Thalamic Microstructural Alterations as Revealed by the T1/T2 Ratio in Chronic Pain Patients
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics
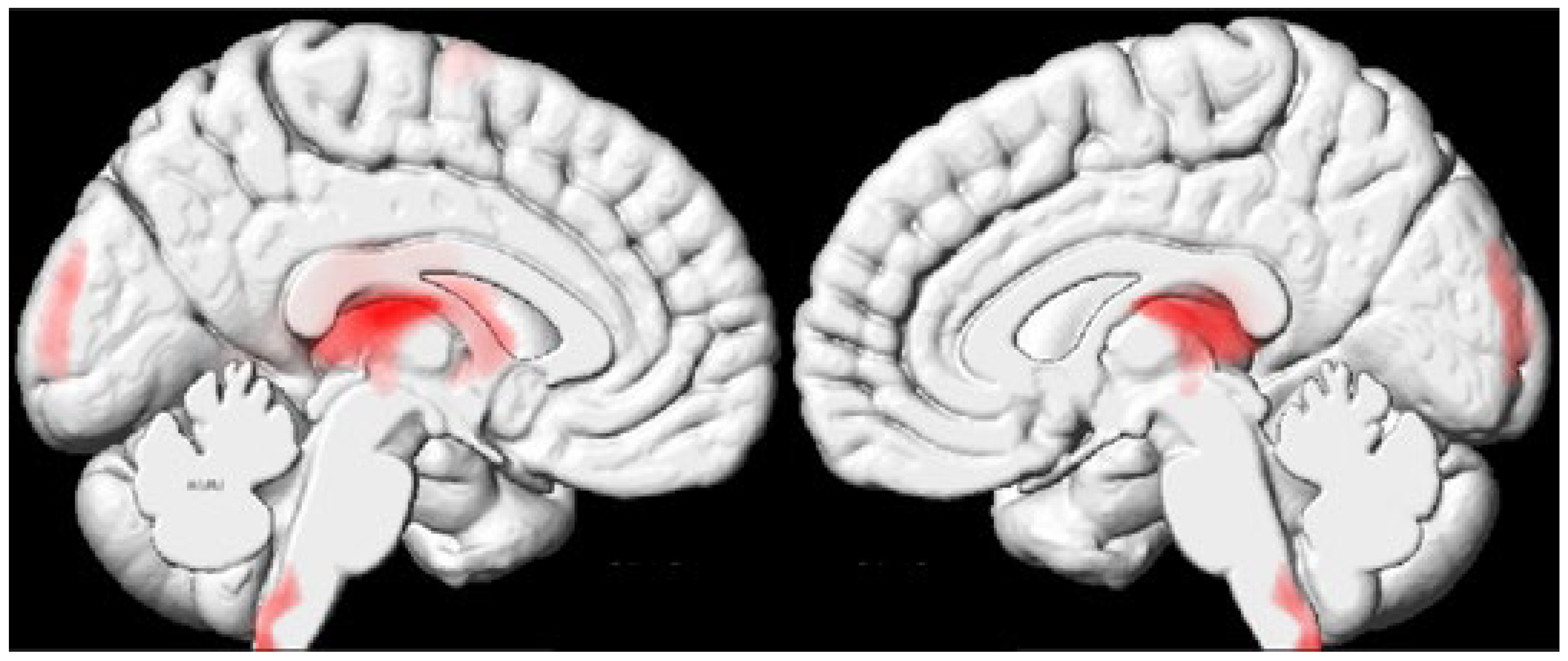
2.2. Whole-Brain Analysis Results
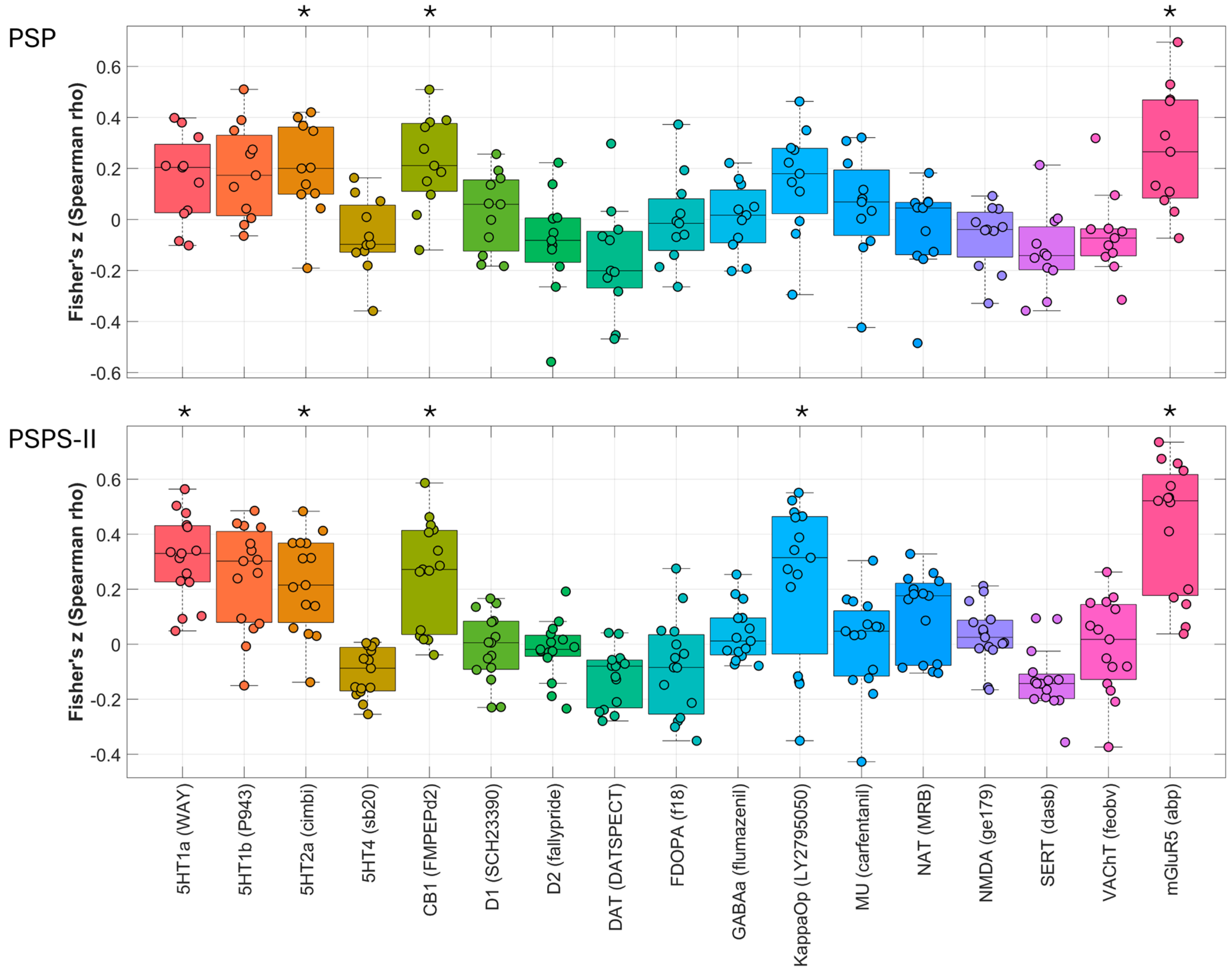
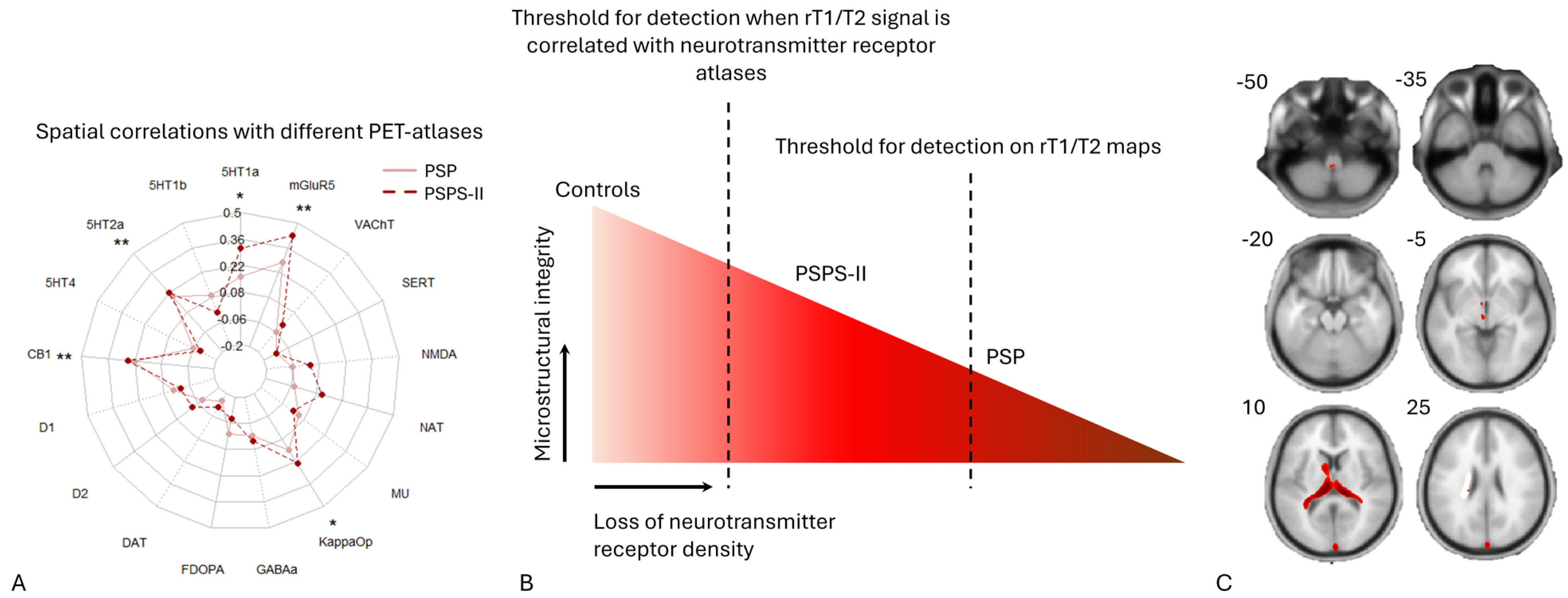
2.3. Spatial Correlations with Neurotransmitter Maps
3. Discussion
3.1. Decreased Microstructural Integrity of the Thalamus
3.2. Evidence for Microstructural Alterations in the Dorsal Cervicomedullary Junction
3.3. Evidence for Hypothetical Microstructural Changes in PSPS-II Patients
3.4. Spatial Coupling of Microstructural Alterations with Neurotransmitter Systems
3.5. Strengths and Limitations
4. Materials and Methods
4.1. Ethics Approval
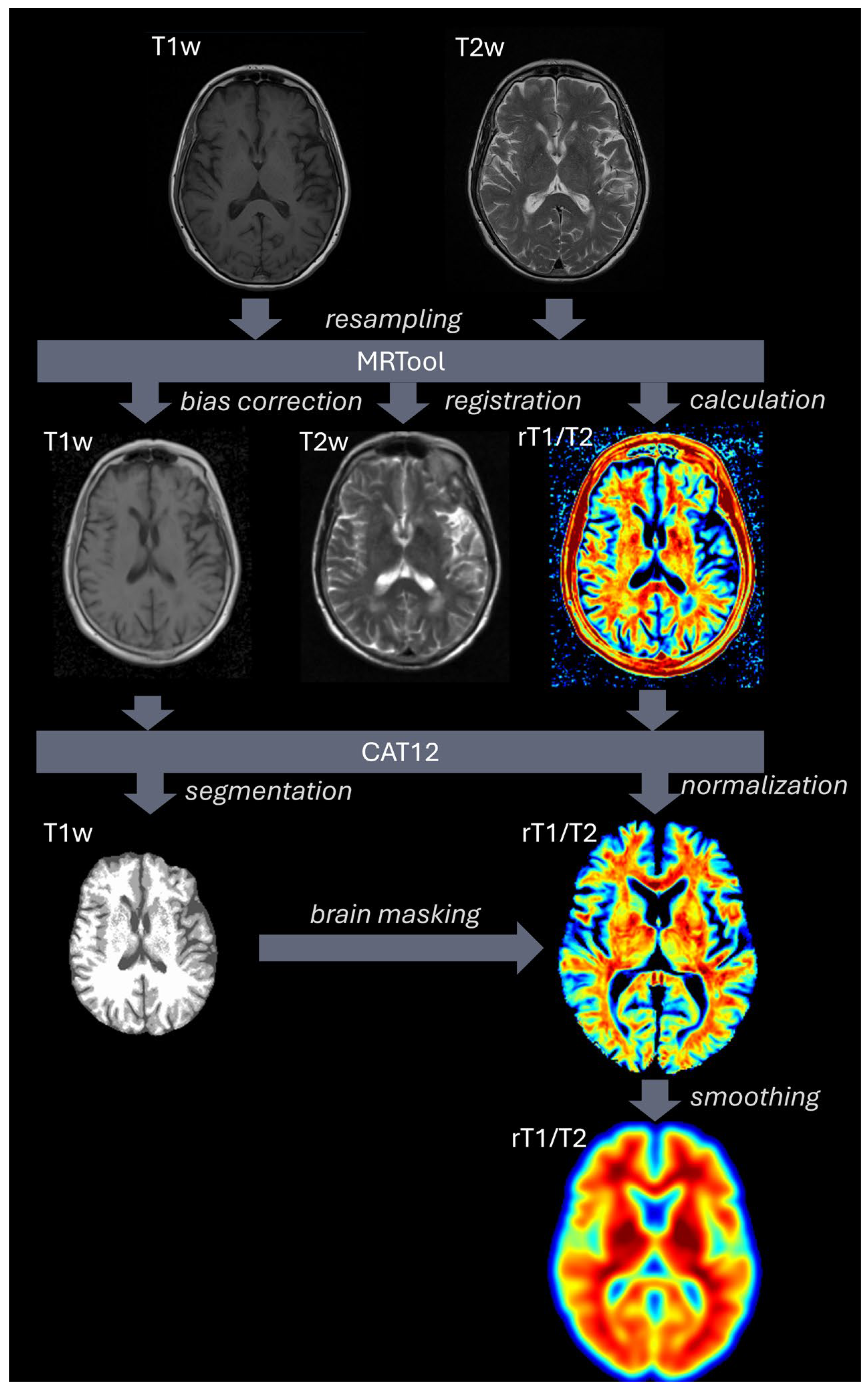
4.2. Data and MRI Processing
4.3. Spatial Correlation of rT1/T2 Signal with Neurotransmitter Maps
4.4. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fayaz, A.; Croft, P.; Langford, R.M.; Donaldson, L.J.; Jones, G.T. Prevalence of chronic pain in the UK: A systematic review and meta-analysis of population studies. BMJ Open 2016, 6, e010364. [Google Scholar] [CrossRef] [PubMed]
- Rikard, S.M.; Strahan, A.E.; Schmit, K.M.; Guy, G.P., Jr. Chronic Pain Among Adults—United States, 2019–2021. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Sá, K.N.; Moreira, L.; Baptista, A.F.; Yeng, L.T.; Teixeira, M.J.; Galhardoni, R.; de Andrade, D.C. Prevalence of chronic pain in developing countries: Systematic review and meta-analysis. Pain Rep. 2019, 4, e779. [Google Scholar] [CrossRef] [PubMed]
- Dydyk, A.M.; Conermann, T. Chronic Pain. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2024. [Google Scholar]
- Mangnus, T.J.P.; Bharwani, K.D.; Dirckx, M.; Huygen, F. From a Symptom-Based to a Mechanism-Based Pharmacotherapeutic Treatment in Complex Regional Pain Syndrome. Drugs 2022, 82, 511–531. [Google Scholar] [CrossRef]
- Tracey, I. Neuroimaging enters the pain biomarker arena. Sci. Transl. Med. 2021, 13, eabj7358. [Google Scholar] [CrossRef]
- van der Miesen, M.M.; Lindquist, M.A.; Wager, T.D. Neuroimaging-based biomarkers for pain: State of the field and current directions. Pain Rep. 2019, 4, e751. [Google Scholar] [CrossRef]
- Park, J.G.; Hong, B.Y.; Park, H.Y.; Yoo, Y.J.; Yoon, M.J.; Kim, J.S.; Lim, S.H. Alteration of White Matter in Patients with Central Post-Stroke Pain. J. Pers. Med. 2021, 11, 417. [Google Scholar] [CrossRef]
- Lee, S.N.; Woo, S.H.; Lee, E.J.; Kim, K.K.; Kim, H.R. Association between T1w/T2w ratio in white matter and cognitive function in Alzheimer’s disease. Sci. Rep. 2024, 14, 7228. [Google Scholar] [CrossRef]
- Pelkmans, W.; Dicks, E.; Barkhof, F.; Vrenken, H.; Scheltens, P.; van der Flier, W.M.; Tijms, B.M. Gray matter T1-w/T2-w ratios are higher in Alzheimer’s disease. Hum. Brain Mapp. 2019, 40, 3900–3909. [Google Scholar] [CrossRef]
- Iwatani, J.; Ishida, T.; Donishi, T.; Ukai, S.; Shinosaki, K.; Terada, M.; Kaneoke, Y. Use of T1-weighted/T2-weighted magnetic resonance ratio images to elucidate changes in the schizophrenic brain. Brain Behav. 2015, 5, e00399. [Google Scholar] [CrossRef]
- Yamamoto, S.; Sanada, T.; Sakai, M.; Arisawa, A.; Kagawa, N.; Shimosegawa, E.; Nakanishi, K.; Kanemura, Y.; Kinoshita, M.; Kishima, H. Prediction and Visualization of Non-Enhancing Tumor in Glioblastoma via T1w/T2w-Ratio Map. Brain Sci. 2022, 12, 99. [Google Scholar] [CrossRef] [PubMed]
- Sanada, T.; Yamamoto, S.; Sakai, M.; Umehara, T.; Sato, H.; Saito, M.; Mitsui, N.; Hiroshima, S.; Anei, R.; Kanemura, Y.; et al. Correlation of T1- to T2-weighted signal intensity ratio with T1- and T2-relaxation time and IDH mutation status in glioma. Sci. Rep. 2022, 12, 18801. [Google Scholar] [CrossRef] [PubMed]
- Rowley, C.D.; Tabrizi, S.J.; Scahill, R.I.; Leavitt, B.R.; Roos, R.A.C.; Durr, A.; Bock, N.A. Altered Intracortical T1-Weighted/T2-Weighted Ratio Signal in Huntington’s Disease. Front. Neurosci. 2018, 12, 805. [Google Scholar] [CrossRef] [PubMed]
- Glasser, M.F.; Van Essen, D.C. Mapping human cortical areas in vivo based on myelin content as revealed by T1- and T2-weighted MRI. J. Neurosci. 2011, 31, 11597–11616. [Google Scholar] [CrossRef]
- Preziosa, P.; Bouman, P.M.; Kiljan, S.; Steenwijk, M.D.; Meani, A.; Pouwels, P.J.; Rocca, M.A.; Filippi, M.; Geurts, J.J.G.; Jonkman, L.E. Neurite density explains cortical T1-weighted/T2-weighted ratio in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2021, 92, 790–792. [Google Scholar] [CrossRef]
- Righart, R.; Biberacher, V.; Jonkman, L.E.; Klaver, R.; Schmidt, P.; Buck, D.; Berthele, A.; Kirschke, J.S.; Zimmer, C.; Hemmer, B.; et al. Cortical pathology in multiple sclerosis detected by the T1/T2-weighted ratio from routine magnetic resonance imaging. Ann. Neurol. 2017, 82, 519–529. [Google Scholar] [CrossRef]
- Sandrone, S.; Aiello, M.; Cavaliere, C.; Thiebaut de Schotten, M.; Reimann, K.; Troakes, C.; Bodi, I.; Lacerda, L.; Monti, S.; Murphy, D.; et al. Mapping myelin in white matter with T1-weighted/T2-weighted maps: Discrepancy with histology and other myelin MRI measures. Brain Struct. Funct. 2023, 228, 525–535. [Google Scholar] [CrossRef]
- Ganzetti, M.; Wenderoth, N.; Mantini, D. Whole brain myelin mapping using T1- and T2-weighted MR imaging data. Front. Hum. Neurosci. 2014, 8, 671. [Google Scholar] [CrossRef]
- Dusek, P.; Dezortova, M.; Wuerfel, J. Imaging of iron. Int. Rev. Neurobiol. 2013, 110, 195–239. [Google Scholar]
- Lim, K.O.; Helpern, J.A. Neuropsychiatric applications of DTI—A review. NMR Biomed. 2002, 15, 587–593. [Google Scholar] [CrossRef]
- George, K. JMD Neuroanatomy, Thalamocortical Radiations. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2024. [Google Scholar]
- Witkam, R.L.; Burmeister, L.S.; Van Goethem, J.W.M.; van der Kolk, A.G.; Vissers, K.C.P.; Henssen, D.J.H.A. Microstructural Changes in the Spinothalamic Tract of CPSS Patients: Preliminary Results from a Single-Center Diffusion-Weighted Magnetic Resonance Imaging Study. Brain Sci. 2023, 13, 1370. [Google Scholar] [CrossRef] [PubMed]
- Torrado-Carvajal, A.; Toschi, N.; Albrecht, D.S.; Chang, K.; Akeju, O.; Kim, M.; Edwards, R.R.; Zhang, Y.; Hooker, J.M.; Duggento, A.; et al. Thalamic neuroinflammation as a reproducible and discriminating signature for chronic low back pain. Pain 2021, 162, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Amici, S.A.; Dong, J.; Guerau-de-Arellano, M. Molecular Mechanisms Modulating the Phenotype of Macrophages and Microglia. Front. Immunol. 2017, 8, 1520. [Google Scholar] [CrossRef]
- Urrutia, P.J.; Bórquez, D.A.; Núñez, M.T. Inflaming the Brain with Iron. Antioxidants 2021, 10, 61. [Google Scholar] [CrossRef]
- Cogswell, P.M.; Fan, A.P. Multimodal comparisons of QSM and PET in neurodegeneration and aging. Neuroimage 2023, 273, 120068. [Google Scholar] [CrossRef]
- Yen, C.T.; Lu, P.L. Thalamus and pain. Acta Anaesthesiol. Taiwan 2013, 51, 73–80. [Google Scholar] [CrossRef]
- De Ridder, D.; Adhia, D.; Vanneste, S. The anatomy of pain and suffering in the brain and its clinical implications. Neurosci. Biobehav. Rev. 2021, 130, 125–146. [Google Scholar] [CrossRef]
- Tunks, E.R.; Crook, J.; Weir, R. Epidemiology of chronic pain with psychological comorbidity: Prevalence, risk, course, and prognosis. Can. J. Psychiatry 2008, 53, 224–234. [Google Scholar] [CrossRef]
- Han, K.M.; De Berardis, D.; Fornaro, M.; Kim, Y.K. Differentiating between bipolar and unipolar depression in functional and structural MRI studies. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 91, 20–27. [Google Scholar] [CrossRef]
- Baranger, D.A.A.; Halchenko, Y.O.; Satz, S.; Ragozzino, R.; Iyengar, S.; Swartz, H.A.; Manelis, A. Aberrant levels of cortical myelin distinguish individuals with depressive disorders from healthy controls. Neuroimage Clin. 2021, 32, 102790. [Google Scholar] [CrossRef]
- Alper, J.; Seifert, A.C.; Verma, G.; Huang, K.H.; Jacob, Y.; Al Qadi, A.; Rutland, J.W.; Patel, S.; Bederson, J.; Shrivastava, R.K.; et al. Leveraging high-resolution 7-tesla MRI to derive quantitative metrics for the trigeminal nerve and subnuclei of limbic structures in trigeminal neuralgia. J. Headache Pain 2021, 22, 112. [Google Scholar] [CrossRef] [PubMed]
- Henssen, D.; Dijk, J.; Knepflé, R.; Sieffers, M.; Winter, A.; Vissers, K. Alterations in grey matter density and functional connectivity in trigeminal neuropathic pain and trigeminal neuralgia: A systematic review and meta-analysis. Neuroimage Clin. 2019, 24, 102039. [Google Scholar] [CrossRef] [PubMed]
- Froesel, M.; Cappe, C.; Ben Hamed, S. A multisensory perspective onto primate pulvinar functions. Neurosci. Biobehav. Rev. 2021, 125, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Chalil, A.; Santyr, B.G.; Abbass, M.; Lau, J.C.; Staudt, M.D. Spinal Nerve Root Stimulation for Chronic Pain: A Systematic Review. Neuromodulation 2024, 27, 36–46. [Google Scholar] [CrossRef]
- Krames, E.S.; Foreman, R. Spinal Cord Stimulation Modulates Visceral Nociception and Hyperalgesia via the Spinothalamic Tracts and the Postsynaptic Dorsal Column Pathways: A Literature Review and Hypothesis. Neuromodulation 2007, 10, 224–237. [Google Scholar] [CrossRef]
- Connell, L.A.; Lincoln, N.B.; Radford, K.A. Somatosensory impairment after stroke: Frequency of different deficits and their recovery. Clin. Rehabil. 2008, 22, 758–767. [Google Scholar] [CrossRef]
- Dong, D.; Hosomi, K.; Mori, N.; Kamijo, Y.I.; Furotani, Y.; Yamagami, D.; Ohnishi, Y.-I.; Watanabe, Y.; Nakamura, T.; Tajima, F.; et al. White matter microstructural alterations in patients with neuropathic pain after spinal cord injury: A diffusion tensor imaging study. Front. Neurol. 2023, 14, 1241658. [Google Scholar] [CrossRef]
- Guo, Y.; Gao, F.; Liu, Y.; Guo, H.; Yu, W.; Chen, Z.; Yang, M.; Du, L.; Yang, D.; Li, J. White Matter Microstructure Alterations in Patients With Spinal Cord Injury Assessed by Diffusion Tensor Imaging. Front. Hum. Neurosci. 2019, 13, 11. [Google Scholar] [CrossRef]
- Ramaswamy, S.; Wodehouse, T.; Langford, R.; Thomson, S.; Taylor, R.; Mehta, V. Characterizing the Somatosensory Profile of Patients With Failed Back Surgery Syndrome With Unilateral Lumbar Radiculopathy Undergoing Spinal Cord Stimulation: A Single Center Prospective Pilot Study. Neuromodulation 2019, 22, 333–340. [Google Scholar] [CrossRef]
- Nijs, J.; Kosek, E.; Chiarotto, A.; Cook, C.; Danneels, L.A.; Fernández-de-Las-Peñas, C.; Hodges, P.W.; Koes, B.; Louw, A.; Ostelo, R.; et al. Nociceptive, neuropathic, or nociplastic low back pain? The low back pain phenotyping (BACPAP) consortium’s international and multidisciplinary consensus recommendations. Lancet Rheumatol. 2024, 6, e178–e188. [Google Scholar] [CrossRef]
- Milligan, A.L.; Szabo-Pardi, T.A.; Burton, M.D. Cannabinoid Receptor Type 1 and Its Role as an Analgesic: An Opioid Alternative? J. Dual Diagn. 2020, 16, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Shi, W.; Liu, W.; Chen, Q.Y.; Zhuo, M. Multiple modulatory roles of serotonin in chronic pain and injury-related anxiety. Front. Synaptic Neurosci. 2023, 15, 1122381. [Google Scholar] [CrossRef] [PubMed]
- Chiechio, S. Modulation of Chronic Pain by Metabotropic Glutamate Receptors. Adv. Pharmacol. 2016, 75, 63–89. [Google Scholar] [PubMed]
- Ito, H.; Navratilova, E.; Vagnerova, B.; Watanabe, M.; Kopruszinski, C.; Moreira de Souza, L.H.; Yue, X.; Ikegami, D.; Moutal, A.; Patwardhan, A.; et al. Chronic pain recruits hypothalamic dynorphin/kappa opioid receptor signalling to promote wakefulness and vigilance. Brain 2023, 146, 1186–1199. [Google Scholar] [CrossRef]
- Ganzetti, M.; Wenderoth, N.; Mantini, D. Mapping pathological changes in brain structure by combining T1- and T2-weighted MR imaging data. Neuroradiology 2015, 57, 917–928. [Google Scholar] [CrossRef]
- Gaser, C.; Dahnke, R.; Thompson, P.M.; Kurth, F.; Luders, E.; Alzheimer’s Disease Neuroimaging Initiative. CAT—A Computational Anatomy Toolbox for the Analysis of Structural MRI Data. bioRxiv 2023. [Google Scholar] [CrossRef]
- Ashburner, J. A fast diffeomorphic image registration algorithm. Neuroimage 2007, 38, 95–113. [Google Scholar] [CrossRef]
- Klein, A.; Andersson, J.; Ardekani, B.A.; Ashburner, J.; Avants, B.; Chiang, M.C.; Christensen, G.E.; Collins, D.L.; Gee, J.; Hellier, P.; et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage 2009, 46, 786–802. [Google Scholar] [CrossRef]
- Xia, M.; Wang, J.; He, Y. BrainNet Viewer: A network visualization tool for human brain connectomics. PLoS ONE 2013, 8, e68910. [Google Scholar] [CrossRef]
- Kiebel, S.J.; Kherif, A.P.; Holmes, C. Chapter 8—The General Linear Model. In Statistical Parametric Mapping; Friston, K., Ashburner, J., Kiebel, S., Nichols, T., Penny, W., Eds.; Academic Press: London, UK, 2007; pp. 101–125. [Google Scholar]
- Poline, J.; Kherif, F.; Pallier, C.; Penny, W. Chapter 9—Contrasts and Classical Inference. In Statistical Parametric Mapping; Friston, K., Ashburner, J., Kiebel, S., Nichols, T., Penny, W., Eds.; Academic Press: London, UK, 2007; pp. 126–139. [Google Scholar]
| Group | Sample Size (n) | Age (at Onset) in Years | Number of Males/Females |
|---|---|---|---|
| PSP | 11 | 64.3 (± 12.5) | 4/7 |
| PSPS-II | 15 | 57.9 (± 9.7) | 8/7 |
| Controls | 18 | 60.5 (± 13.0) | 9/9 |
| Cluster | Peak MNI | Centre of Gravity MNI | Number of Voxels | Best Corresponding Location | ||||
|---|---|---|---|---|---|---|---|---|
| x | y | z | x | y | z | |||
| 1 | −3 | −47 | −57 | −1 | −47 | −61 | 1138 | Cervicomedullary junction/caudal medulla (bilateral) |
| 2 | −10 | −20 | 14 | −8 | −23 | 12 | 17533 | Thalamus (bilateral) and caudate nucleus (left) |
| 3 | 3 | −100 | 5 | 2 | −97 | 13 | 1522 | Occipital lobe/cuneus (bilateral) |
| 4 | −28 | −4 | 71 | −27 | 0 | 73 | 1333 | Superior frontal gyrus (left) |
| Receptor Atlas | PSPS-II | PSP | ||
|---|---|---|---|---|
| Mean Fisher’s z (Spearman’s rho) | FDR-Corrected p-Value | Mean Fisher’s z (Spearman’s rho) | FDR-Corrected p-Value | |
| 5HT1a | 0.3116 | 1.70 × 10−3 | 0.1581 | 0.117572 |
| 5HT1b | −0.0045 | 0.94431 | 0.0886 | 0.33736 |
| 5HT2a | 0.221 | 1.70 × 10−3 | 0.1933 | 0.027197 |
| 5HT4 | −0.1018 | 0.1491 | −0.0646 | 0.539189 |
| CB1 | 0.2537 | 0.00255 | 0.2234 | 0.002833 |
| D1 | −0.0138 | 0.94431 | 0.0264 | 0.853679 |
| D2 | −0.0221 | 0.853679 | −0.0901 | 0.456083 |
| DAT | −0.12 | 0.113424 | −0.1545 | 0.125525 |
| FDOPA | −0.0853 | 0.349784 | −0.0048 | 0.94431 |
| GABAa | 0.0373 | 0.652741 | 0.0049 | 0.94431 |
| KappaOp | 0.2339 | 0.00272 | 0.1512 | 0.080573 |
| MU | 0.0078 | 0.94431 | 0.0483 | 0.739996 |
| NAT | 0.1068 | 0.229054 | −0.0436 | 0.853679 |
| NMDA | 0.0328 | 0.798782 | −0.0656 | 0.635897 |
| SERT | −0.13 | 0.067237 | −0.1257 | 0.162957 |
| VAChT | −0.0073 | 0.94431 | −0.06 | 0.643311 |
| mGluR5 | 0.4265 | 0.002266 | 0.2749 | 0.033572 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Grinsven, M.; Witkam, R.; Kurt, E.; Özkan, S.; van der Kolk, A.; Vissers, K.; Henssen, D. Thalamic Microstructural Alterations as Revealed by the T1/T2 Ratio in Chronic Pain Patients. J. Clin. Med. 2025, 14, 2888. https://doi.org/10.3390/jcm14092888
van Grinsven M, Witkam R, Kurt E, Özkan S, van der Kolk A, Vissers K, Henssen D. Thalamic Microstructural Alterations as Revealed by the T1/T2 Ratio in Chronic Pain Patients. Journal of Clinical Medicine. 2025; 14(9):2888. https://doi.org/10.3390/jcm14092888
Chicago/Turabian Stylevan Grinsven, Max, Richard Witkam, Erkan Kurt, Sezai Özkan, Anja van der Kolk, Kris Vissers, and Dylan Henssen. 2025. "Thalamic Microstructural Alterations as Revealed by the T1/T2 Ratio in Chronic Pain Patients" Journal of Clinical Medicine 14, no. 9: 2888. https://doi.org/10.3390/jcm14092888
APA Stylevan Grinsven, M., Witkam, R., Kurt, E., Özkan, S., van der Kolk, A., Vissers, K., & Henssen, D. (2025). Thalamic Microstructural Alterations as Revealed by the T1/T2 Ratio in Chronic Pain Patients. Journal of Clinical Medicine, 14(9), 2888. https://doi.org/10.3390/jcm14092888






