Improving the Reliability of Muscle Tissue Characterization Post-Stroke: A Secondary Statistical Analysis of Echotexture Features
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants and Data Collection
2.3. Echotexture Feature Extraction
2.4. Ultrasound Imaging Assessment
2.5. Statistical Analysis
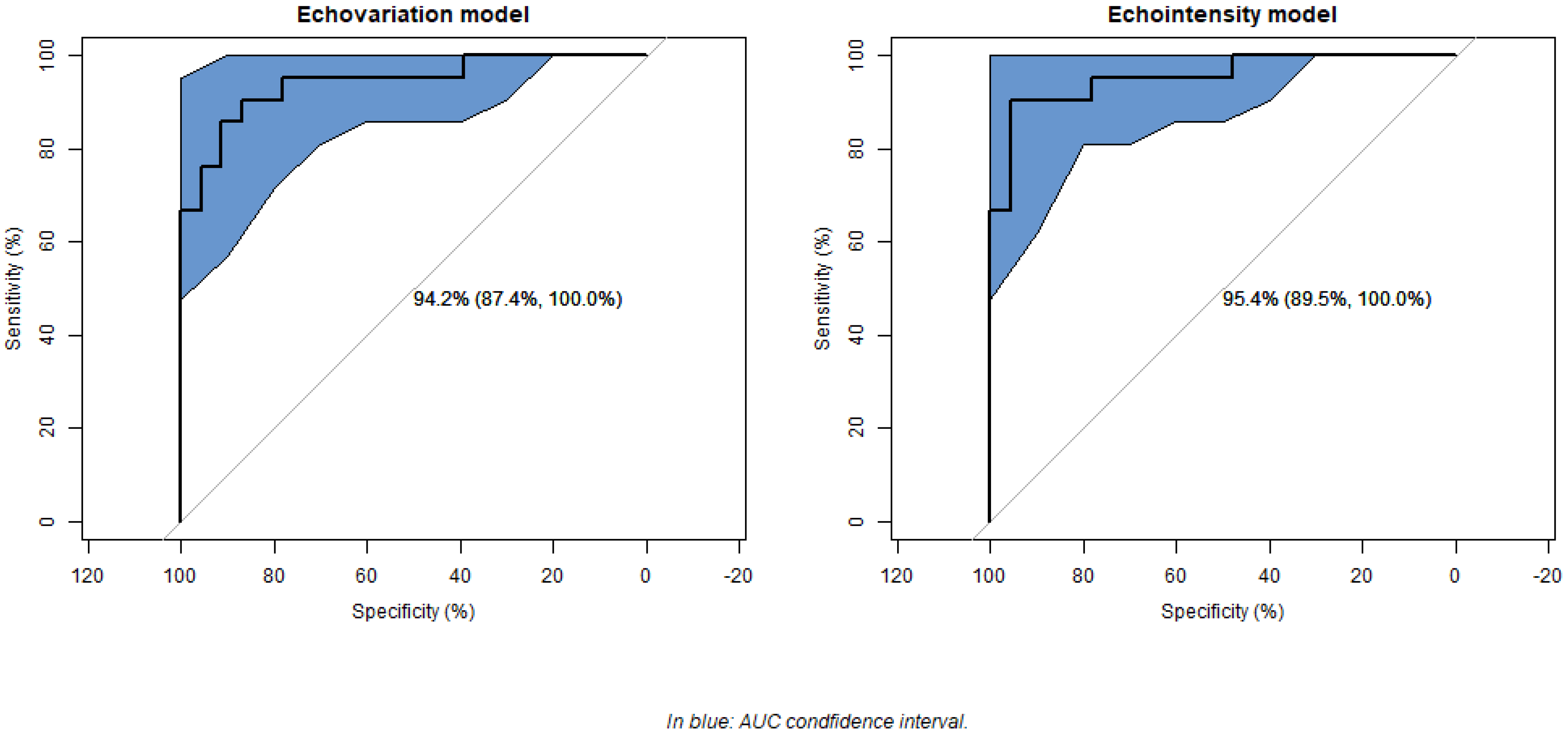
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dougherty, G. Medical Image Processing: Techniques and Applications; Biological and Medical Physics, Biomedical Engineering; Springer: New York, NY, USA, 2011; ISBN 978-1-4419-9769-2. [Google Scholar]
- Chowdhary, C.L.; Acharjya, D.P. Segmentation and Feature Extraction in Medical Imaging: A Systematic Review. Procedia Comput. Sci. 2020, 167, 26–36. [Google Scholar] [CrossRef]
- Ker, J.; Wang, L.; Rao, J.; Lim, T. Deep Learning Applications in Medical Image Analysis. IEEE Access 2017, 6, 9375–9379. [Google Scholar] [CrossRef]
- Hilbert, A.; Ramos, L.A.; van Os, H.J.A.; Olabarriaga, S.D.; Tolhuisen, M.L.; Wermer, M.J.H.; Barros, R.S.; van der Schaaf, I.; Dippel, D.; Roos, Y.B.W.E.M.; et al. Data-Efficient Deep Learning of Radiological Image Data for Outcome Prediction after Endovascular Treatment of Patients with Acute Ischemic Stroke. Comput. Biol. Med. 2019, 115, 103516. [Google Scholar] [CrossRef]
- Kazerouni, A.S.; Gadde, M.; Gardner, A.; Hormuth, D.A.; Jarrett, A.M.; Johnson, K.E.; Lima, E.A.B.F.; Lorenzo, G.; Phillips, C.; Brock, A.; et al. Integrating Quantitative Assays with Biologically Based Mathematical Modeling for Predictive Oncology. iScience 2020, 23, 101807. [Google Scholar] [CrossRef]
- Niederer, S.A.; Lumens, J.; Trayanova, N.A. Computational Models in Cardiology. Nat. Rev. Cardiol. 2019, 16, 100. [Google Scholar] [CrossRef]
- Carlier, P.G.; Marty, B.; Scheidegger, O.; Loureiro De Sousa, P.; Baudin, P.Y.; Snezhko, E.; Vlodavets, D. Skeletal Muscle Quantitative Nuclear Magnetic Resonance Imaging and Spectroscopy as an Outcome Measure for Clinical Trials. J. Neuromuscul. Dis. 2016, 3, 1–28. [Google Scholar] [CrossRef]
- Kamiya, N. Deep Learning Technique for Musculoskeletal Analysis. Adv. Exp. Med. Biol. 2020, 1213, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Paris, M.T.; Mourtzakis, M. Muscle Composition Analysis of Ultrasound Images: A Narrative Review of Texture Analysis. Ultrasound Med. Biol. 2021, 47, 880–895. [Google Scholar] [CrossRef]
- Haralick, R.M.; Dinstein, I.; Shanmugam, K. Textural Features for Image Classification. IEEE Trans. Syst. Man. Cybern. 1973, SMC-3, 610–621. [Google Scholar] [CrossRef]
- Haralick, R.M. Statistical and Structural Approaches to Texture. Proc. IEEE 1979, 67, 786–804. [Google Scholar] [CrossRef]
- Galloway, M.M. Texture Analysis Using Grey Level Run Lengths. STIN 1974, 75, 18555. [Google Scholar]
- Correa-de-Araujo, R.; Harris-Love, M.O.; Miljkovic, I.; Fragala, M.S.; Anthony, B.W.; Manini, T.M. The Need for Standardized Assessment of Muscle Quality in Skeletal Muscle Function Deficit and Other Aging-Related Muscle Dysfunctions: A Symposium Report. Front. Physiol. 2017, 8, 87. [Google Scholar] [CrossRef] [PubMed]
- Asadi, B.; Pujol-Fuentes, C.; Carcasona-Otal, A.; Calvo, S.; Herrero, P.; Lapuente-Hernández, D. Characterizing Muscle Tissue Quality Post-Stroke: Echovariation as a Clinical Indicator. J. Clin. Med. 2024, 13, 7800. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Díaz, J.; del Baño-Aledo, M.E.; Tembl-Ferrairó, J.I.; Chumillas, M.J.; Vázquez-Costa, J.F.; Martínez-Payá, J.J. Quantitative Neuromuscular Ultrasound Analysis as Biomarkers in Amyotrophic Lateral Sclerosis. Eur. Radiol. 2019, 29, 4266–4275. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Payá, J.J.; Ríos-Díaz, J.; Del Baño-Aledo, M.E.; Tembl-Ferrairó, J.I.; Vazquez-Costa, J.F.; Medina-Mirapeix, F. Quantitative Muscle Ultrasonography Using Textural Analysis in Amyotrophic Lateral Sclerosis. Ultrason. Imaging 2017, 39, 357–368. [Google Scholar] [CrossRef]
- Behr, M.; Noseworthy, M.; Kumbhare, D. Feasibility of a Support Vector Machine Classifier for Myofascial Pain Syndrome: Diagnostic Case-Control Study. J. Ultrasound Med. 2019, 38, 2119–2132. [Google Scholar] [CrossRef]
- Del-Canto-fernández, A.; Calleja-Martínez, P.; Descalzo-Hoyas, B.; Rodríguez-Posada, S.; Cuenca-Zaldívar, N.; Fernández-Carnero, S.; Naranjo-Cinto, F.; Gallego-Izquierdo, T. The Application of Image Texture Analysis Techniques on the Effects of Dry Needling versus Placebo in Low-Back Pain Patients: A Pilot-Study. Appl. Sci. 2022, 12, 5556. [Google Scholar] [CrossRef]
- Escriche-Escuder, A.; Trinidad-Fernández, M.; Pajares, B.; Iglesias-Campos, M.; Alba, E.; García-Almeida, J.M.; Roldán-Jiménez, C.; Cuesta-Vargas, A.I. Responsiveness of the New Index Muscular Echotexture in Women with Metastatic Breast Cancer: An Exercise Intervention Study. Sci. Rep. 2022, 12, 15148. [Google Scholar] [CrossRef]
- De-La-cruz-torres, B.; Romero-Morales, C. Muscular Echovariation as a New Biomarker for the Classification of Soleus Muscle Pathology: A Cross-Sectional Study. Diagnostics 2021, 11, 1884. [Google Scholar] [CrossRef]
- Molina-Payá, F.J.; Ríos-Díaz, J.; Carrasco-Martínez, F.; Martínez-Payá, J.J. Infrared Thermography, Intratendon Vascular Resistance, and Echotexture in Athletes with Patellar Tendinopathy: A Cross-Sectional Study. Ultrason. Imaging 2023, 45, 47–61. [Google Scholar] [CrossRef]
- Molinari, F.; Caresio, C.; Acharya, U.R.; Mookiah, M.R.K.; Minetto, M.A. Advances in Quantitative Muscle Ultrasonography Using Texture Analysis of Ultrasound Images. Ultrasound Med. Biol. 2015, 41, 2520–2532. [Google Scholar] [CrossRef]
- Sahinis, C.; Kellis, E. Hamstring Muscle Quality Properties Using Texture Analysis of Ultrasound Images. Ultrasound Med. Biol. 2023, 49, 431–440. [Google Scholar] [CrossRef]
- Serra, M.C. STROKE. Innov. Aging 2019, 3, S574. [Google Scholar] [CrossRef]
- Ryan, A.S.; Dobrovolny, C.L.; Smith, G.V.; Silver, K.H.; Macko, R.F. Hemiparetic Muscle Atrophy and Increased Intramuscular Fat in Stroke Patients. Arch. Phys. Med. Rehabil. 2002, 83, 1703–1707. [Google Scholar] [CrossRef] [PubMed]
- Tok, F.; Özçakar, L.; Safaz, I.; Alaca, R. Effects of Botulinum Toxin-A on the Muscle Architecture of Stroke Patients: The First Ultrasonographic Study. J. Rehabil. Med. 2011, 43, 1016–1019. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.B.; Zhang, J.; Leng, Z.P.; Chen, X.; Song, W.Q. Evaluation of Spasticity after Stroke by Using Ultrasound to Measure the Muscle Architecture Parameters: A Clinical Study. Int. J. Clin. Exp. Med. 2014, 7, 2712. [Google Scholar] [PubMed]
- Hadi, S.; Khadijeh, O.; Hadian, M.; Niloofar, A.Y.; Olyaei, G.; Hossein, B.; Calvo, S.; Herrero, P. The Effect of Dry Needling on Spasticity, Gait and Muscle Architecture in Patients with Chronic Stroke: A Case Series Study. Top. Stroke Rehabil. 2018, 25, 326–332. [Google Scholar] [CrossRef]
- Picelli, A.; Tamburin, S.; Cavazza, S.; Scampoli, C.; Manca, M.; Cosma, M.; Berto, G.; Vallies, G.; Roncari, L.; Melotti, C.; et al. Relationship between Ultrasonographic, Electromyographic, and Clinical Parameters in Adult Stroke Patients with Spastic Equinus: An Observational Study. Arch. Phys. Med. Rehabil. 2014, 95, 1564–1570. [Google Scholar] [CrossRef]
- Heckmatt, J.Z.; Leeman, S.; Dubowitz, V. Ultrasound Imaging in the Diagnosis of Muscle Disease. J. Pediatr. 1982, 101, 656–660. [Google Scholar] [CrossRef]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M.; Blettner, M.; et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and Elaboration. Int. J. Surg. 2014, 12, 1500–1524. [Google Scholar] [CrossRef]
- Shrestha, B.; Dunn, L. The Declaration of Helsinki on Medical Research Involving Human Subjects: A Review of Seventh Revision. J. Nepal. Health Res. Counc. 2020, 17, 548–552. [Google Scholar] [CrossRef]
- Tsai, D.-M.; Hsiao, B. Automatic surface inspection using wavelet reconstruction. Pattern Recognit. 2001, 34, 1285–1305. [Google Scholar] [CrossRef]
- Chu, A.; Sehgal, C.M.; Greenleaf, J.F. Use of gray value distribution of run lengths for texture analysis. Pattern Recognit. Lett. 1990, 11, 415–419. [Google Scholar] [CrossRef]
- Amadasun, M.; King, R. Textural features corresponding to textural properties. IEEE Trans. Syst. Man Cybern. 1989, 19, 1264–1274. [Google Scholar] [CrossRef]
- Moreta, M.C.; Fleet, A.; Reebye, R.; McKernan, G.; Berger, M.; Farag, J.; Munin, M.C. Reliability and Validity of the Modified Heckmatt Scale in Evaluating Muscle Changes With Ultrasound in Spasticity. Arch. Rehabil. Res. Clin. Transl. 2020, 2, 100071. [Google Scholar] [CrossRef] [PubMed]
- Fleis, L.; Levin, B.; Paik, M.C. The Measurement of Interrater Agreement. In Statistical Methods for Rates and Proportions, 2nd ed.; John Wiley: New York, NY, USA, 1981. [Google Scholar]
- Mavuto, M. Statistics Corner: A Guide to Appropriate Use of Correlation Coefficient in Medical Research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar]
- Canosa-Carro, L.; López-López, D.; García-Sanz, F.; Díaz-Meco-conde, R.; García-Bermejo, P.; De-La-cruz-torres, B.; Marszalek, J.; Romero-Morales, C. Features of Extrinsic Plantar Muscles in Patients with Plantar Fasciitis by Ultrasound Imaging: A Retrospective Case Control Research. Diagnostics 2022, 12, 897. [Google Scholar] [CrossRef]
- Martínez-Payá, J.J.; del Baño-Aledo, M.E.; Ríos-Díaz, J.; Tembl-Ferrairó, J.I.; Vázquez-Costa, J.F.; Medina-Mirapeix, F. Muscular Echovariation: A New Biomarker in Amyotrophic Lateral Sclerosis. Ultrasound Med. Biol. 2017, 43, 1153–1162. [Google Scholar] [CrossRef]
- Pillen, S.; Arts, I.M.P.; Zwarts, M.J. Muscle Ultrasound in Neuromuscular Disorders. Muscle Nerve 2008, 37, 679–693. [Google Scholar] [CrossRef]
- Akazawa, N.; Harada, K.; Okawa, N.; Kishi, M.; Tamura, K.; Moriyama, H. Changes in Quadriceps Thickness and Echo Intensity in Chronic Stroke Survivors: A 3-Year Longitudinal Study. J. Stroke Cerebrovasc. Dis. 2021, 30, 105543. [Google Scholar] [CrossRef]
- Park, B.E.; Jang, W.S.; Yoo, S.K. Texture Analysis of Supraspinatus Ultrasound Image for Computer Aided Diagnostic System. Healthc. Inform. Res. 2016, 22, 299–304. [Google Scholar] [CrossRef]
- Sarwal, A.; Parry, S.M.; Berry, M.J.; Hsu, F.C.; Lewis, M.T.; Justus, N.W.; Morris, P.E.; Denehy, L.; Berney, S.; Dhar, S.; et al. Interobserver Reliability of Quantitative Muscle Sonographic Analysis in the Critically Ill Population. J. Ultrasound Med. 2015, 34, 1191–1200. [Google Scholar] [CrossRef] [PubMed]
- Picelli, A.; Bonetti, P.; Fontana, C.; Barausse, M.; Dambruoso, F.; Gajofatto, F.; Girardi, P.; Manca, M.; Gimigliano, R.; Smania, N. Is Spastic Muscle Echo Intensity Related to the Response to Botulinum Toxin Type A in Patients with Stroke? A Cohort Study. Arch. Phys. Med. Rehabil. 2012, 93, 1253–1258. [Google Scholar] [CrossRef] [PubMed]
| Overall | Low Impairment | High Impairment | a p-Value | ||
|---|---|---|---|---|---|
| N | 44 | 21 | 23 | ||
| Main outcomes | |||||
| Echovariation | 46.26 ± 16.69 | 56.84 ± 16.52 | 36.60 ± 9.58 | <0.001 * | |
| Echointensity | 82.89 ± 23.60 | 66.56 ± 17.92 | 97.80 ± 17.58 | <0.001 * | |
| Secondary outcomes | |||||
| Heckmatt scale, n (%) | Grade 1 | 3 (6.8) | 3 (14.3) | 0 (0.0) | <0.001 * |
| Grade 2 | 21 (47.7) | 18 (85.7) | 3 (13.0) | ||
| Grade 3 | 12 (27.3) | 0 (0.0) | 12 (52.2) | ||
| Grade 4 | 8 (18.2) | 0 (0.0) | 8 (34.8) | ||
| Variance | 1237.73 ± 324.37 | 1267.46 ± 301.08 | 1210.59 ± 348.76 | 0.568 | |
| Standard deviation | 34.87 ± 4.71 | 35.34 ± 4.42 | 34.45 ± 5.02 | 0.536 | |
| Skewness | 0.50 ± 0.34 | 0.65 ± 0.32 | 0.37 ± 0.31 | 0.005 * | |
| Kurtosis | 0.03 ± 0.65 | 0.22 ± 0.81 | −0.15 ± 0.40 | 0.056 | |
| Correlation | 0.97 ± 0.01 | 0.97 ± 0.01 | 0.98 ± 0.01 | 0.103 | |
| Dissimilarity | 5.97 ± 0.74 | 6.21 ± 0.81 | 5.75 ± 0.60 | 0.037 * | |
| Energy | 0.02 ± 0.01 | 0.03 ± 0.02 | 0.02 ± 0.00 | 0.042 * | |
| Contrast | 62.00 ± 14.82 | 68.20 ± 15.69 | 56.35 ± 11.65 | 0.007 * | |
| Homogeneity | 0.17 ± 0.03 | 0.18 ± 0.04 | 0.17 ± 0.02 | 0.444 | |
| Angular Second Moment | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.058 | |
| Maximum probability | 0.01 ± 0.02 | 0.01 ± 0.02 | 0.00 ± 0.00 | 0.013 * | |
| Entropy | 7.04 ± 0.22 | 7.00 ± 0.24 | 7.07 ± 0.20 | 0.275 | |
| Cluster Shade | 8.03 ± 0.23 | 7.99 ± 0.25 | 8.07 ± 0.20 | 0.234 | |
| Cluster Prominence | 10.87 ± 0.36 | 10.81 ± 0.42 | 10.92 ± 0.28 | 0.294 | |
| Short-Run Emphasis | 0.73 ± 0.08 | 0.73 ± 0.07 | 0.72 ± 0.08 | 0.49 | |
| Long-Run Emphasis | 440.64 ± 134.70 | 447.98 ± 173.53 | 433.94 ± 89.38 | 0.734 | |
| Gray-Level Uniformity | 10,957.52 ± 2970.64 | 10,237.13 ± 2319.04 | 11,615.27 ± 3378.09 | 0.126 | |
| Run-Length Uniformity | 17,268.72 ± 4566.54 | 17,109.75 ± 2938.33 | 17,413.87 ± 5732.68 | 0.828 | |
| Run Percentage | 15.27 ± 3.28 | 15.37 ± 2.45 | 15.17 ± 3.95 | 0.837 | |
| Odds Ratio (95% CI) | Coefficient (SE) | 95% CI | Z-Value (a p-Value) | Variable Importance | |
|---|---|---|---|---|---|
| Echovariation main outcome model | |||||
| (Intercept) | 7.77 × 1031 (111,740.12, 1.63 × 1073) | 73.43 (SE = 38.61) | 11.62, 168.57 | 0.057 | |
| Echovariation | 1.275 (1.1, 1.62) | 0.24 (SE = 0.09) | 0.09, 0.48 | 0.012 * | 2.52 |
| Dissimilarity | 17.856 (2.61, 360) | 2.88 (SE = 1.19) | 0.96, 5.88 | 0.016 * | 2.40 |
| Entropy | <0.001 (0.001, 0.01) | −13.75 (SE = 6.31) | −29.66, −3.98 | 0.029 * | 2.17 |
| Gray-Level Uniformity | 1 (0.99, 1) | 0 (SE = 0) | −0.001, 0 | 0.154 | 1.42 |
| Echointensity main outcome model | |||||
| (Intercept) | 3.64 × 1020 (0, 5.00 × 1054) | 47.34 (SE = 32.57) | −8.6, 125.95 | 0.146 | |
| Echointensity | 0.86 (0.74, 0.93) | −0.14 (SE = 0.05) | −0.29, −0.06 | 0.009 * | 2.62 |
| Dissimilarity | 15.65 (2.33, 287.63) | 2.75 (SE = 1.17) | 0.84, 5.66 | 0.019 * | 2.33 |
| Entropy | 0.001 (0, 5.02) | −6.69 (SE = 4.87) | −18.43, 1.61 | 0.170 | 1.37 |
| Gray-Level Uniformity | 1 (0.99, 1) | 0 (SE = 0) | −0.001, 0 | 0.142 | 1.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asadi, B.; Cuenca-Zaldívar, J.N.; Carcasona-Otal, A.; Herrero, P.; Lapuente-Hernández, D. Improving the Reliability of Muscle Tissue Characterization Post-Stroke: A Secondary Statistical Analysis of Echotexture Features. J. Clin. Med. 2025, 14, 2902. https://doi.org/10.3390/jcm14092902
Asadi B, Cuenca-Zaldívar JN, Carcasona-Otal A, Herrero P, Lapuente-Hernández D. Improving the Reliability of Muscle Tissue Characterization Post-Stroke: A Secondary Statistical Analysis of Echotexture Features. Journal of Clinical Medicine. 2025; 14(9):2902. https://doi.org/10.3390/jcm14092902
Chicago/Turabian StyleAsadi, Borhan, Juan Nicolás Cuenca-Zaldívar, Alberto Carcasona-Otal, Pablo Herrero, and Diego Lapuente-Hernández. 2025. "Improving the Reliability of Muscle Tissue Characterization Post-Stroke: A Secondary Statistical Analysis of Echotexture Features" Journal of Clinical Medicine 14, no. 9: 2902. https://doi.org/10.3390/jcm14092902
APA StyleAsadi, B., Cuenca-Zaldívar, J. N., Carcasona-Otal, A., Herrero, P., & Lapuente-Hernández, D. (2025). Improving the Reliability of Muscle Tissue Characterization Post-Stroke: A Secondary Statistical Analysis of Echotexture Features. Journal of Clinical Medicine, 14(9), 2902. https://doi.org/10.3390/jcm14092902







