Updates in Diagnostic Tools for ILD
Abstract
1. Introduction
2. Genomic Classifier
3. Transbronchial Cryobiopsy
4. Surgical Lung Biopsy
5. Genetic Testing
6. Endobronchial Ultrasound-Guided Cryobiopsy
7. Speckled Transthoracic Echocardiography
8. Putting Everything Together
9. Future Directions
Funding
Conflicts of Interest
References
- Raghu, G.; Remy-Jardin, M.; Richeldi, L.; Thomson, C.C.; Antoniou, K.M.; Bissell, B.D.; Bouros, D.; Buendia-Roldan, I.; Caro, F.; Crestani, B.; et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2022, 205, E18–E47. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, K.R.; Wells, A.U.; Cottin, V.; Devaraj, A.; Walsh, S.L.F.; Inoue, Y.; Richeldi, L.; Kolb, M.; Tetzlaff, K.; Stowasser, S.; et al. Nintedanib in Progressive Fibrosing Interstitial Lung Diseases. N. Engl. J. Med. 2019, 381, 1718–1727. [Google Scholar] [CrossRef]
- Maher, T.M.; Corte, T.J.; Fischer, A.; Kreuter, M.; Lederer, D.J.; Molina-Molina, M.; Axmann, J.; Kirchgaessler, K.U.; Samara, K.; Gilberg, F.; et al. Pirfenidone in patients with unclassifiable progressive fibrosing interstitial lung disease: A double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir. Med. 2020, 8, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Idiopathic Pulmonary Fibrosis Clinical Research Network; Raghu, G.; Anstrom, K.J.; King, T.E., Jr.; Lasky, J.A.; Martinez, F.J. Prednisone, Azathioprine, and N-Acetylcysteine for Pulmonary Fibrosis. N. Engl. J. Med. 2012, 366, 1968–1977. [Google Scholar] [PubMed]
- Spagnolo, P.; Rossi, G.; Trisolini, R.; Sverzellati, N.; Baughman, R.P.; Wells, A.U. Pulmonary sarcoidosis. Lancet Respir. Med. 2018, 6, 389–402. [Google Scholar] [CrossRef]
- Salisbury, M.L.; Myers, J.L.; Belloli, E.A.; Kazerooni, E.A.; Martinez, F.J.; Flaherty, K.R. Diagnosis and treatment of fibrotic hypersensitivity pneumonia: Where we stand and where we need to go. Am. J. Respir. Crit. Care Med. 2017, 196, 690–699. [Google Scholar] [CrossRef]
- Distler, O.; Highland, K.B.; Gahlemann, M.; Azuma, A.; Fischer, A.; Mayes, M.D.; Raghu, G.; Sauter, W.; Girard, M.; Alves, M.; et al. Nintedanib for Systemic Sclerosis–Associated Interstitial Lung Disease. N. Engl. J. Med. 2019, 380, 2518–2528. [Google Scholar] [CrossRef]
- Mathai, S.C.; Danoff, S.K. Management of interstitial lung disease associated with connective tissue disease. BMJ 2016, 352, h6819. [Google Scholar] [CrossRef] [PubMed]
- Tomassetti, S.; Ravaglia, C.; Puglisi, S.; Ryu, J.H.; Colby, T.V.; Cavazza, A.; Wells, A.U.; Pavone, M.; Vancheri, C.; Lavorini, F.; et al. Impact of Lung Biopsy Information on Treatment Strategy of Patients with Interstitial Lung Diseases. Ann. Am. Thorac. Soc. 2022, 19, 737–745. [Google Scholar] [CrossRef]
- Ribeiro Neto, M.L.; Arrossi, A.V.; Yadav, R.; Culver, D.A.; Mukhopadhyay, S.; Parambil, J.G.; Southern, B.D.; Tolle, L.; Pande, A.; Almeida, F.A.; et al. Prospective cohort of cryobiopsy in interstitial lung diseases: A single center experience. BMC Pulm. Med. 2022, 22, 215. [Google Scholar] [CrossRef]
- Cottin, V.; Tomassetti, S.; Valenzuela, C.; Walsh, S.L.F.; Antoniou, K.M.; Bonella, F.; Brown, K.K.; Collard, H.R.; Corte, T.J.; Flaherty, K.R.; et al. Integrating Clinical Probability into the Diagnostic Approach to Idiopathic Pulmonary Fibrosis An International Working Group Perspective. Am. J. Respir. Crit. Care Med. 2022, 206, 247–259. [Google Scholar] [CrossRef]
- Hariri, L.P.; Roden, A.C.; Chung, J.H.; Danoff, S.K.; Manjarres, D.C.G.; Hartwig, M.; Kheir, F.; King, C.; Kreider, M.; Lynch, D.A.; et al. The Role of Surgical Lung Biopsy in the Diagnosis of Perspective from the Pulmonary Fibrosis Foundation. Ann. Am. Thorac. Soc. 2021, 18, 1601–1609. [Google Scholar] [CrossRef]
- Ravaglia, C.; Nicholson, A.G. Biopsy in interstitial lung disease: Specific diagnosis and the identification of the progressive fibrotic phenotype. Curr. Opin. Pulm. Med. 2021, 27, 355–362. [Google Scholar] [CrossRef]
- Korevaar, D.A.; Colella, S.; Fally, M.; Camuset, J.; Colby, T.V.; Hagmeyer, L.; Hetzel, J.; Maldonado, F.; Morais, A.; Ravaglia, C.; et al. European Respiratory Society guidelines on transbronchial lung cryobiopsy in the diagnosis of interstitial lung diseases. Eur. Respir. J. 2022, 60, 2200425. [Google Scholar] [CrossRef] [PubMed]
- Goobie, G.C.; Guler, S.A. The alternative approach: Genomic classifiers for prognostication in interstitial lung disease. Eur. Respir. J. 2023, 61, 2300033. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, J.P.; McKeever, T.M.; Fogarty, A.W.; Navaratnam, V.; Hubbard, R.B. Surgical lung biopsy for the diagnosis of interstitial lung disease in England: 1997–2008. Eur. Respir. J. 2016, 48, 1453–1461. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.H.; Shapera, S.; To, T.; Marras, T.K.; Gershon, A.; Dell, S. Procedure volume and mortality after surgical lung biopsy in interstitial lung disease. Eur. Respir. J. 2019, 53, 1801164. [Google Scholar] [CrossRef]
- Radu, D.; Freynet, O.; Kambouchner, M.; Boubaya, M.; Nunes, H.; Uzunhan, Y.; Brillet, P.Y.; Guiraudet, P.; Noorah, M.Z.; Israël-Biet, D.; et al. Diagnosis Yield and Safety of Surgical Biopsy in Interstitial Lung Diseases: A Prospective Study. Ann. Thorac. Surg. 2022, 114, 1911–1917. [Google Scholar] [CrossRef]
- Katzenstein, A.A.; Mukhopadhyay, S.; Myers, J.L. Erratum to “Diagnosis of usual interstitial pneumonia and distinction from other fibrosing interstitial lung diseases” [Hum Pathol 39 (2008) 1275-1294]. Hum. Pathol. 2008, 39, 1562–1581. [Google Scholar] [CrossRef]
- Tomassetti, S.; Cavazza, A.; Colby, T.V.; Ryu, J.H.; Nanni, O.; Scarpi, E.; Tantalocco, P.; Buccioli, M.; Dubini, A.; Piciucchi, S.; et al. Transbronchial biopsy is useful in predicting UIP pattern. Respir. Res. 2012, 13, 96. [Google Scholar] [CrossRef]
- Shim, H.S.; Park, M.S.; Park, I.K. Histopathologic findings of transbronchial biopsy in usual interstitial pneumonia.pdf. Pathol. Int. 2010, 60, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Berbescu, E.A.; Katzenstein, A.-L.A.; Snow, J.L.; Zisman, D.A. Transbronchial Biopsy in Usual Interstitial Pneumonia. Chest 2006, 129, 1126–1131. [Google Scholar] [CrossRef]
- Pankratz, D.G.; Choi, Y.; Imtiaz, U.; Fedorowicz, M.; Anderson, J.D.; Colby, T.V.; Myers, J.L.; Lynch, D.A.; Brown, K.K.; Flaherty, K.R.; et al. Usual Interstitial Pneumonia Can Be Detected in Transbronchial Biopsies Using Machine Learning. Ann. Am. Thorac. Soc. 2017, 14, 1646–1654. [Google Scholar] [CrossRef]
- Raghu, G.; Flaherty, K.R.; Lederer, D.J.; Lynch, D.A.; Colby, T.V.; Myers, J.L.; Groshong, S.D.; Larsen, B.T.; Chung, J.H.; Steele, M.P.; et al. Use of a molecular classifier to identify usual interstitial pneumonia in conventional transbronchial lung biopsy samples: A prospective validation study. Lancet Respir. 2024, 7, 487–496. [Google Scholar] [CrossRef]
- Richeldi, L.; Scholand, M.B.; Lynch, D.A.; Colby, T.V.; Myers, J.L.; Groshong, S.D.; Chung, J.H.; Benzaquen, S.; Nathan, S.D.; Davis, J.R.; et al. Utility of a Molecular Classifier as a Complement to High-Resolution Computed Tomography to Identify Usual Interstitial Pneumonia. Am. J. Respir. Crit. Care Med. 2021, 203, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Kheir, F.; Becerra, J.P.U.; Bissell, B.; Ghazipura, M.; Herman, D.; Hon, S.M.; Hossain, T.; Khor, Y.H.; Knight, S.L.; Kreuter, M.; et al. Use of a Genomic Classifier in Patients with Interstitial Lung Disease A Systematic Review and Meta-Analysis. Ann. Am. Thorac. Soc. 2022, 19, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Lasky, J.A.; Case, A.; Unterman, A.; Kreuter, M.; Scholand, M.B.; Chaudhary, S.; Lofaro, L.R.; Johnson, M.; Huang, J.; Bhorade, S.M.; et al. The Impact of the Envisia Genomic Classifier in the Diagnosis and Management of Patients with Idiopathic Pulmonary Fibrosis. Ann. Am. Thorac. Soc. 2022, 19, 916–924. [Google Scholar] [CrossRef]
- Goobie, G.C.; Kass, D.J. Genomic Classifiers in Diagnosing Interstitial Lung Disease: Finding the Right Place at the Right Time. Ann. Am. Thorac. Soc. 2022, 19, 895–897. [Google Scholar] [CrossRef] [PubMed]
- Arya, R.; Boujaoude, Z.; Rafferty, W.J.; Abouzgheib, W. Usefulness and safety of transbronchial biopsy with large forceps during flexible bronchoscopy. Baylor Univ. Med. Cent. Proc. 2020, 34, 232–236. [Google Scholar] [CrossRef]
- Galli, J.A.; Panetta, N.L.; Gaeckle, N.; Martinez, F.J.; Moore, B.; Moore, T.; Courey, A.; Flaherty, K.; Criner, G.J. Pneumothorax After Transbronchial Biopsy in Pulmonary Fibrosis: Lessons from the Multicenter COMET Trial. Lung 2017, 195, 537–543. [Google Scholar] [CrossRef]
- Pajares, V.; Núñez-Delgado, M.; Bonet, G.; Pérez-Pallarés, J.; Martínez, R.; Cubero, N.; Zabala, T.; Cordovilla, R.; Flandes, J.; Disdier, C.; et al. Transbronchial biopsy results according to diffuse interstitial lung disease classification. Cryobiopsy versus forceps: MULTICRIO study. PLoS ONE 2020, 15, e0239114. [Google Scholar] [CrossRef] [PubMed]
- Sethi, J.; Ali, M.S.; Mohananey, D.; Nanchal, R.; Maldonado, F.; Musani, A. Are Transbronchial Cryobiopsies Ready for Prime Time? J. Bronchol. Interv. Pulmonol. 2019, 26, 22–32. [Google Scholar] [CrossRef]
- Iftikhar, I.H.; Alghothani, L.; Sardi, A.; Berkowitz, D.; Musani, A.I. Transbronchial Lung Cryobiopsy and Video-assisted Thoracoscopic Lung Biopsy in the Diagnosis of Diffuse Parenchymal Lung Disease A Meta-analysis of Diagnostic Test Accuracy. Ann. Am. Thorac. Soc. 2017, 14, 1197–1211. [Google Scholar] [CrossRef]
- Johannson, K.A.; Marcoux, V.S.; Ronksley, P.E.; Ryerson, C.J. Diagnostic Yield and Complications of Transbronchial Lung Cryobiopsy for Interstitial Lung Disease. Ann. Am. Thorac. Soc. 2016, 13, 1828–1838. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, M.; Colby, T.V.; Berthet, J.; Gamez, A.S.; Mallet, J.; Serre, I.; Cancellieri, A.; Cavazza, A.; Solovei, L.; Amore, A.D.; et al. Poor Concordance between Sequential Transbronchial Lung Cryobiopsy and Surgical Lung Biopsy in the Diagnosis of Diffuse Interstitial Lung Diseases. Am. J. Respir. Crit. Care Med. 2019, 199, 1249–1256. [Google Scholar] [CrossRef]
- Troy, L.K.; Grainge, C.; Corte, T.J.; Williamson, J.P.; Vallely, M.P.; Cooper, W.A.; Mahar, A.; Myers, J.L.; Lai, S.; Mulyadi, E.; et al. Diagnostic accuracy of transbronchial lung cryobiopsy for interstitial lung disease diagnosis (COLDICE): A prospective, comparative study. Lancet Respir. Med. 2020, 8, 171–181. [Google Scholar] [CrossRef]
- Kalverda, K.A.; Ninaber, M.K.; Wijmans, L.; von der Thüsen, J.; Jonkers, R.E.; Daniels, J.M.; Miedema, J.R.; Dickhoff, C.; Hölters, J.; Heineman, D.; et al. Transbronchial cryobiopsy followed by as-needed surgical lung biopsy versus immediate surgical lung biopsy for diagnosing interstitial lung disease (the COLD study): A randomised controlled trial. Lancet Respir. Med. 2024, 12, 513–522. [Google Scholar] [CrossRef]
- Fortin, M.; Liberman, M.; Delage, A.; Dion, G.; Martel, S.; Rolland, F.; Soumagne, T.; Trahan, S.; Assayag, D.; Albert, E.; et al. Transbronchial Lung Cryobiopsy and Surgical LungBiopsy: A Prospective Multi-Centre Agreement Clinical Trial(CAN-ICE). Am. J. Respir. Crit. Care Med. 2023, 207, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- Kheir, F.; Alkhatib, A.; Berry, G.J.; Daroca, P.; Diethelm, L.; Rampolla, R.; Saito, S.; Smith, D.L.; Weill, D.; Bateman, M.; et al. Using Bronchoscopic Lung Cryobiopsy and a Genomic Classifier in the Multidisciplinary Diagnosis of Diffuse Interstitial Lung Diseases. Chest 2020, 158, 2015–2025. [Google Scholar] [CrossRef]
- Rodrigues, I.; Gomes, R.E.; Coutinho, L.M.; Rego, M.T.; Machado, F.; Morais, A.; Bastos, H.N. Diagnostic yield and safety of transbronchial lung cryobiopsy and surgical lung biopsy in interstitial lung diseases: A systematic review and meta-analysis. Eur. Respir. Rev. 2022, 31, 210280. [Google Scholar] [CrossRef]
- Han, Q.; Luo, Q.; Xie, J.; Wu, L.; Liao, L. Diagnostic yield and postoperative mortality associated with surgical lung biopsy for evaluation of interstitial lung diseases: A systematic review and meta-analysis. J. Thorac. Cardiovasc. Surg. 2015, 149, 1394–1401. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, J.P.; Fogarty, A.W.; Mckeever, T.M.; Hubbard, R.B. In-Hospital Mortality after Surgical Lung Biopsy for Interstitial Lung Disease in the United States 2000 to 2011. Am. J. Respir. Crit. Care Med. 2016, 193, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Sigurdsson, M.I.; Isaksson, H.J.; Gudmundsson, G.; Gudbjartsson, T. Diagnostic Surgical Lung Biopsies for Suspected Interstitial Lung Diseases: A Retrospective Study. Ann. Thorac. Surg. 2009, 88, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, G.; Estrada, A.; Pedroza, J.; Aragón, B.; Mejía, M.; Navarro, C.; Selman, M. Preoperative Risk Factors Associated With Mortality in Lung Biopsy Patients With Interstitial Lung Disease. J. Investig. Surg. 2005, 18, 39–45. [Google Scholar] [CrossRef]
- Pastre, J.; Khandhar, S.; Barnett, S.; Ksovreli, I.; Mani, H.; Brown, A.W.; Shlobin, O.A.; Ahmad, K.; Khangoora, V.; Aryal, S.; et al. Surgical Lung Biopsy for Interstitial Lung Disease. Ann. Am. Thorac. Soc. 2021, 18, 460–467. [Google Scholar] [CrossRef]
- Hutchinson, J.; Hubbard, R.; Raghu, G. Surgical lung biopsy for interstitial lung disease: When considered necessary, should these be done in larger and experienced centres only? Eur. Respir. J. 2019, 53, 1900023. [Google Scholar] [CrossRef]
- Ravaglia, C.; Bonifazi, M.; Wells, A.U.; Tomassetti, S.; Gurioli, C.; Piciucchi, S.; Dubini, A.; Tantalocco, P.; Sanna, S.; Negri, E.; et al. Safety and Diagnostic Yield of Transbronchial Lung Cryobiopsy in Diffuse Parenchymal Lung Diseases: A Comparative Study versus Video-Assisted Thoracoscopic Lung Biopsy and a Systematic Review of the Literature. Respiration 2016, 91, 215–227. [Google Scholar] [CrossRef]
- Adegunsoye, A.; Kropski, J.A.; Behr, J.; Blackwell, T.S.; Corte, T.J. Genetics and Genomics of Pulmonary Fibrosis Charting the Molecular Landscape and Shaping Precision Medicine. Am. J. Respir. Crit. Care Med. 2024, 210, 401–423. [Google Scholar] [CrossRef]
- Al, R.B.E.T.; Borie, R.; Kannengiesser, C.; Antoniou, K.; Bonella, F.; Crestani, B.; Fabre, A.; Froidure, A.; Galvin, L.; Griese, M.; et al. European Respiratory Society statement on familial pulmonary fibrosis. Eur. Respir. J. 2023, 61, 2201383. [Google Scholar] [CrossRef]
- Zhang, D.; Newton, C.A. Familial Pulmonary Fibrosis Genetic Features and Clinical Implications. Chest 2021, 160, 1764–1773. [Google Scholar] [CrossRef]
- Savage, S.A.; Alter, B.P. Dyskeratosis Congenita. Hematol. Oncol. Clin. N. Am. 2009, 23, 215–231. [Google Scholar] [CrossRef] [PubMed]
- Borie, R.; Kannengiesser, C.; De Fontbrune, F.S.; Gouya, L.; Nathan, N.; Crestani, B. Management of suspected monogenic lung fibrosis in a specialised centre. Eur. Respir. Rev. 2017, 26, 160122. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kuan, P.J.; Xing, C.; Cronkhite, J.T.; Torres, F.; Rosenblatt, R.L.; DiMaio, J.M.; Kinch, L.N.; Grishin, N.V.; Garcia, C.K. Genetic Defects in Surfactant Protein A2 Are Associated with Pulmonary Fibrosis and Lung Cancer. Am. J. Hum. Genet. 2009, 84, 52–59. [Google Scholar] [CrossRef]
- Alder, J.K.; Hanumanthu, V.S.; Strong, M.A.; DeZern, A.E.; Stanley, S.E.; Takemoto, C.M.; Danilova, L.; Applegate, C.D.; Bolton, S.G.; Mohr, D.W.; et al. Diagnostic utility of telomere length testing in a hospital-based setting. Proc. Natl. Acad. Sci. USA 2018, 115, E2358–E2365. [Google Scholar] [CrossRef]
- Van Moorsel, C.H.M.; Van Oosterhout, M.F.M.; Barlo, N.P.; De Jong, P.A.; Van Der Vis, J.J.; Ruven, H.J.T.; Van Es, H.W.; Van Den Bosch, J.M.; Grutters, J.C. Surfactant Protein C Mutations Are the Basis ofa Significant Portion of Adult Familial PulmonaryFibrosis in a Dutch Cohort. Am. J. Respir. Crit. Care Med. 2010, 182, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Peljto, A.L.; Zhang, Y.; Fingerlin, T.E.; Shwu-Fan, M.; Garcia, J.G.N.; Richards, T.J.; Silveira, L.J.; Lindell, K.O.; Steele, M.P.; Loyd, J.E.; et al. Association between the MUC5B promoter polymorphism and survival in patients with idiopathic pulmonary fibrosis. JAMA 2013, 309, 2232–2239. [Google Scholar] [CrossRef]
- Poletti, V.; Petrarulo, S.; Piciucchi, S.; Dubini, A.; De Grauw, A.J.; Sultani, F.; Martinello, S.; Gonunguntla, H.K.; Ravaglia, C. EBUS-guided cryobiopsy in the diagnosis of thoracic disorders. Pulmonology 2024, 30, 459–465. [Google Scholar] [CrossRef]
- Mangold, M.S.; Franzen, D.P.; Hetzel, J.; Latshang, T.D.; Roeder, M.; Vesenbeckh, S.M.; Ulrich, S.; Gaisl, T.; Steinack, C. Ultrasound-guided transbronchial cryobiopsy of mediastinal and hilar lesions: A multicenter pragmatic cohort study with real-world evidence. BMJ Open Respir. Res. 2024, 11, e002617. [Google Scholar] [CrossRef]
- Ariza-Prota, M.; Pérez-Pallarés, J.; Fernández-Fernández, A.; García-Alfonso, L.; Cascón, J.A.; Torres-Rivas, H.; Fernández-Fernández, L.; Sánchez, I.; Gil, M.; García-Clemente, M.; et al. Endobronchial ultrasound-guided transbronchial mediastinal cryobiopsy in the diagnosis of mediastinal lesions: Safety, feasibility and diagnostic yield—Experience in 50 cases. ERJ Open Res. 2023, 9, 00448–2022. [Google Scholar] [CrossRef]
- Chemla, D.; Castelain, V.; Hoette, S.; Creuzé, N.; Provencher, S.; Zhu, K.; Humbert, M.; Herve, P. Strong linear relationship between heart rate and mean pulmonary artery pressure in exercising patients with severe precapillary pulmonary hypertension. Am. J. Physiol. Hear. Circ. Physiol. 2013, 305, 769–777. [Google Scholar] [CrossRef]
- Santoro, C.; Buonauro, A.; Canora, A.; Rea, G.; Canonico, M.E.; Esposito, R.; Sanduzzi, A.; Esposito, G.; Bocchino, M. Non-Invasive Assessment of Right Ventricle to Arterial Coupling for Prognosis Stratification of Fibrotic Interstitial Lung Diseases. J. Clin. Med. 2022, 11, 6115. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, A.; Stanziola, A.A.; Saggar, R.; Saggar, R.; Sperlongano, S.; Conte, M.; D’Alto, M.; Ferrara, F.; Gargani, L.; Lancellotti, P.; et al. Right Ventricular Functional Reserve in Early-Stage Idiopathic Pulmonary Fibrosis: An Exercise Two-Dimensional Speckle Tracking Doppler Echocardiography Study. Chest 2019, 155, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Buonauro, A.; Santoro, C.; Galderisi, M.; Canora, A.; Sorrentino, R.; Esposito, R.; Lembo, M.; Canonico, M.E.; Ilardi, F.; Fazio, V.; et al. Impaired right and left ventricular longitudinal function in patients with fibrotic interstitial lung diseases. J. Clin. Med. 2020, 9, 587. [Google Scholar] [CrossRef]
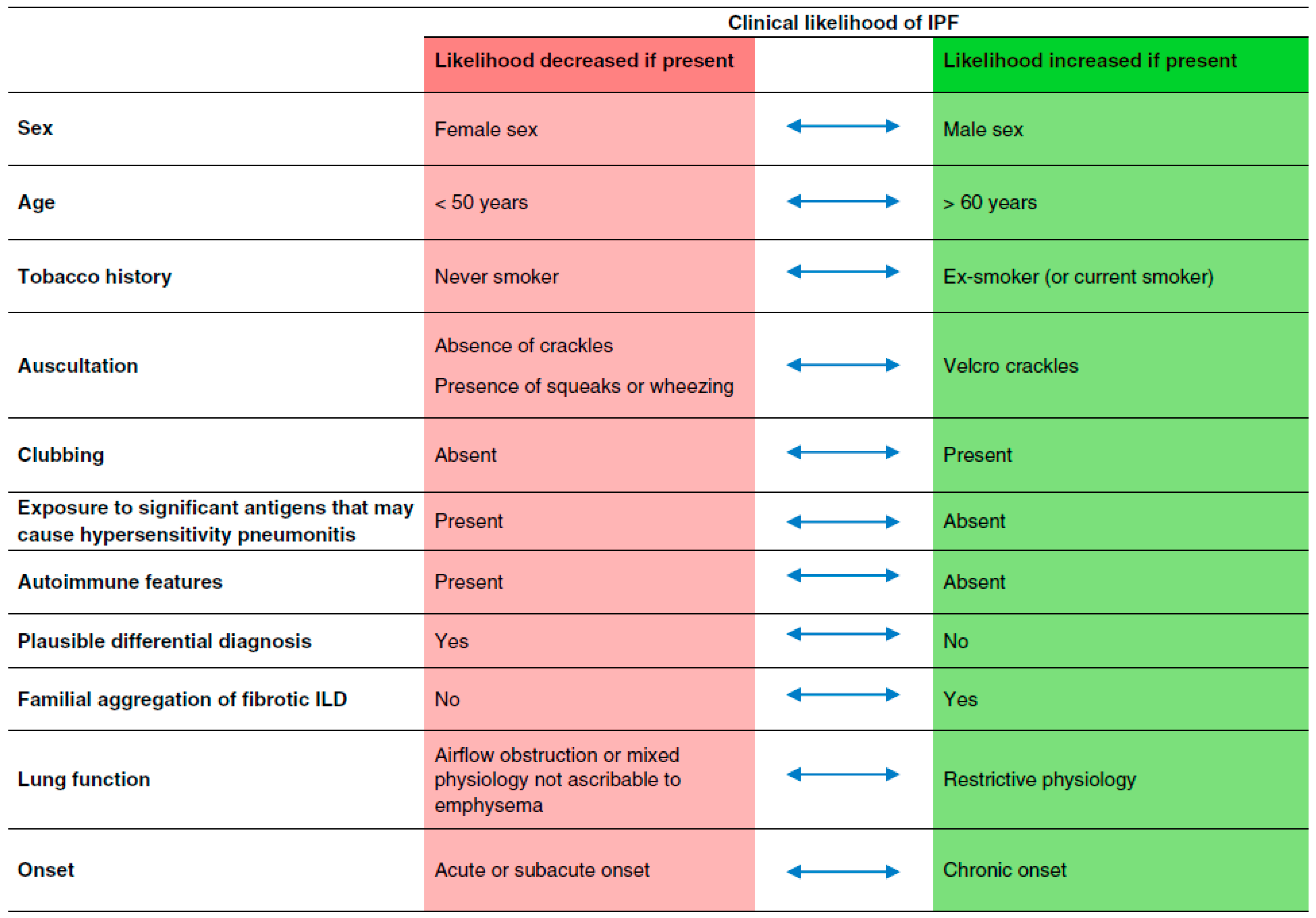

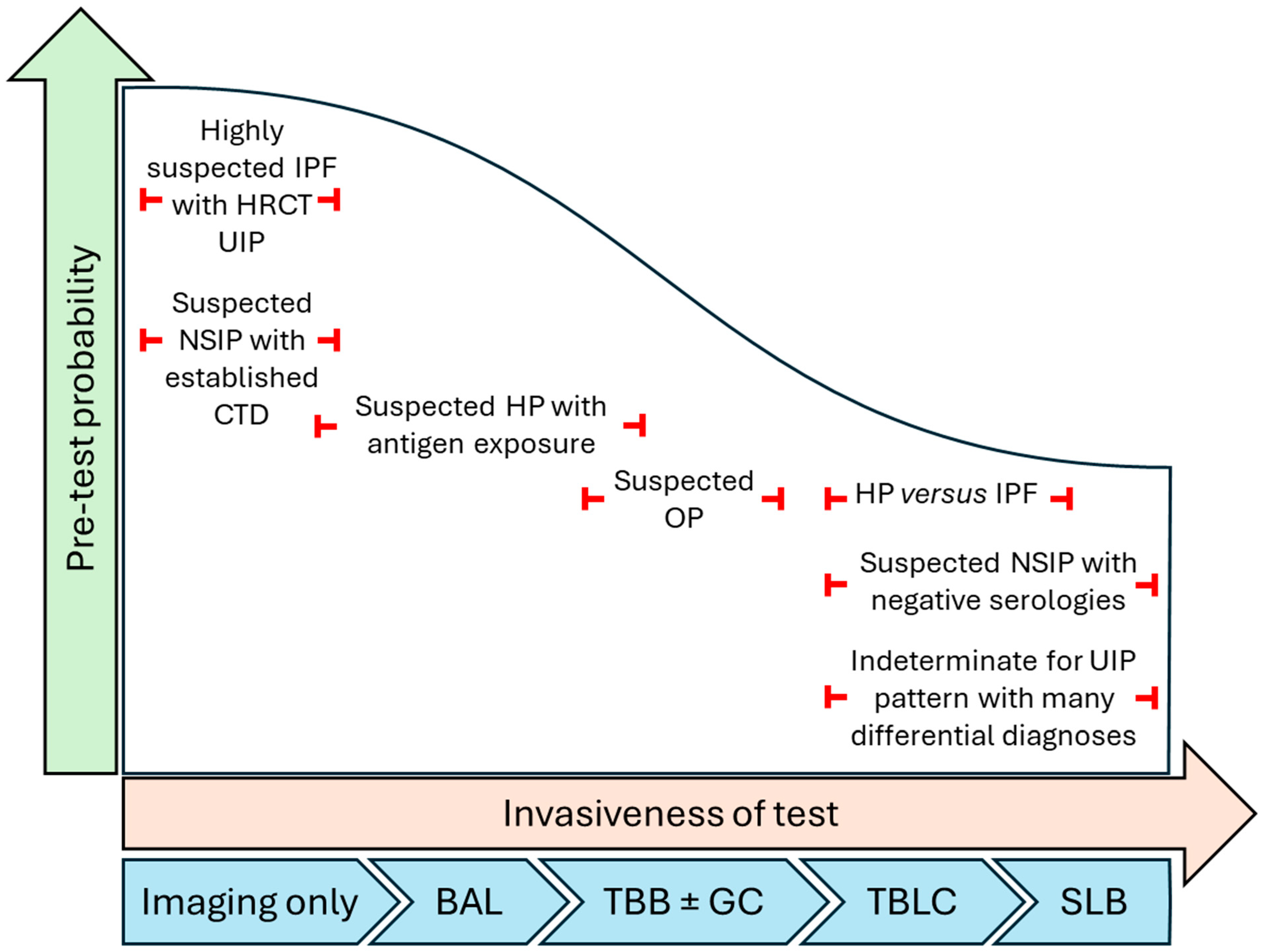
| Diagnostic Tool | Pros | Cons |
|---|---|---|
| GC |
|
|
| TBLC |
|
|
| SLB |
|
|
| Genetic testing |
|
|
| EBUS-C |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tharwani, A.; Ribeiro Neto, M.L. Updates in Diagnostic Tools for ILD. J. Clin. Med. 2025, 14, 2924. https://doi.org/10.3390/jcm14092924
Tharwani A, Ribeiro Neto ML. Updates in Diagnostic Tools for ILD. Journal of Clinical Medicine. 2025; 14(9):2924. https://doi.org/10.3390/jcm14092924
Chicago/Turabian StyleTharwani, Arsal, and Manuel L. Ribeiro Neto. 2025. "Updates in Diagnostic Tools for ILD" Journal of Clinical Medicine 14, no. 9: 2924. https://doi.org/10.3390/jcm14092924
APA StyleTharwani, A., & Ribeiro Neto, M. L. (2025). Updates in Diagnostic Tools for ILD. Journal of Clinical Medicine, 14(9), 2924. https://doi.org/10.3390/jcm14092924






