Skeletal Muscle Density as a Predictor of Prognosis and Physical Reserve in Patients with Cancer of Unknown Primary
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients Sample
2.3. Inclusion Criteria
2.4. Exclusion Criteria
2.5. Data Collection
2.5.1. Charlson Comorbidity Index
2.5.2. ECOG Performance Status
2.6. Skeletal Muscle Evaluation
2.7. Statistical Analysis
2.8. Study Approval
3. Results
3.1. Baseline Characteristics
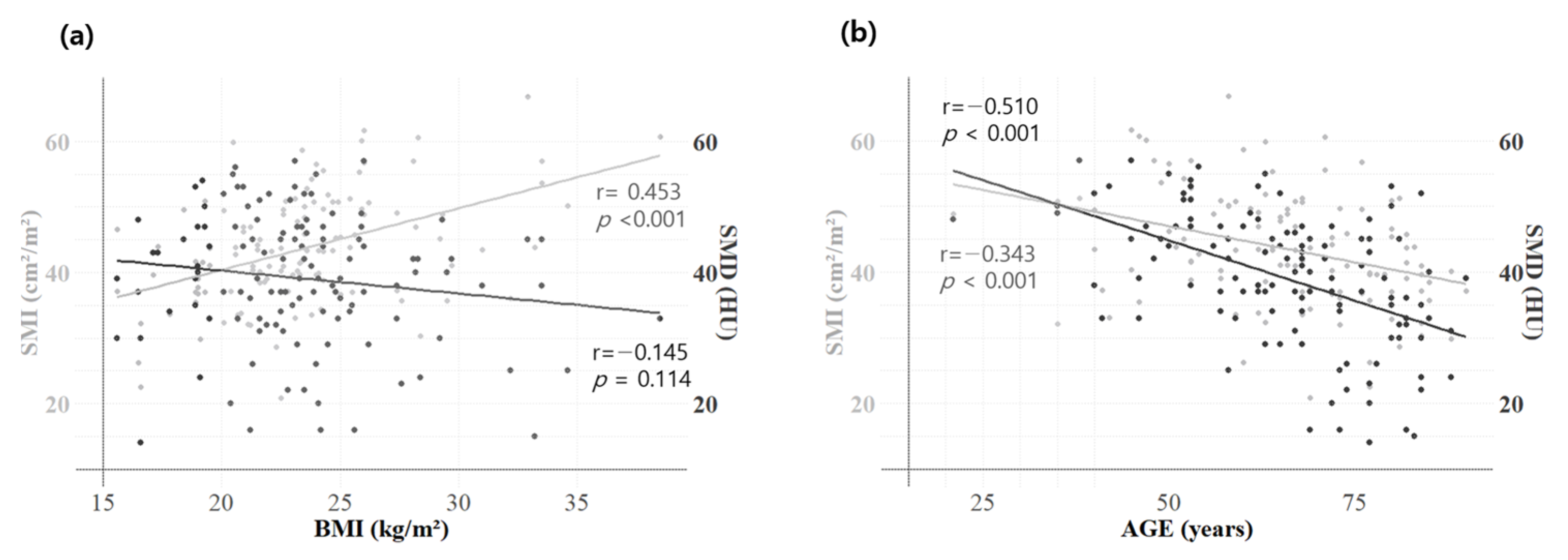
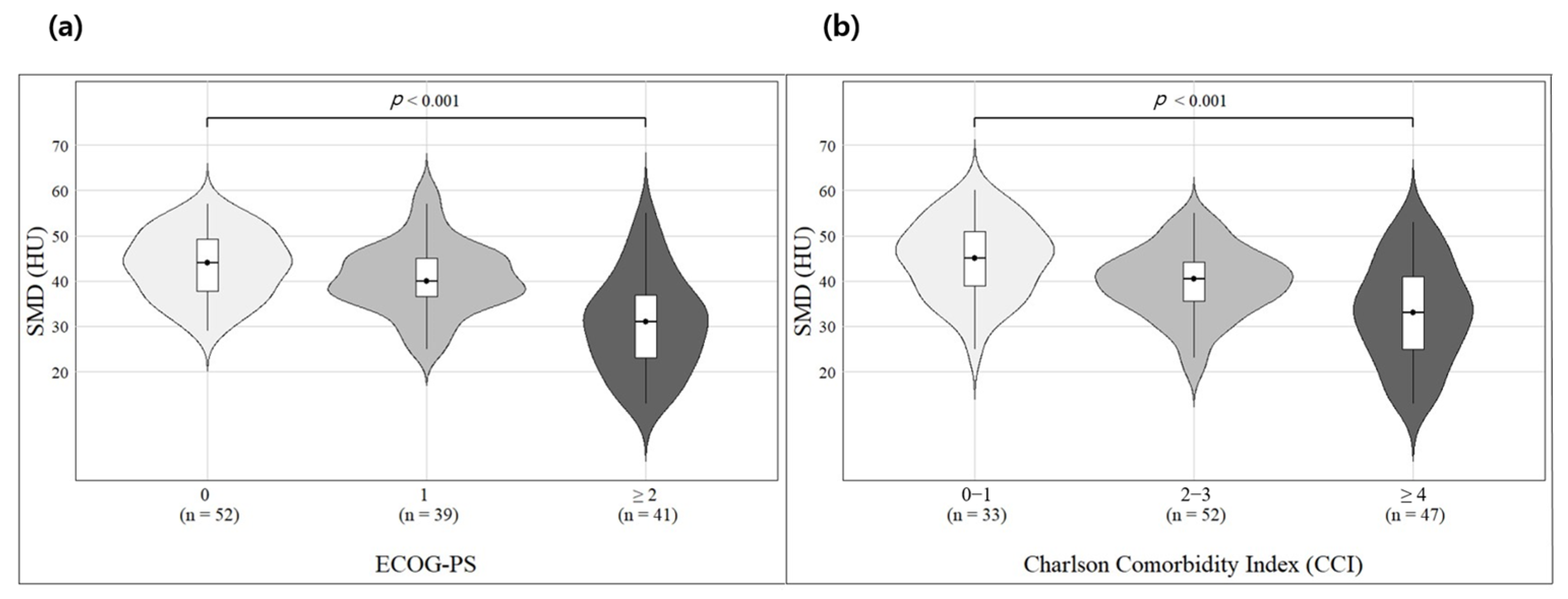
3.2. Correlation Analysis—Skeletal Muscle Indicators and Other Clinical Factors
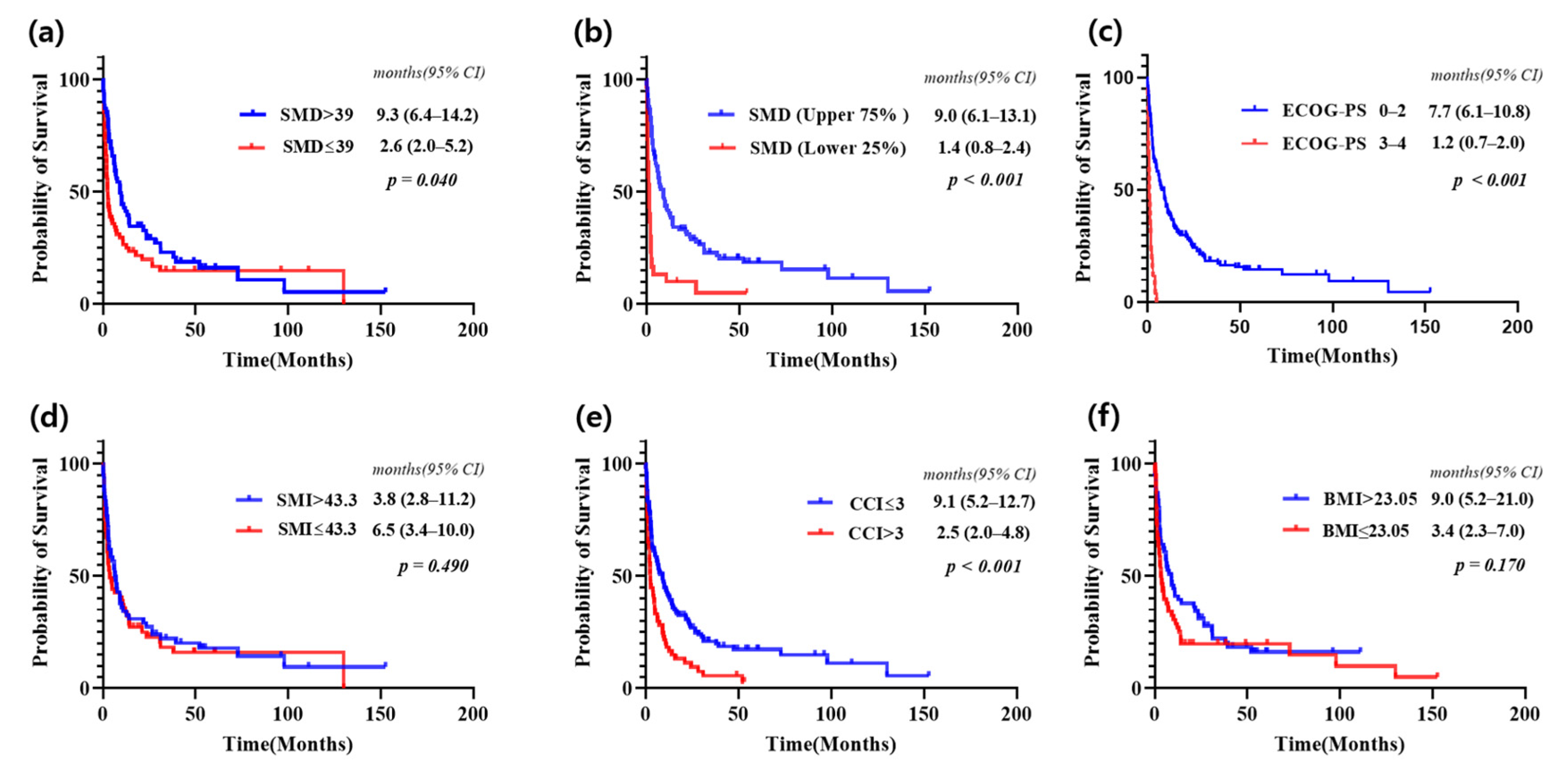
3.3. Survival Analysis Based on SMD and Other Clinical Factors
3.4. Univariate and Multivariate Survival Analysis: Identifying Prognostic Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Cancer of Unknown Primary origin | CUP |
| Eastern Cooperative Oncology Group-Performance Status | ECOG-PS |
| Lactate dehydrogenase | LDH |
| Neutrophil-to-lymphocyte ratio | NLR |
| Computed tomography | CT |
| Skeletal muscle index | SMI |
| Skeletal muscle density | SMD |
| Hounsfield units | HU |
| Overall survival | OS |
| Charlson Comorbidity Index | CCI |
| Median overall survival | mOS |
References
- Lee, M.S.; Sanoff, H.K. Cancer of unknown primary. BMJ 2020, 371, m4050. [Google Scholar] [CrossRef] [PubMed]
- Rassy, E.; Pavlidis, N. The currently declining incidence of cancer of unknown primary. Cancer Epidemiol. 2019, 61, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.; Bochtler, T.; Pauli, C.; Baciarello, G.; Delorme, S.; Hemminki, K.; Mileshkin, L.; Moch, H.; Oien, K.; Olivier, T.; et al. Cancer of unknown primary: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 228–246. [Google Scholar] [CrossRef]
- Fizazi, K.; Greco, F.A.; Pavlidis, N.; Daugaard, G.; Oien, K.; Pentheroudakis, G.; Committee, E.G. Cancers of unknown primary site: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26 (Suppl. 5), v133–v138. [Google Scholar] [CrossRef]
- Rassy, E.; Pavlidis, N. Progress in refining the clinical management of cancer of unknown primary in the molecular era. Nat. Rev. Clin. Oncol. 2020, 17, 541–554. [Google Scholar] [CrossRef]
- Beauchamp, K.; Moran, B.; O’Brien, T.; Brennan, D.; Crown, J.; Sheahan, K.; Cotter, M.B. Carcinoma of unknown primary (CUP): An update for histopathologists. Cancer Metastasis Rev. 2023, 42, 1189–1200. [Google Scholar] [CrossRef]
- Moon, I.; LoPiccolo, J.; Baca, S.C.; Sholl, L.M.; Kehl, K.L.; Hassett, M.J.; Liu, D.; Schrag, D.; Gusev, A. Machine learning for genetics-based classification and treatment response prediction in cancer of unknown primary. Nat. Med. 2023, 29, 2057–2067. [Google Scholar] [CrossRef]
- Abbruzzese, J.L.; Abbruzzese, M.C.; Hess, K.R.; Raber, M.N.; Lenzi, R.; Frost, P. Unknown primary carcinoma: Natural history and prognostic factors in 657 consecutive patients. J. Clin. Oncol. 1994, 12, 1272–1280. [Google Scholar] [CrossRef]
- Choi, J.; Nahm, J.H.; Kim, S.K. Prognostic clinicopathologic factors in carcinoma of unknown primary origin: A study of 106 consecutive cases. Oncotarget 2017, 8, 62630–62640. [Google Scholar] [CrossRef]
- Pavlidis, N.; Pentheroudakis, G.; Plataniotis, G. Cervical lymph node metastases of squamous cell carcinoma from an unknown primary site: A favourable prognosis subset of patients with CUP. Clin. Transl. Oncol. 2009, 11, 340–348. [Google Scholar] [CrossRef]
- Huey, R.W.; Smaglo, B.G.; Estrella, J.S.; Matamoros, A.; Overman, M.J.; Varadhachary, G.R.; Raghav, K.P.S. Cancer of Unknown Primary Presenting as Bone-Predominant or Lymph Node-Only Disease: A Clinicopathologic Portrait. Oncologist 2021, 26, e650–e657. [Google Scholar] [CrossRef] [PubMed]
- Templeton, A.J.; McNamara, M.G.; Šeruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocaña, A.; Leibowitz-Amit, R.; Sonpavde, G.; Knox, J.J.; Tran, B.; et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2014, 106, dju124. [Google Scholar] [CrossRef] [PubMed]
- Culine, S.; Kramar, A.; Saghatchian, M.; Bugat, R.; Lesimple, T.; Lortholary, A.; Merrouche, Y.; Laplanche, A.; Fizazi, K. Development and validation of a prognostic model to predict the length of survival in patients with carcinomas of an unknown primary site. J. Clin. Oncol. 2002, 20, 4679–4683. [Google Scholar] [CrossRef]
- Raghav, K.; Hwang, H.; Jácome, A.A.; Bhang, E.; Willett, A.; Huey, R.W.; Dhillon, N.P.; Modha, J.; Smaglo, B.; Matamoros, A., Jr.; et al. Development and Validation of a Novel Nomogram for Individualized Prediction of Survival in Cancer of Unknown Primary. Clin. Cancer Res. 2021, 27, 3414–3421. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Anderson, D.E.; D’Agostino, J.M.; Bruno, A.G.; Demissie, S.; Kiel, D.P.; Bouxsein, M.L. Variations of CT-based trunk muscle attenuation by age, sex, and specific muscle. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 317–323. [Google Scholar] [CrossRef]
- Aubrey, J.; Esfandiari, N.; Baracos, V.E.; Buteau, F.A.; Frenette, J.; Putman, C.T.; Mazurak, V.C. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol. 2014, 210, 489–497. [Google Scholar] [CrossRef]
- Lee, J.; Park, J.S.; Heo, J.E.; Ahn, H.K.; Jang, W.S.; Ham, W.S.; Rha, K.H.; Choi, Y.D. Muscle Characteristics Obtained Using Computed Tomography as Prognosticators in Patients with Castration-Resistant Prostate Cancer. Cancers 2020, 12, 1864. [Google Scholar] [CrossRef]
- Kroenke, C.H.; Prado, C.M.; Meyerhardt, J.A.; Weltzien, E.K.; Xiao, J.; Cespedes Feliciano, E.M.; Caan, B.J. Muscle radiodensity and mortality in patients with colorectal cancer. Cancer 2018, 124, 3008–3015. [Google Scholar] [CrossRef]
- Caan, B.J.; Cespedes Feliciano, E.M.; Prado, C.M.; Alexeeff, S.; Kroenke, C.H.; Bradshaw, P.; Quesenberry, C.P.; Weltzien, E.K.; Castillo, A.L.; Olobatuyi, T.A.; et al. Association of Muscle and Adiposity Measured by Computed Tomography with Survival in Patients with Nonmetastatic Breast Cancer. JAMA Oncol. 2018, 4, 798–804. [Google Scholar] [CrossRef]
- Kim, Y.Y.; Lee, J.; Jeong, W.K.; Kim, S.T.; Kim, J.H.; Hong, J.Y.; Kang, W.K.; Kim, K.M.; Sohn, I.; Choi, D. Prognostic significance of sarcopenia in microsatellite-stable gastric cancer patients treated with programmed death-1 inhibitors. Gastric Cancer 2021, 24, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Ballinger, T.J.; Marques, H.S.; Xue, G.; Hoffman, R.; Gatsonis, C.; Zhao, F.; Miller, K.D.; Sparano, J.; Connolly, R.M. Impact of Muscle Measures on Outcome in Patients Receiving Endocrine Therapy for Metastatic Breast Cancer: Analysis of ECOG-ACRIN E2112. J. Natl. Compr. Cancer Netw. 2023, 21, 915–923.e911. [Google Scholar] [CrossRef] [PubMed]
- Aleixo, G.F.P.; Shachar, S.S.; Nyrop, K.A.; Muss, H.B.; Malpica, L.; Williams, G.R. Myosteatosis and prognosis in cancer: Systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2020, 145, 102839. [Google Scholar] [CrossRef]
- Ebadi, M.; Martin, L.; Ghosh, S.; Field, C.J.; Lehner, R.; Baracos, V.E.; Mazurak, V.C. Subcutaneous adiposity is an independent predictor of mortality in cancer patients. Br. J. Cancer 2017, 117, 148–155. [Google Scholar] [CrossRef]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef]
- Prado, C.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef]
- Charlson, M.E.; Carrozzino, D.; Guidi, J.; Patierno, C. Charlson Comorbidity Index: A Critical Review of Clinimetric Properties. Psychother. Psychosom. 2022, 91, 8–35. [Google Scholar] [CrossRef]
- Simcock, R.; Wright, J. Beyond Performance Status. Clin. Oncol. (R. Coll. Radiol.) 2020, 32, 553–561. [Google Scholar] [CrossRef]
- Martin, L.; Birdsell, L.; Macdonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef]
- Shachar, S.S.; Williams, G.R.; Muss, H.B.; Nishijima, T.F. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur. J. Cancer 2016, 57, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Antoun, S.; Lanoy, E.; Iacovelli, R.; Albiges-Sauvin, L.; Loriot, Y.; Merad-Taoufik, M.; Fizazi, K.; di Palma, M.; Baracos, V.E.; Escudier, B. Skeletal muscle density predicts prognosis in patients with metastatic renal cell carcinoma treated with targeted therapies. Cancer 2013, 119, 3377–3384. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Baek, S.; Han, S.; Kim, G.M.; Sohn, J.; Rhee, Y.; Hong, N.; Kim, M.H. Low Skeletal Muscle Radiodensity Predicts Response to CDK4/6 Inhibitors Plus Aromatase Inhibitors in Advanced Breast Cancer. J. Cachexia Sarcopenia Muscle 2025, 16, e13666. [Google Scholar] [CrossRef]
- Kim, I.H.; Choi, M.H.; Lee, I.S.; Hong, T.H.; Lee, M.A. Clinical significance of skeletal muscle density and sarcopenia in patients with pancreatic cancer undergoing first-line chemotherapy: A retrospective observational study. BMC Cancer 2021, 21, 77. [Google Scholar] [CrossRef]
- Hayashi, N.; Ando, Y.; Gyawali, B.; Shimokata, T.; Maeda, O.; Fukaya, M.; Goto, H.; Nagino, M.; Kodera, Y. Low skeletal muscle density is associated with poor survival in patients who receive chemotherapy for metastatic gastric cancer. Oncol. Rep. 2016, 35, 1727–1731. [Google Scholar] [CrossRef]
| Variables | Number (Total n = 184, %) |
|---|---|
| Age (years) | |
| <65 | 72 (39.1%) |
| ≥65 | 112 (60.9%) |
| Sex | |
| Female | 82 (44.6%) |
| Male | 102 (55.4%) |
| Histology | |
| Adenocarcinoma | 58 (31.5%) |
| Squamous cell carcinoma | 35 (19.0%) |
| Poorly differentiated carcinoma | 33 (17.9%) |
| Neuroendocrine cell carcinoma | 16 (8.7%) |
| Small cell carcinoma | 4 (2.2%) |
| Undifferentiated carcinoma | 3 (1.6%) |
| Others | 35 (19.0%) |
| ECOG-PS | |
| 0–2 | 157 (85.3%) |
| 3–4 | 27 (14.7%) |
| CCI (Charlson Comorbidity Index) * | |
| ≤3 | 119 (64.7%) |
| >3 | 65 (35.3%) |
| NLR (Neutrophil-to-lymphocyte ratio) | |
| <5 | 100 (54.3%) |
| ≥5 | 84 (45.7%) |
| Disease extent | |
| Single or oligometastasis | 79 (42.9%) |
| Multiple | 105 (57.1%) |
| SMD (Skeletal Muscle Density) | |
| ≤39 | 69 (37.5%) |
| >39 | 63 (34.2%) |
| Not available | 52 (28.3%) |
| SMI (Skeletal Muscle Mass Index) | |
| ≤43.3 | 63 (34.2%) |
| >43.3 | 62 (33.7%) |
| Not available | 59 (32.1%) |
| BMI (Body Mass Index) | |
| ≤23.05 | 62 (33.7%) |
| >23.05 | 63 (34.2%) |
| Not available | 59 (32.1%) |
| Treatment | |
| Chemotherapy group | 60 (32.6%) |
| Local treatment group | 48 (26.1%) |
| No (supportive care group) | 76 (41.3%) |
| Correlation | All (n = 132) | Male (n = 74) | Female (n = 58) | ||||
|---|---|---|---|---|---|---|---|
| r | p Value | r | p Value | r | p Value | ||
| Pearson | SMD vs. Age | −0.510 | <0.001 | −0.511 | <0.001 | −0.542 | <0.001 |
| SMD vs. NLR | −0.205 | 0.019 | −0.262 | 0.024 | −0.134 | 0.317 | |
| SMD vs. SMI | 0.341 | <0.001 | 0.418 | <0.001 | −0.006 | 0.967 | |
| SMD vs. BMI | −0.145 | 0.114 | −0.039 | 0.750 | −0.174 | 0.217 | |
| SMI vs. Age | −0.343 | <0.001 | −0.441 | <0.001 | −0.177 | 0.204 | |
| SMI vs. NLR | −0.273 | 0.002 | −0.329 | 0.005 | −0.214 | 0.124 | |
| SMI vs. BMI | 0.453 | <0.001 | 0.609 | <0.001 | 0.497 | <0.001 | |
| BMI vs. Age | −0.228 | 0.011 | −0.373 | 0.001 | −0.078 | 0.578 | |
| BMI vs. NLR | −0.246 | 0.006 | −0.358 | 0.002 | −0.160 | 0.254 | |
| Spearman | SMD vs. CCI | −0.443 | <0.001 | −0.476 | <0.001 | −0.457 | <0.001 |
| SMD vs. ECOG-PS | −0.488 | <0.001 | −0.523 | <0.001 | −0.486 | <0.001 | |
| SMI vs. CCI | −0.309 | 0.001 | −0.441 | <0.001 | −0.109 | 0.435 | |
| SMI vs. ECOG-PS | −0.224 | 0.013 | −0.349 | 0.003 | −0.107 | 0.446 | |
| BMI vs. CCI | −0.143 | 0.113 | −0.228 | 0.054 | −0.003 | 0.984 | |
| BMI vs. ECOG-PS | −0.131 | 0.147 | −0.259 | 0.028 | 0.052 | 0.710 | |
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Sex (male) | 1.209 (0.880–1.659) | 0.241 | ||
| Age * | 1.030 (1.016–1.044) | <0.001 | 0.992 (0.964–1.021) | 0.590 |
| ECOG-PS (0–4) | 1.935 (1.658–2.258) | <0.001 | 1.275 (0.989–1.644) | 0.061 |
| Histology | 1.775 (1.271–2.478) | 0.001 | 1.498 (0.925–2.427) | 0.100 |
| (adenocarcinoma) | ||||
| Disease extent | 3.800 (2.686–5.375) | <0.001 | 2.254 (1.331–3.819) | 0.003 |
| (multiple) | ||||
| NLR * | 1.051 (1.024–1.078) | <0.001 | 1.041 (0.999–1.085) | 0.058 |
| SMD * | 0.954 (0.934–0.975) | <0.001 | 0.962 (0.937–0.987) | 0.004 |
| CCI (0~8) | 1.222 (1.113–1.341) | <0.001 | 0.965 (0.790–1.180) | 0.731 |
| SMI * | 0.987 (0.965–1.011) | 0.283 | ||
| BMI * | 0.950 (0.897–1.006) | 0.082 | 0.966 (0.909–1.026) | 0.264 |
| Treatment (no treatment) | 1 (reference) | 1 (reference) | ||
| Local treatment | 0.126 (0.069–0.232) | <0.001 | 0.230 (0.100–0.528) | <0.001 |
| Chemotherapy | 0.195 (0.136–0.279) | <0.001 | 0.357 (0.177–0.720) | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.; Park, S.J.; Kim, J.; Hong, S.H.; Kim, I.-H.; Lee, J.; Lee, M.A.; Shin, K.; Mun, H.S. Skeletal Muscle Density as a Predictor of Prognosis and Physical Reserve in Patients with Cancer of Unknown Primary. J. Clin. Med. 2025, 14, 2947. https://doi.org/10.3390/jcm14092947
Lee K, Park SJ, Kim J, Hong SH, Kim I-H, Lee J, Lee MA, Shin K, Mun HS. Skeletal Muscle Density as a Predictor of Prognosis and Physical Reserve in Patients with Cancer of Unknown Primary. Journal of Clinical Medicine. 2025; 14(9):2947. https://doi.org/10.3390/jcm14092947
Chicago/Turabian StyleLee, Kwonjae, Se Jun Park, Joori Kim, Sook Hee Hong, In-Ho Kim, Jieun Lee, Myung Ah Lee, Kabsoo Shin, and Han Song Mun. 2025. "Skeletal Muscle Density as a Predictor of Prognosis and Physical Reserve in Patients with Cancer of Unknown Primary" Journal of Clinical Medicine 14, no. 9: 2947. https://doi.org/10.3390/jcm14092947
APA StyleLee, K., Park, S. J., Kim, J., Hong, S. H., Kim, I.-H., Lee, J., Lee, M. A., Shin, K., & Mun, H. S. (2025). Skeletal Muscle Density as a Predictor of Prognosis and Physical Reserve in Patients with Cancer of Unknown Primary. Journal of Clinical Medicine, 14(9), 2947. https://doi.org/10.3390/jcm14092947






