0.662 (0.588–0.730) | 0.161 | >47.6 |
| eGFR | 0.761 (0.692–0.821) | 0.001 | ≤70.09 |
| RGR | 0.746 (0.676–0.808) | 0.023 | >0.67 |
| NT-proBNP | 0.958 (0.918–0.982) | <0.001 | >3.58 |
| Parameter | Entire Cohort n = 627 | HFrEF n = 275 | HFmrEF n = 170 | HFpEF n = 182 | ||||
|---|---|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value | |
| Harrell’s C-Index (95% CI) | Harrell’s C-Index (95% CI) | Harrell’s C-Index (95% CI) | Harrell’s C-Index (95% CI) | |||||
| Age | 1.036 (1.002–1.071) | 0.024 | 1.032 (0.984–1.082) | 0.169 | 1.027 (0.967–1.090) | 0.361 | 1.068 (0.977–1.168) | 0.118 |
| 0.562 (0.444–0.679) | - | - | - | |||||
| Heart rate | 1.015 (1.002–1.028) | 0.028 | 1.009 (0.987–1.031) | 0.413 | 1.020 (0.999–1.041) | 0.069 | 1.023 (0.992–1.054) | 0.171 |
| 0.670 (0.571–0.768) | - | - | - | |||||
| SBP | 0.970 (0.953–0.987) | <0.001 | 0.961 (0.933–0.990) | 0.004 | 0.979 (0.950–1.008) | 0.141 | 0.963 (0.924–1.004) | 0.067 |
| 0.687 (0.566–0.807) | 0.765 (0.584–0.945) | - | - | |||||
| DBP | 0.939 (0.912–0.967) | <0.001 | 0.932 (0.893–0.972) | 0.001 | 0.946 (0.901–0.993) | 0.019 | 0.963 (0.898–1.011) | 0.094 |
| 0.717 (0.603–0.831) | 0.717 (0.487–0.946) | 0.716 (0.594–0.838) | - | |||||
| NYHA-FC | 5.121 (2.500–10.492) | <0.001 | 5.814 (1.720–19.653) | 0.004 | 2.864 (0.495–16.565) | 0.239 | 29.917 (5.727–156.269) | <0.001 |
| 0.697 (0.596–0.798) | 0.752 (0.605–0.899) | - | 0.856 (0.706–1.000) | |||||
| Comorbidities, n | 1.153 (0.931–1.426) | 0.191 | 1.623 (1.145–2.300) | 0.005 | 0.948 (0.661–1.361) | 0.774 | 0.779 (0.475–1.277) | 0.322 |
| - | 0.709 (0.537–0.881) | - | - | |||||
| RDW | 1.066 (1.032–1.102) | <0.001 | 1.077 (1.020–1.137) | 0.006 | 1.137 (1.073–1.203) | <0.001 | 1.006 (0.929–1.090) | 0.872 |
| 0.702 (0.571–0.834) | 0.722 (0.505–0.938) | 0.789 (0.625–0.953) | - | |||||
| eGFR | 0.958 (0.943–0.974) | <0.001 | 0.928 (0.898–0.958) | <0.001 | 0.981 (0.959–1.004) | 0.096 | 0.966 (0.936–0.997) | 0.036 |
| 0.792 (0.693–0.892) | 0.900 (0.810–0.991) | - | 0.756 (0.552–0.959) | |||||
| RGR | 1.703 (1.485–1.953) | <0.001 | 1.673 (1.410–1.984) | <0.001 | 2.700 (1.235–5.902) | 0.030 | 2.527 (1.206–5.298) | 0.046 |
| 0.809 (0.696–0.922) | 0.931 (0.877–0.984) | 0.758 (0.594–0.923) | 0.689 (0.368–1.000) | |||||
| NT-proBNP | 8.750 (3.326–23.018) | <0.001 | 33.739 (4.632–245.753) | <0.001 | 2.477 (0.724–8.472) | 0.096 | 108.181 (11.561–1012.243) | <0.001 |
| 0.791 (0.725–0.858) | 0.804 (0.700–0.909) | - | 0.954 (0.900–1.000) | |||||
| 0.809 (0.696–0.922) | 0.931 (0.877–0.984) | 0.758 (0.594–0.923) | 0.689 (0.368–1.000) | |||||
| Parameter | Entire Cohort n = 627 | HFrEF n = 275 | HFmrEF n = 170 | HFpEF n = 182 | ||||
|---|---|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value | |
| Age | 1.044 (1.010–1.079) | 0.010 | - | - | - | - | - | - |
| Heart rate | - | - | - | - | - | - | - | - |
| SBP | - | - | - | - | - | - | - | - |
| DBP | - | - | - | - | - | - | - | - |
| NYHA-FC | 3.050 (1.445–6.438) | 0.003 | - | - | - | - | 17.521 (2.404–127.660) | 0.004 |
| Comorbidities, n | - | - | 1.946 (1.300–2.912) | 0.001 | - | - | - | - |
| RDW | - | - | - | - | 1.137 (1.073–1.203) | <0.001 | - | - |
| eGFR | - | - | - | - | - | - | - | - |
| RGR | 1.661 (1.391–1.984) | <0.001 | 1.958 (1.502–2.552) | <0.001 | - | - | - | - |
| NT-proBNP | 4.200 (1.533–11.503) | 0.005 | 28.510 (3.641–223.201) | 0.001 | - | - | 342.596 (12.203–9618.118) | <0.001 |
| Model’s overall model fit | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| Model’s Harrell’s C-index | 0.847 (0.768–0.927) | 0.963 (0.942–0.985) | 0.789 (0.625–0.953) | 0.979 (0.966–0.992) | ||||
| Parameter | Dependent Expected Value (6-Month All-Cause Mortality) | ||
|---|---|---|---|
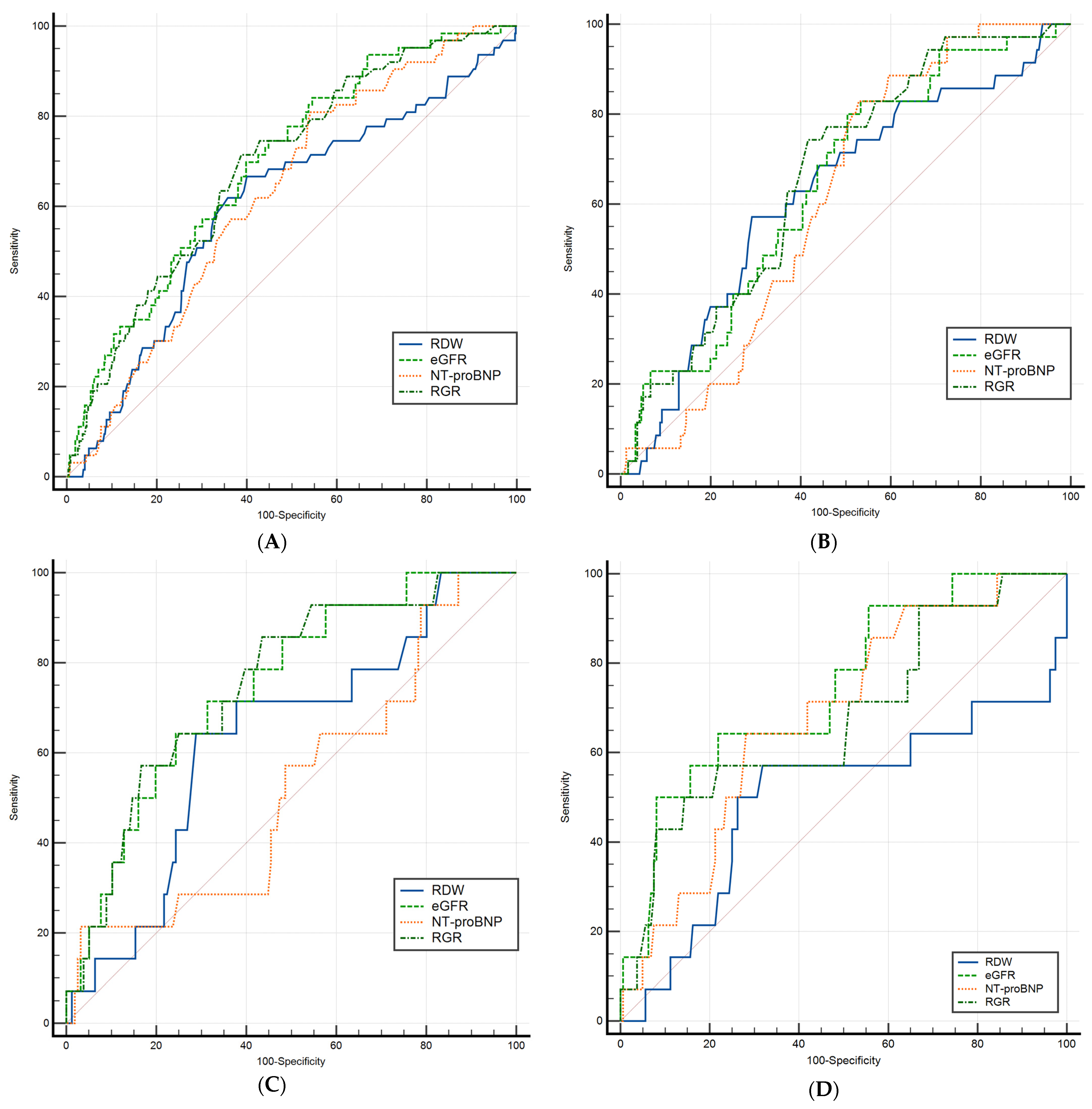
| Entire Cohort (n = 595) | AUC (95% CI) | p Value | Cut-Off Point |
| RDW | 0.619 (0.579–0.658) | 0.002 | >47.4 |
| eGFR | 0.715 (0.677–0.751) | <0.001 | ≤69.84 |
| NT-proBNP (pg/mL) | 0.649 (0.610–0.688) | 0.001 | >3.34 |
| RGR | 0.713 (0.675–0.749) | <0.001 | >0.69 |
| HFrEF (n = 262) | |||
| RDW | 0.639 (0.578–0.697) | 0.006 | >49.5 |
| eGFR | 0.671 (0.610–0.727) | <0.001 | ≤75.29 |
| NT-proBNP (pg/mL) | 0.631 (0.570–0.690) | 0.001 | >3.64 |
| RGR | 0.694 (0.634–0.749) | <0.001 | >0.69 |
| HFmrEF (n = 159) | |||
| RDW | 0.660 (0.581–0.733) | 0.040 | >48.1 |
| eGFR | 0.767 (0.693–0.830) | <0.001 | ≤55.19 |
| NT-proBNP (pg/mL) | 0.537 (0.457–0.617) | 0.651 | >4.01 |
| RGR | 0.777 (0.704–0.839) | <0.001 | >0.66 |
| HFpEF (n = 174) | |||
| RDW | 0.501 (0.424–0.577) | 0.994 | >47.9 |
| eGFR | 0.747 (0.675–0.809) | <0.001 | ≤58.45 |
| NT-proBNP (pg/mL) | 0.683 (0.608–0.751) | 0.008 | >3.32 |
| RGR | 0.678 (0.603–0.747) | 0.031 | >0.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varga, A.; Cristescu, L.; Marusteri, M.-S.; Mares, R.G.; Iancu, D.-G.; Suteu, R.A.; Tilinca, R.-M.; Tilea, I. Prognostic Value of the Red Cell Distribution Width-to-eGFR Ratio (RGR) Across Chronic Heart Failure Phenotypes: A Retrospective Observational Pilot Study. J. Clin. Med. 2025, 14, 2852. https://doi.org/10.3390/jcm14082852
Varga A, Cristescu L, Marusteri M-S, Mares RG, Iancu D-G, Suteu RA, Tilinca R-M, Tilea I. Prognostic Value of the Red Cell Distribution Width-to-eGFR Ratio (RGR) Across Chronic Heart Failure Phenotypes: A Retrospective Observational Pilot Study. Journal of Clinical Medicine. 2025; 14(8):2852. https://doi.org/10.3390/jcm14082852
Chicago/Turabian StyleVarga, Andreea, Liviu Cristescu, Marius-Stefan Marusteri, Razvan Gheorghita Mares, Dragos-Gabriel Iancu, Radu Adrian Suteu, Raluca-Maria Tilinca, and Ioan Tilea. 2025. "Prognostic Value of the Red Cell Distribution Width-to-eGFR Ratio (RGR) Across Chronic Heart Failure Phenotypes: A Retrospective Observational Pilot Study" Journal of Clinical Medicine 14, no. 8: 2852. https://doi.org/10.3390/jcm14082852
APA StyleVarga, A., Cristescu, L., Marusteri, M.-S., Mares, R. G., Iancu, D.-G., Suteu, R. A., Tilinca, R.-M., & Tilea, I. (2025). Prognostic Value of the Red Cell Distribution Width-to-eGFR Ratio (RGR) Across Chronic Heart Failure Phenotypes: A Retrospective Observational Pilot Study. Journal of Clinical Medicine, 14(8), 2852. https://doi.org/10.3390/jcm14082852








