A Pragmatic Approach to Acute Cardiorenal Syndrome: Diagnostic Strategies and Targeted Therapies to Overcome Diuretic Resistance
Abstract
:1. Introduction
2. Common Misconceptions in Cardiorenal Syndrome
2.1. Arterial Underfilling Versus Venous Congestion
2.2. Rising Serum Creatinine Versus Worsening Renal Function with Diuresis
3. Initial Assessment and Diagnostic Workup
3.1. Rule out Intrinsic Renal Disease
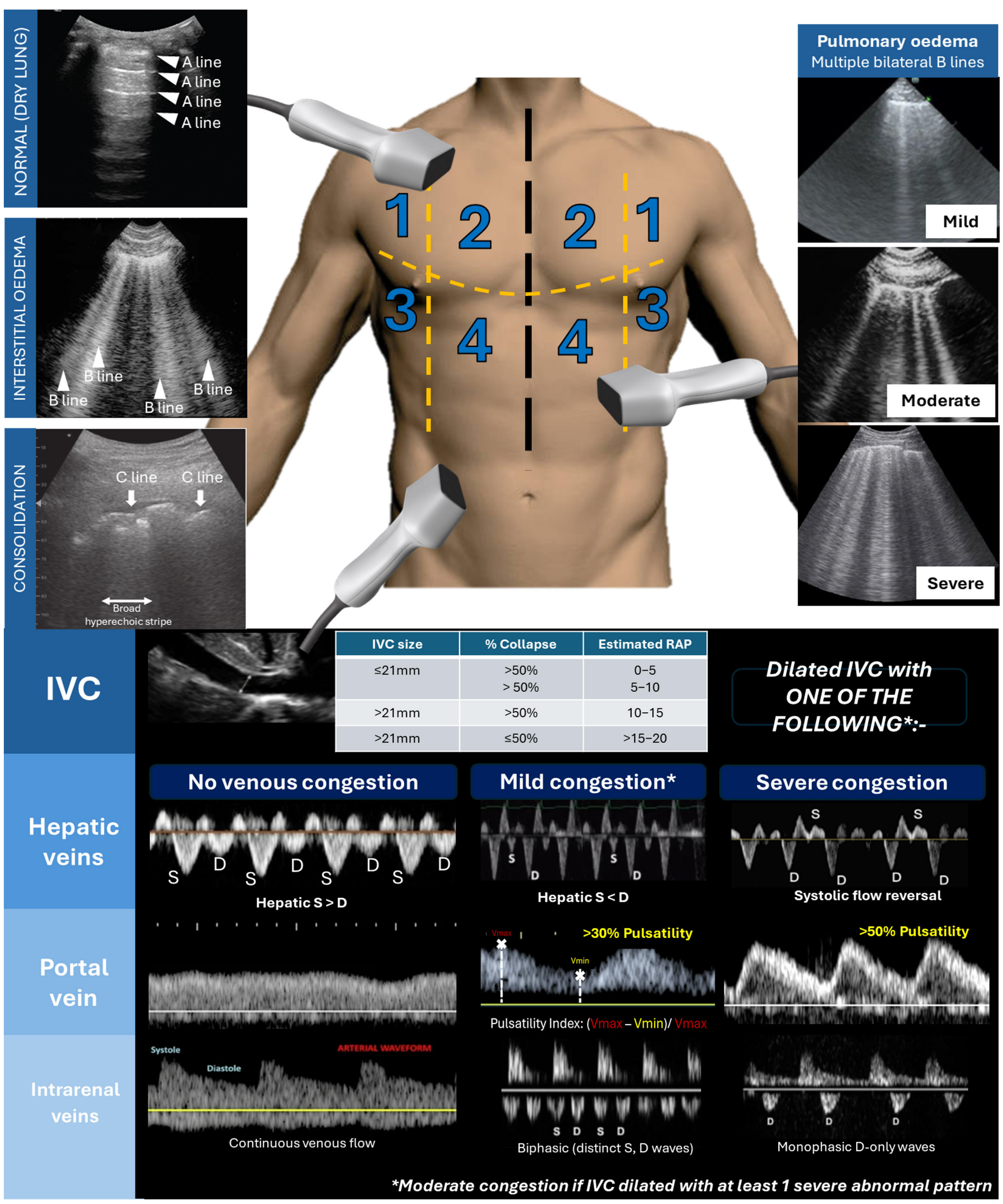
3.2. Ultrasound Assessment of Congestion
4. Decongestive Strategies in Diuretic Resistance
4.1. Furosemide Dose Titration Guided by Spot Urinary Sodium
4.2. Continuous Versus Bolus Furosemide
4.3. Sequential Nephron Blockade
4.4. Hypertonic Saline Solution (HSS)
4.5. Vasopressin-2 Receptor Antagonist
5. Renal Replacement Therapies in Diuretic Resistance
5.1. Peritoneal Dialysis
5.2. Ultrafiltration
6. Role of Inotropic Agents in Acute CRS
7. Long-Term HF Management in CRS
8. Future Directions in CRS Management
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sohal, S.; Uppal, D.; Mathai, S.V.; Wats, K.; Uppal, N.N. Acute Cardiorenal Syndrome: An Update. Cardiol. Rev. 2024, 32, 489–498. [Google Scholar] [CrossRef]
- Kim, J.A.; Wu, L.; Rodriguez, M.; Lentine, K.L.; Virk, H.U.H.; Hachem, K.E.; Lerma, E.V.; Kiernan, M.S.; Rangaswami, J.; Krittanawong, C. Recent Developments in the Evaluation and Management of Cardiorenal Syndrome: A Comprehensive Review. Curr. Probl. Cardiol. 2023, 48, 101509. [Google Scholar] [CrossRef]
- Gallo, G.; Lanza, O.; Savoia, C. New Insight in Cardiorenal Syndrome: From Biomarkers to Therapy. Int. J. Mol. Sci. 2023, 24, 5089. [Google Scholar] [CrossRef]
- Ronco, C.; Haapio, M.; House, A.A.; Anavekar, N.; Bellomo, R. Cardiorenal syndrome. J. Am. Coll. Cardiol. 2008, 52, 1527–1539. [Google Scholar] [CrossRef]
- Heywood, J.T.; Fonarow, G.C.; Costanzo, M.R.; Mathur, V.S.; Wigneswaran, J.R.; Wynne, J. ADHERE Scientific Advisory Committee and Investigators. High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: A report from the ADHERE database. J. Card. Fail. 2007, 13, 422–430. [Google Scholar] [CrossRef]
- Hillege, H.L.; Nitsch, D.; Pfeffer, M.A.; Swedberg, K.; McMurray, J.J.; Yusuf, S.; Granger, C.B.; Michelson, E.L.; Ostergren, J.; Cornel, J.H.; et al. Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation 2006, 113, 671–678. [Google Scholar] [CrossRef]
- Hall, J.E.; Hall, M.E.; Guyton, A.C. Guyton and Hall Textbook of Medical Physiology, 14th ed.; Elsevier: Philadelphia, PA, USA, 2021. [Google Scholar]
- Fallick, C.; Sobotka, P.A.; Dunlap, M.E. Sympathetically mediated changes in capacitance: Redistribution of the venous reservoir as a cause of decompensation. Circ. Heart Fail. 2011, 4, 669–675. [Google Scholar] [CrossRef]
- Binanay, C.; Califf, R.M.; Hasselblad, V.; O’Connor, C.M.; Shah, M.R.; Sopko, G.; Stevenson, L.W. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: The ESCAPE trial. JAMA 2005, 294, 1625–1633. [Google Scholar]
- Anisman, S.D.; Erickson, S.B.; Morden, N.E. How to prescribe loop diuretics in oedema. BMJ 2019, 364, l359. [Google Scholar] [CrossRef]
- Costanzo, M.R. Verdict In: Congestion Guilty! JACC Heart Fail. 2015, 3, 762–764. [Google Scholar] [CrossRef]
- Umanath, K.; Testani, J.M.; Lewis, J.B. “Dip” in eGFR: Stay the Course With SGLT-2 Inhibition. Circulation 2022, 146, 463–465. [Google Scholar] [CrossRef]
- Damman, K.; Beldhuis, I.E.; van der Meer, P.; Krikken, J.A.; Coster, J.E.; Nieuwland, W.; van Veldhuisen, D.J.; Voors, A.A.; Ter Maaten, J.M. Renal function and natriuresis-guided diuretic therapy—A pre-specified analysis from the PUSH-AHF trial. Eur. J. Heart Fail. 2024, 26, 1347–1357. [Google Scholar] [CrossRef]
- Ostermann, M.; Legrand, M.; Meersch, M.; Srisawat, N.; Zarbock, A.; Kellum, J.A. Biomarkers in acute kidney injury. Ann. Intensive Care 2024, 14, 145. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Ahmad, T.; Jackson, K.; Rao, V.S.; Tang, W.H.W.; Brisco-Bacik, M.A.; Chen, H.H.; Felker, G.M.; Hernandez, A.F.; O’Connor, C.M.; Sabbisetti, V.S.; et al. Worsening Renal Function in Patients With Acute Heart Failure Undergoing Aggressive Diuresis Is Not Associated With Tubular Injury. Circulation 2018, 137, 2016–2028. [Google Scholar] [CrossRef]
- Garcia-Pavia, P.; Rapezzi, C.; Adler, Y.; Arad, M.; Basso, C.; Brucato, A.; Burazor, I.; Caforio, A.L.P.; Damy, T.; Eriksson, U.; et al. Diagnosis and treatment of cardiac amyloidosis: A position statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2021, 42, 1554–1568. [Google Scholar] [CrossRef]
- Assavapokee, T.; Rola, P.; Assavapokee, N.; Koratala, A. Decoding VExUS: A practical guide for excelling in point-of-care ultrasound assessment of venous congestion. Ultrasound J. 2024, 16, 48. [Google Scholar] [CrossRef]
- Koratala, A.; Romero-González, G.; Soliman-Aboumarie, H.; Kazory, A. Unlocking the Potential of VExUS in Assessing Venous Congestion: The Art of Doing It Right. Cardiorenal Med. 2024, 14, 350–374. [Google Scholar] [CrossRef]
- Wilcox, C.S.; Testani, J.M.; Pitt, B. Pathophysiology of Diuretic Resistance and Its Implications for the Management of Chronic Heart Failure. Hypertension 2020, 76, 1045–1054. [Google Scholar] [CrossRef]
- Felker, G.M.; Lee, K.L.; Bull, D.A.; Redfield, M.M.; Stevenson, L.W.; Goldsmith, S.R.; LeWinter, M.M.; Deswal, A.; Rouleau, J.L.; Ofili, E.O.; et al. Diuretic strategies in patients with acute decompensated heart failure. N. Engl. J. Med. 2011, 364, 797–805. [Google Scholar] [CrossRef]
- Dauw, J.; Charaya, K.; Lelonek, M.; Zegri-Reiriz, I.; Nasr, S.; Paredes-Paucar, C.P.; Borbély, A.; Erdal, F.; Benkouar, R.; Cobo-Marcos, M.; et al. Protocolized Natriuresis-Guided Decongestion Improves Diuretic Response: The Multicenter ENACT-HF Study. Circ. Heart Fail. 2024, 17, e011105. [Google Scholar] [CrossRef] [PubMed]
- Cuthbert, J.J.; Pellicori, P.; Rigby, A.; Pan, D.; Kazmi, S.; Shah, P.; Clark, A.L. Low serum chloride in patients with chronic heart failure: Clinical associations and prognostic significance. Eur. J. Heart Fail. 2018, 20, 1426–1435. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Sinha, A.D.; Tu, W. Chlorthalidone for Resistant Hypertension in Advanced Chronic Kidney Disease. Circulation 2022, 146, 718–720. [Google Scholar] [CrossRef]
- Cox, Z.L.; Hung, R.; Lenihan, D.J.; Testani, J.M. Diuretic Strategies for Loop Diuretic Resistance in Acute Heart Failure: The 3T Trial. JACC Heart Fail. 2020, 8, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Fudim, M.; Brooksbank, J.; Giczewska, A.; Greene, S.J.; Grodin, J.L.; Martens, P.; Ter Maaten, J.M.; Sharma, A.; Verbrugge, F.H.; Chakraborty, H.; et al. Ultrafiltration in Acute Heart Failure: Implications of Ejection Fraction and Early Response to Treatment From CARRESS-HF. J. Am. Heart Assoc. 2020, 9, e015752. [Google Scholar] [CrossRef]
- Ellison, D.H. Diuretic resistance: Physiology and therapeutics. Semin. Nephrol. 1999, 19, 581–597. [Google Scholar]
- Verbrugge, F.H.; Martens, P.; Ameloot, K.; Haemels, V.; Penders, J.; Dupont, M.; Tang, W.H.W.; Droogné, W.; Mullens, W. Acetazolamide to increase natriuresis in congestive heart failure at high risk for diuretic resistance. Eur. J. Heart Fail. 2019, 21, 1415–1422. [Google Scholar] [CrossRef]
- Martens, P.; Verbrugge, F.H.; Dauw, J.; Nijst, P.; Meekers, E.; Augusto, S.N.; Ter Maaten, J.M.; Heylen, L.; Damman, K.; Mebazaa, A.; et al. Pre-treatment bicarbonate levels and decongestion by acetazolamide: The ADVOR trial. Eur. Heart J. 2023, 44, 1995–2005. [Google Scholar] [CrossRef]
- Yeoh, S.E.; Osmanska, J.; Petrie, M.C.; Brooksbank, K.J.M.; Clark, A.L.; Docherty, K.F.; Foley, P.W.; Guha, K.; Halliday, C.A.; Jhund, P.S.; et al. Dapagliflozin vs metolazone in heart failure resistant to loop diuretics. Eur. Heart J. 2023, 44, 2966–2977. [Google Scholar] [CrossRef]
- Butler, J.; Anstrom, K.J.; Felker, G.M.; Givertz, M.M.; Kalogeropoulos, A.P.; Konstam, M.A.; Mann, D.L.; Margulies, K.B.; McNulty, S.E.; Mentz, R.J.; et al. Efficacy and Safety of Spironolactone in Acute Heart Failure: The ATHENA-HF Randomized Clinical Trial. JAMA Cardiol. 2017, 2, 950–958. [Google Scholar] [CrossRef]
- Cobo Marcos, M.; de la Espriella, R.; Comín-Colet, J.; Zegrí-Reiriz, I.; Rubio Gracia, J.; Morales-Rull, J.L.; Llàcer, P.; Díez-Villanueva, P.; Jiménez-Marrero, S.; de Juan Bagudá, J.; et al. Efficacy and safety of hypertonic saline therapy in ambulatory patients with heart failure: The SALT-HF trial. Eur. J. Heart Fail. 2024, 26, 2118–2128. [Google Scholar] [CrossRef]
- Paterna, S.; Fasullo, S.; Parrinello, G.; Cannizzaro, S.; Basile, I.; Vitrano, G.; Terrazzino, G.; Maringhini, G.; Ganci, F.; Scalzo, S.; et al. Short-term effects of hypertonic saline solution in acute heart failure and long-term effects of a moderate sodium restriction in patients with compensated heart failure with New York Heart Association class III (Class C) (SMAC-HF Study). Am. J. Med. Sci. 2011, 342, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Rossi, J.; Bayram, M.; Udelson, J.E.; Lloyd-Jones, D.; Adams, K.F.; Oconnor, C.M.; Stough, W.G.; Ouyang, J.; Shin, D.D.; Orlandi, C.; et al. Improvement in hyponatremia during hospitalization for worsening heart failure is associated with improved outcomes: Insights from the Acute and Chronic Therapeutic Impact of a Vasopressin Antagonist in Chronic Heart Failure (ACTIV in CHF) trial. Acute Card. Care 2007, 9, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Kazory, A.; Koratala, A.; Ronco, C. Customization of Peritoneal Dialysis in Cardiorenal Syndrome by Optimization of Sodium Extraction. Cardiorenal. Med. 2019, 9, 117–124. [Google Scholar] [CrossRef]
- Grossekettler, L.; Schmack, B.; Meyer, K.; Brockmann, C.; Wanninger, R.; Kreusser, M.M.; Frankenstein, L.; Kihm, L.P.; Zeier, M.; Katus, H.A.; et al. Peritoneal dialysis as therapeutic option in heart failure patients. ESC Heart Fail. 2019, 6, 271–279. [Google Scholar] [CrossRef]
- Lu, R.; Muciño-Bermejo, M.J.; Ribeiro, L.C.; Tonini, E.; Estremadoyro, C.; Samoni, S.; Sharma, A.; Zaragoza Galván, J.d.J.; Crepaldi, C.; Brendolan, A.; et al. Peritoneal dialysis in patients with refractory congestive heart failure: A systematic review. Cardiorenal. Med. 2015, 5, 145–156. [Google Scholar] [CrossRef]
- Mullens, W.; Abrahams, Z.; Francis, G.S.; Taylor, D.O.; Starling, R.C.; Tang, W.H. Prompt reduction in intra-abdominal pressure following large-volume mechanical fluid removal improves renal insufficiency in refractory decompensated heart failure. J. Card. Fail. 2008, 14, 508–514. [Google Scholar] [CrossRef]
- Mullens, W.; Schulze, P.C.; Westphal, J.; Bogoviku, J.; Bauersachs, J. Great debate: In patients with decompensated heart failure, acetazolamide in addition to loop diuretics is the first choice. Eur. Heart J. 2023, 44, 2159–2169. [Google Scholar] [CrossRef]
- Kazory, A.; Ejaz, A.A.; Ross, E.A. The UNLOAD trial: A “nephrologic” standpoint. J. Am. Coll. Cardiol. 2007, 50, 820–821. [Google Scholar] [CrossRef]
- Bart, B.A.; Boyle, A.; Bank, A.J.; Anand, I.; Olivari, M.T.; Kraemer, M.; Mackedanz, S.; Sobotka, P.A.; Schollmeyer, M.; Goldsmith, S.R. Ultrafiltration versus usual care for hospitalized patients with heart failure: The Relief for Acutely Fluid-Overloaded Patients with Decompensated Congestive Heart Failure (RAPID-CHF) trial. J. Am. Coll. Cardiol. 2005, 46, 2043–2046. [Google Scholar] [CrossRef]
- Costanzo, M.R.; Negoianu, D.; Jaski, B.E.; Bart, B.A.; Heywood, J.T.; Anand, I.S.; Smelser, J.M.; Kaneshige, A.M.; Chomsky, D.B.; Adler, E.D.; et al. Aquapheresis Versus Intravenous Diuretics and Hospitalizations for Heart Failure. JACC Heart Fail. 2016, 4, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Villegas-Gutiérrez, L.Y.; Núñez, J.; Kashani, K.; Chávez-Iñiguez, J.S. Kidney Replacement Therapies and Ultrafiltration in Cardiorenal Syndrome. Cardiorenal. Med. 2024, 14, 320–333. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.H.; Anstrom, K.J.; Givertz, M.M.; Stevenson, L.W.; Semigran, M.J.; Goldsmith, S.R.; Bart, B.A.; Bull, D.A.; Stehlik, J.; LeWinter, M.M.; et al. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: The ROSE acute heart failure randomized trial. JAMA 2013, 310, 2533–2543. [Google Scholar] [CrossRef] [PubMed]
- Follath, F.; Cleland, J.G.; Just, H.; Papp, J.G.; Scholz, H.; Peuhkurinen, K.; Harjola, V.P.; Mitrovic, V.; Abdalla, M.; Sandell, E.P.; et al. Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low-output heart failure (the LIDO study): A randomised double-blind trial. Lancet 2002, 360, 196–202. [Google Scholar] [CrossRef]
- Boerrigter, G.; Costello-Boerrigter, L.C.; Abraham, W.T.; Sutton, M.G.; Heublein, D.M.; Kruger, K.M.; Hill, M.R.; McCullough, P.A.; Burnett, J.C., Jr. Cardiac resynchronization therapy improves renal function in human heart failure with reduced glomerular filtration rate. J. Card. Fail. 2008, 14, 539–546. [Google Scholar] [CrossRef]


| Type of CRS | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Acute vs. chronic | Acute | Chronic | Acute | Chronic | Secondary |
| Primary dysfunction | Heart | Heart | Kidney | Kidney | Both |
| Secondary disease | Kidneys | Kidneys | Heart | Heart | Both |
| Example of causes | Acute MI ADHF cardiogenic shock | Chronic congestive cardiac failure | AKI, Acute tubular necrosis, Acute GN | Chronic glomerular disease | Sepsis, infiltration, diabetes, vasculitis, liver cirrhosis |
| Mechanism | Acutely low CO, suddenly high CVP, RAAS/SNS activation | Chronically low CO, high CVP, RAAS activation, endothelial dysfunction | Electrolyte imbalance, hypertension, increased preload to RV, RAAS and SNS activation | Electrolyte imbalance, anaemia, chronic inflammation RAAS/SNS activation | Oxidative stress, Cytokine Inflammation |
| Drug/Intervention | Trials | eGFR Exclusion | Main Finding(s) |
|---|---|---|---|
| Beta-blockers | CIBIS-II MERIT-HF | No limit | Reduce mortality and HFH |
| ACE inhibitors (e.g., enalapril) | CONSENSUS SOLVD | SCr > 300 µmol/L (3.4 mg/dL) SCr > 176.5 µmol/L (2 mg/dL) | Reduce HF-related mortality and slow CKD progression |
| Angiotensin-receptor blockers (e.g., candesartan, valsartan) | CHARM ValHEFT | SCr > 265 µmol (3 mg/dL) | Reduce CV mortality and HFH |
| Sacubitril/Valsartan | PARADIGM-HF | eGFR ≤ 30 | Reduced risk of CV death and HFH; slowed eGFR decline and ESRD |
| SGLT2 inhibitors | DAPA-CKD DAPA-HF EMPEROR-Reduced | eGFR ≤ 25 eGFR ≤ 30 eGFR ≤ 20 | Slowed CKD progression Reduced CV death and HFH |
| Mineralocorticoid antagonists | RALES EMPHASIS-HF | SCr > 221 µmol (>2.5 mg/dL) eGFR ≤ 30 | Reduce mortality and HFH |
| Cardiac re-synchronisation therapy | MIRACLE MADIT-CRT | eGFR < 30 in MIRACLE No cut-off in MADIT-CRT | Increased eGFR by 2.7 ± 1.2 Reduce HFH and mortality |
| Finerenone | FIDELIO-DKD FIGARO-DKD FINEARTS | eGFR ≤ 25 eGFR ≤ 25 | Reduced risk of renal failure, eGFR decline or renal-related deaths Reduced risk of worsening HF (EF > 40%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, P.; Khweir, L.; Kuehl, M.; Joshi, M.; Appunu, K.; Ayub, W.; Banerjee, P. A Pragmatic Approach to Acute Cardiorenal Syndrome: Diagnostic Strategies and Targeted Therapies to Overcome Diuretic Resistance. J. Clin. Med. 2025, 14, 2996. https://doi.org/10.3390/jcm14092996
Tran P, Khweir L, Kuehl M, Joshi M, Appunu K, Ayub W, Banerjee P. A Pragmatic Approach to Acute Cardiorenal Syndrome: Diagnostic Strategies and Targeted Therapies to Overcome Diuretic Resistance. Journal of Clinical Medicine. 2025; 14(9):2996. https://doi.org/10.3390/jcm14092996
Chicago/Turabian StyleTran, Patrick, Laith Khweir, Michael Kuehl, Mithilesh Joshi, Krishna Appunu, Waqar Ayub, and Prithwish Banerjee. 2025. "A Pragmatic Approach to Acute Cardiorenal Syndrome: Diagnostic Strategies and Targeted Therapies to Overcome Diuretic Resistance" Journal of Clinical Medicine 14, no. 9: 2996. https://doi.org/10.3390/jcm14092996
APA StyleTran, P., Khweir, L., Kuehl, M., Joshi, M., Appunu, K., Ayub, W., & Banerjee, P. (2025). A Pragmatic Approach to Acute Cardiorenal Syndrome: Diagnostic Strategies and Targeted Therapies to Overcome Diuretic Resistance. Journal of Clinical Medicine, 14(9), 2996. https://doi.org/10.3390/jcm14092996






