Cognitive Brain Networks and Enlarged Perivascular Spaces: Implications for Symptom Severity and Support Needs in Children with Autism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. MRI Processing
2.3. Mapping and Measurement of Perivascular Spaces
- Temporal lobe: entorhinal cortex, parahippocampal gyrus, fusiform gyrus, superior temporal gyrus, middle temporal gyrus, inferior temporal gyrus, transverse temporal gyrus, and temporal pole (8 areas).
- Frontal lobe: superior frontal, middle frontal gyrus (rostral and caudal), inferior frontal gyrus (pars opercularis, pars triangularis, and pars orbitalis), orbitofrontal gyrus (lateral and medial), precentral gyrus, paracentral lobule, and frontal pole (11 areas).
- Parietal lobe: postcentral gyrus, supramarginal gyrus, superior parietal lobule, inferior parietal lobule, and precuneus (5 areas).
- Occipital lobe: lingual gyrus, pericalcarine cortex, cuneus cortex, and lateral occipital cortex (4 areas).
- Cingulate cortex: rostral anterior, caudal anterior, posterior, and isthmus (4 areas).
- BG areas were subdivided, for each side, into the followings: thalamus, caudate, putamen, and pallidus (4 areas).
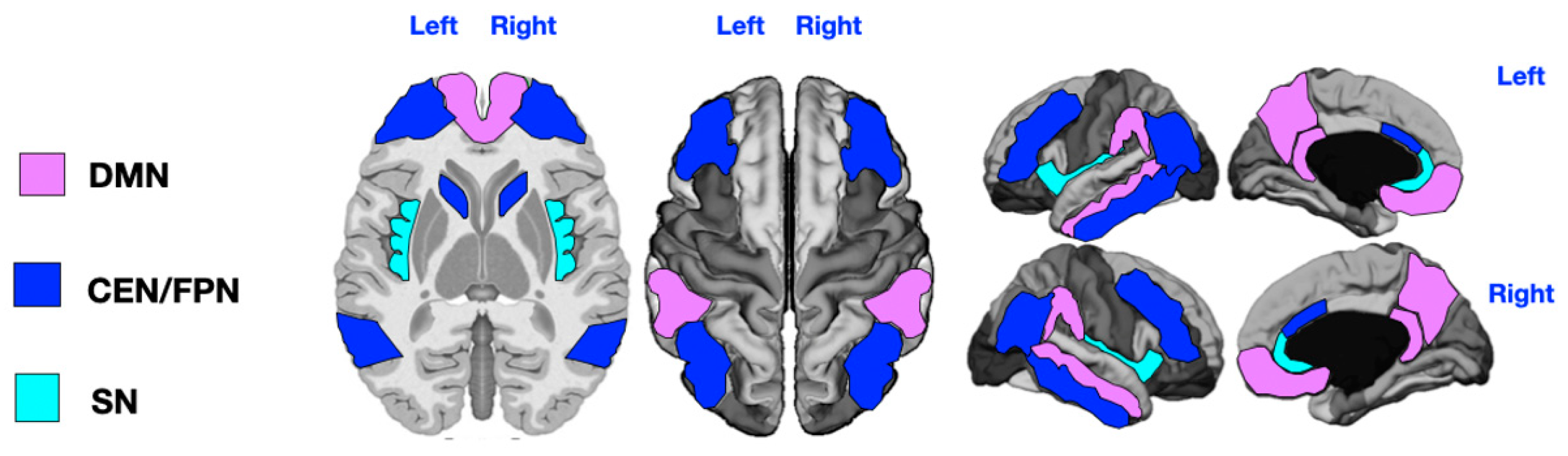
2.4. Brain Functional Networks
2.5. Statistical Analysis
3. Results
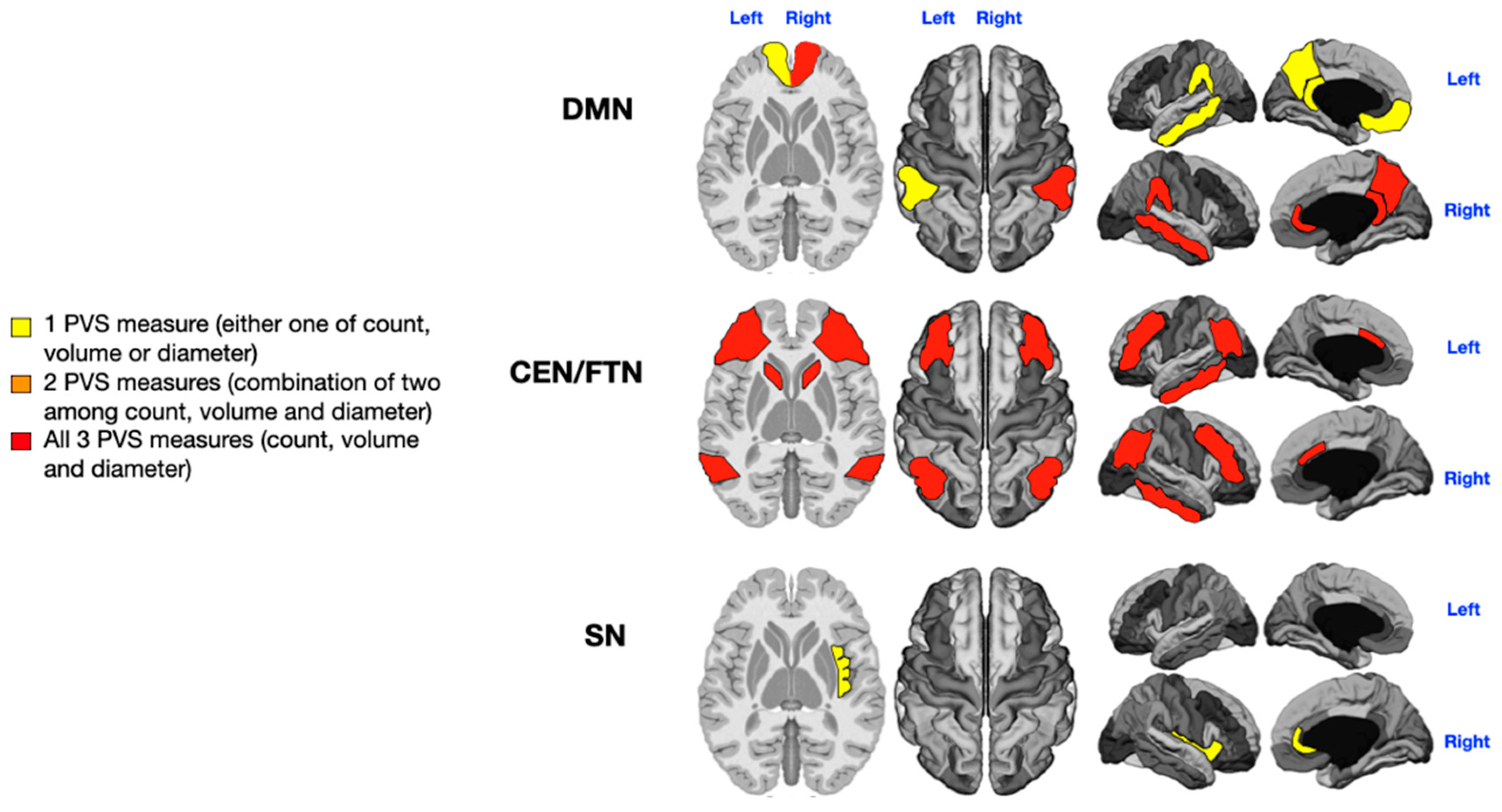
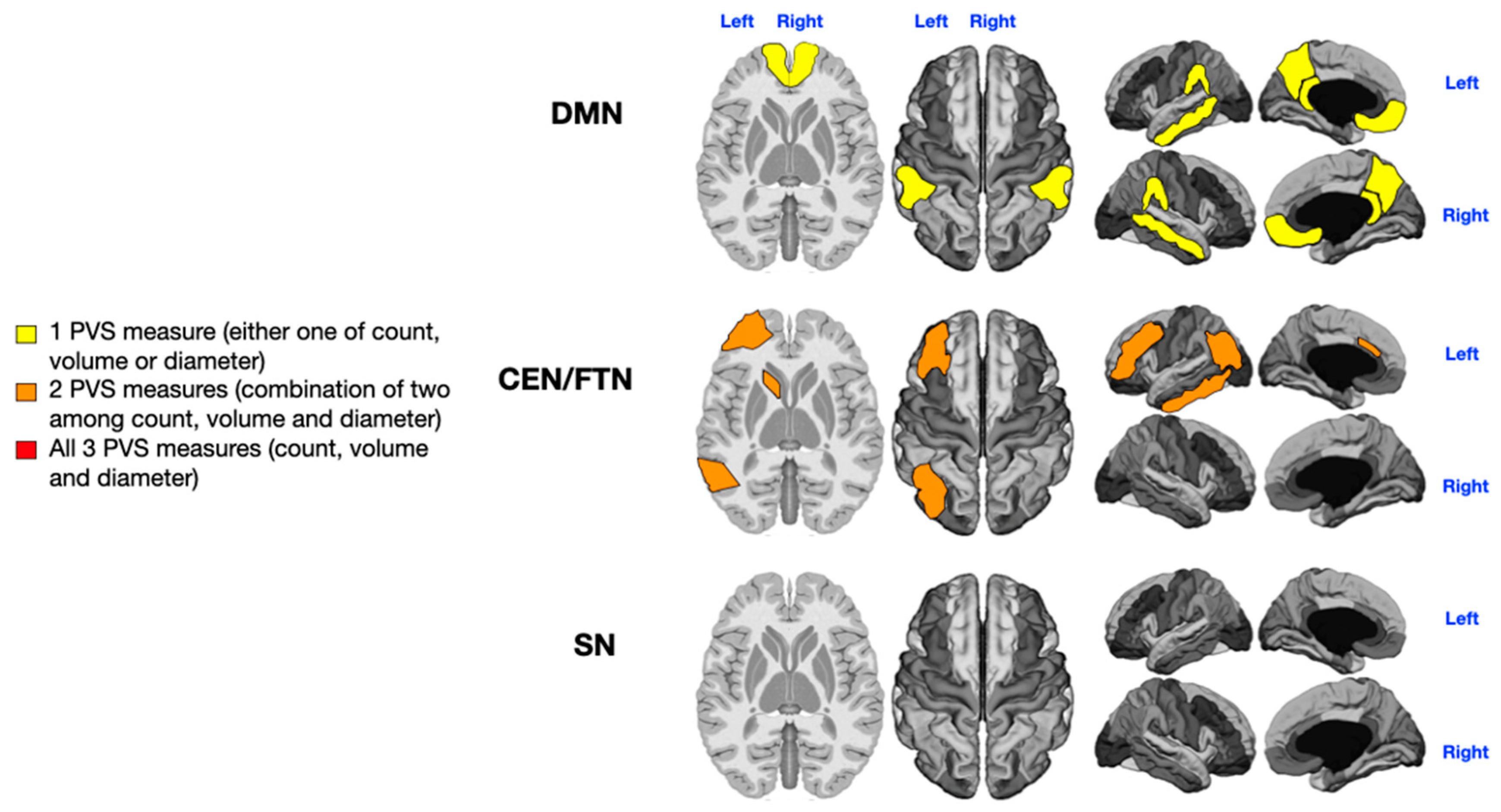
3.1. Language Impairment
3.2. Sensorial Alterations
3.3. Motor Stereotypies
3.4. Level of Rehabilitation Needs
4. Discussion
4.1. Language Impairment
4.2. Sensorial Issues
4.3. Motor Stereotypes
4.4. Level of Support
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADHD | Attention deficit hyperactivity disorder |
| ADOS-2 | Autism Diagnostic Observation Schedule, second edition |
| ASD | Autism spectrum disorder |
| CEN | Central Executive Network |
| CSF | Cerebrospinal fluid |
| DLPFC | Dorsolateral prefrontal cortex |
| DMN | Default Mode Network |
| DSM-5 | Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition |
| ePVS | Enlarged perivascular space |
| fMRI | Functional magnetic resonance imaging |
| FPN | Frontoparietal Network |
| MRI | Magnetic resonance imaging |
| PVS | Perivascular space |
| SN | Salience Network |
| WM | White matter |
References
- American Psychiatric Association aAPAD-TF. Diagnostic and Statistical Manual of Mental Disorders: DSM-5; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Lord, C.; Charman, T.; Havdahl, A.; Carbone, P.; Anagnostou, E.; Boyd, B.; Carr, T.; de Vries, P.J.; Dissanayake, C.; Divan, G.; et al. The Lancet Commission on the future of care and clinical research in autism. Lancet 2022, 399, 271–334. [Google Scholar] [CrossRef] [PubMed]
- Zuckerman, K.E.; Broder-Fingert, S.; Sheldrick, R.C. To reduce the average age of autism diagnosis, screen preschoolers in primary care. Autism 2021, 25, 593–596. [Google Scholar] [CrossRef]
- Hadders-Algra, M. Emerging signs of autism spectrum disorder in infancy: Putative neural substrate. Dev. Med. Child Neurol. 2022, 64, 1344–1350. [Google Scholar] [CrossRef]
- McCrimmon, A.; Rostad, K. Test Review: Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) Manual (Part II): Toddler Module. J. Psychoeduc. Assess. 2013, 32, 88–92. [Google Scholar] [CrossRef]
- dos Santos, C.L.; Barreto, I.I.; Floriano, I.; Tristão, L.S.; Silvinato, A.; Bernardo, W.M. Screening and diagnostic tools for autism spectrum disorder: Systematic review and meta-analysis. Clinics 2024, 79, 100323. [Google Scholar] [CrossRef]
- Lord, C.; Rutter, M.; Le Couteur, A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J. Autism Dev. Disord. 1994, 24, 659–685. [Google Scholar] [CrossRef] [PubMed]
- Schopler, E.; Reichler, R.J.; DeVellis, R.F.; Daly, K. Childhood Autism Rating Scale (CARS, CPRS). APA PsycTests 1980. [Google Scholar] [CrossRef]
- Carta, A.; Fucà, E.; Guerrera, S.; Napoli, E.; Valeri, G.; Vicari, S. Characterization of Clinical Manifestations in the Co-occurring Phenotype of Attention Deficit/Hyperactivity Disorder and Autism Spectrum Disorder. Front. Psychol. 2020, 11, 861. [Google Scholar] [CrossRef]
- Lai, M.C.; Kassee, C.; Besney, R.; Bonato, S.; Hull, L.; Mandy, W.; Szatmari, P.P.; Ameis, S.H. Prevalence of co-occurring mental health diagnoses in the autism population: A systematic review and meta-analysis. Lancet Psychiatry 2019, 6, 819–829. [Google Scholar] [CrossRef]
- Brambilla, P.; Hardan, A.; di Nemi, S.U.; Perez, J.; Soares, J.C.; Barale, F. Brain anatomy and development in autism: Review of structural MRI studies. Brain Res. Bull. 2003, 61, 557–569. [Google Scholar] [CrossRef]
- Nordahl, C.W.; Lange, N.; Li, D.D.; Barnett, L.A.; Lee, A.; Buonocore, M.H.; Simon, T.J.; Rogers, S.; Ozonoff, S.; Amaral, D.G. Brain enlargement is associated with regression in preschool-age boys with autism spectrum disorders. Proc. Natl. Acad. Sci. USA 2011, 108, 20195–20200. [Google Scholar] [CrossRef]
- Donovan, A.P.A.; Basson, M.A. The neuroanatomy of autism—A developmental perspective. Am. J. Anat. 2016, 230, 4–15. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lamanna, J.; Meldolesi, J. Autism Spectrum Disorder: Brain Areas Involved, Neurobiological Mechanisms, Diagnoses and Therapies. Int. J. Mol. Sci. 2024, 25, 2423. [Google Scholar] [CrossRef]
- Shen, M.D.; Nordahl, C.W.; Li, D.D.; Lee, A.; Angkustsiri, K.; Emerson, R.W.; Rogers, S.J.; Ozonoff, S.; Amaral, D.G. Extra-axial cerebrospinal fluid in high-risk and normal-risk children with autism aged 2–4 years: A case-control study. Lancet Psychiatry 2018, 5, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Li, X.B.; Ruan, C.M.; Zibrila, A.I.; Musa, M.; Wu, Y.M.; Zhang, Z.M.; Liu, H.; Salimeen, M. Children with autism spectrum disorder present glymphatic system dysfunction evidenced by diffusion tensor imaging along the perivascular space. Medicine 2022, 101, e32061. [Google Scholar] [CrossRef] [PubMed]
- Wardlaw, J.M.; Benveniste, H.; Nedergaard, M.; Zlokovic, B.V.; Mestre, H.; Lee, H.; Doubal, F.N.; Brown, R.; Ramirez, J.; MacIntosh, B.J.; et al. Perivascular spaces in the brain: Anatomy, physiology and pathology. Nat. Rev. Neurol. 2020, 16, 137–153. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Hu, X.; Li, H.; Zhao, Y. Perivascular Spaces, Glymphatic System and MR. Front. Neurol. 2022, 13, 844938. [Google Scholar] [CrossRef]
- Kamagata, K.; Saito, Y.; Andica, C.; Uchida, W.; Takabayashi, K.; Yoshida, S.; Hagiwara, A.; Fujita, S.; Nakaya, M.; Akashi, T.; et al. Noninvasive Magnetic Resonance Imaging Measures of Glymphatic System Activity. J. Magn. Reson. Imaging 2022, 59, 1476–1493. [Google Scholar] [CrossRef]
- Duering, M.; Biessels, G.J.; Brodtmann, A.; Chen, C.; Cordonnier, C.; de Leeuw, F.-E.; Debette, S.; Frayne, R.; Jouvent, E.; Rost, N.S.; et al. Neuroimaging standards for research into small vessel disease—Advances since 2013. Lancet Neurol. 2023, 22, 602–618, Erratum in Lancet Neurol. 2023, 22, e10. [Google Scholar] [CrossRef]
- Granberg, T.; Moridi, T.; Brand, J.S.; Neumann, S.; Hlavica, M.; Piehl, F.; Ineichen, B.V. Enlarged perivascular spaces in multiple sclerosis on magnetic resonance imaging: A systematic review and meta-analysis. J. Neurol. 2020, 267, 3199–3212. [Google Scholar] [CrossRef]
- Taber, K.H.; Shaw, J.B.; Loveland, K.A.; Pearson, D.A.; Lane, D.M.; Hayman, L.A. Accentuated Virchow-Robin spaces in the centrum semiovale in children with autistic disorder. J. Comput. Assist. Tomogr. 2004, 28, 263–268. [Google Scholar] [CrossRef]
- Zeegers, M.; Van Der Grond, J.; Durston, S.; Nievelstein, R.J.; Witkamp, T.; Van Daalen, E.; Buitelaar, J.; Van Engeland, H. Radiological findings in autistic and developmentally delayed children. Brain Dev. 2006, 28, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Boddaert, N.; Zilbovicius, M.; Philipe, A.; Robel, L.; Bourgeois, M.; Barthélemy, C.; Seidenwurm, D.; Meresse, I.; Laurier, L.; Desguerre, I.; et al. MRI findings in 77 children with non-syndromic autistic disorder. PLoS ONE 2009, 4, e4415. [Google Scholar] [CrossRef]
- Garic, D.; McKinstry, R.C.; Rutsohn, J.; Slomowitz, R.; Wolff, J.; MacIntyre, L.C.; Weisenfeld, L.A.H.; Kim, S.H.; Pandey, J.; John, T.S.; et al. Enlarged Perivascular Spaces in Infancy and Autism Diagnosis, Cerebrospinal Fluid Volume, and Later Sleep Problems. JAMA Netw. Open 2023, 6, e2348341. [Google Scholar] [CrossRef]
- Hayden, M.R. Brain endothelial cell activation and dysfunction associate with and contribute to the development of enlarged perivascular spaces and cerebral small vessel disease. Histol. Histopathol. 2024, 39, 1565–1586. [Google Scholar] [CrossRef] [PubMed]
- Uddin, L.Q.; Castellanos, F.X.; Menon, V. Resting state functional brain connectivity in child and adolescent psychiatry: Where are we now? Neuropsychopharmacology 2024, 50, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Supekar, K.; Musen, M.; Menon, V. Development of large-scale functional brain networks in children. PLoS Biol. 2009, 7, e1000157. [Google Scholar] [CrossRef]
- Uddin, L.Q.; Betzel, R.F.; Cohen, J.R.; Damoiseaux, J.S.; De Brigard, F.; Eickhoff, S.B.; Fornito, A.; Gratton, C.; Gordon, E.M.; Laird, A.R.; et al. Controversies and progress on standardization of large-scale brain network nomenclature. Netw. Neurosci. 2023, 7, 864–903. [Google Scholar] [CrossRef]
- Dadi, K.; Varoquaux, G.; Machlouzarides-Shalit, A.; Gorgolewski, K.J.; Wassermann, D.; Thirion, B.; Mensch, A. Fine-grain atlases of functional modes for fMRI analysis. NeuroImage 2020, 221, 117126. [Google Scholar] [CrossRef]
- Blume, J.; Dhanasekara, C.S.; Kahathuduwa, C.N.; Mastergeorge, A.M. Central Executive and Default Mode Networks: An Appraisal of Executive Function and Social Skill Brain-Behavior Correlates in Youth with Autism Spectrum Disorder. J. Autism Dev. Disord. 2024, 54, 1882–1896. [Google Scholar] [CrossRef]
- Menon, V. 20 years of the default mode network: A review and synthesis. Neuron 2023, 111, 2469–2487. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lin, L.; Zhou, D.; Song, Y.; Stein, A.; Zhou, S.; Xu, H.; Zhao, W.; Cong, F.; Sun, J.; et al. Age-related unstable transient states and imbalanced activation proportion of brain networks in people with autism spectrum disorder: A resting-state fMRI study using coactivation pattern analyses. Netw. Neurosci. 2024, 8, 1173–1191. [Google Scholar] [CrossRef]
- Zhu, J.-S.; Gong, Q.; Zhao, M.-T.; Jiao, Y. Atypical brain network topology of the triple network and cortico-subcortical network in autism spectrum disorder. Neuroscience 2025, 564, 21–30. [Google Scholar] [CrossRef]
- Miller, E.K.; Cohen, J.D. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001, 24, 167–202. [Google Scholar] [CrossRef]
- Turner, K.C.; Frost, L.; Linsenbardt, D.; McIlroy, J.R.; Müller, R.-A. Atypically diffuse functional connectivity between caudate nuclei and cerebral cortex in autism. Behav. Brain Funct. 2006, 2, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Uddin, L.Q. Salience processing and insular cortical function and dysfunction. Nat. Rev. Neurosci. 2015, 16, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Zang, S.; Bai, Y.; Wang, H. Reconfiguration of Brain Network Dynamics in Autism Spectrum Disorder Based on Hidden Markov Model. Front. Hum. Neurosci. 2022, 16, 774921. [Google Scholar] [CrossRef]
- Hong, S.-J.; Vogelstein, J.T.; Gozzi, A.; Bernhardt, B.C.; Yeo, B.T.; Milham, M.P.; Di Martino, A. Toward neurosubtypes in autism. Biol. Psychiatry 2020, 88, 111–128. [Google Scholar] [CrossRef]
- Di Martino, A.; Yan, C.-G.; Li, Q.; Denio, E.; Castellanos, F.X.; Alaerts, K.; Anderson, J.S.; Assaf, M.; Bookheimer, S.Y.; Dapretto, M.; et al. The autism brain imaging data exchange: Towards a large-scale evaluation of the intrinsic brain architecture in autism. Mol. Psychiatry 2013, 19, 659–667. [Google Scholar] [CrossRef]
- Kennedy, D.P.; Courchesne, E. The intrinsic functional organization of the brain is altered in autism. NeuroImage 2008, 39, 1877–1885. [Google Scholar] [CrossRef]
- Guo, X.; Zhai, G.; Liu, J.; Zhang, X.; Zhang, T.; Cui, D.; Zhou, R.; Gao, L. Heterogeneity of dynamic synergetic configurations of salience network in children with autism spectrum disorder. Autism Res. 2023, 16, 2275–2290. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Thomson, P.; Kiar, G.; Castellanos, F.X.; Milham, M.P.; Bernhardt, B.; Di Martino, A. Delineating a Pathway for the Discovery of Functional Connectome Biomarkers of Autism. Adv. Neurobiol. 2024, 40, 511–544. [Google Scholar] [CrossRef] [PubMed]
- Sotgiu, M.A.; Jacono, A.L.; Barisano, G.; Saderi, L.; Cavassa, V.; Montella, A.; Crivelli, P.; Carta, A.; Sotgiu, S. Brain perivascular spaces and autism: Clinical and pathogenic implications from an innovative volumetric MRI study. Front. Neurosci. 2023, 17, 1205489. [Google Scholar] [CrossRef]
- Sotgiu, S.; Cavassa, V.; Puci, M.V.; Sotgiu, M.A.; Turilli, D.; Jacono, A.L.; Nuvoli, A.; Masala, S.; Barisano, G.; Carta, A. Enlarged perivascular spaces under the dorso-lateral prefrontal cortex and severity of autism. Sci. Rep. 2025, 15, 8142. [Google Scholar] [CrossRef] [PubMed]
- NICE—National Institute for Health and Clinical Excellence Autism—Quality Standard (QS51) 2014 (n.d.). Available online: https://www.nice.org.uk/guidance/qs51 (accessed on 1 February 2023).
- Puonti, O.; Iglesias, J.E.; Van Leemput, K. Fast and sequence-adaptive whole-brain segmentation using parametric Bayesian modeling. NeuroImage 2016, 143, 235–249. [Google Scholar] [CrossRef]
- Cox, R.W. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996, 29, 162–173. [Google Scholar] [CrossRef]
- Barisano, G.; Sheikh-Bahaei, N.; Law, M.; Toga, A.W.; Sepehrband, F. Body mass index, time of day and genetics affect perivascular spaces in the white matter. J. Cereb. Blood Flow Metab. 2021, 41, 1563–1578. [Google Scholar] [CrossRef]
- Potter, G.M.; Chappell, F.M.; Morris, Z.; Wardlaw, J.M. Cerebral perivascular spaces visible on magnetic resonance imaging: Development of a qualitative rating scale and its observer reliability. Cerebrovasc. Dis. 2015, 39, 224–231. [Google Scholar] [CrossRef]
- Barisano, G.; Lynch, K.M.; Sibilia, F.; Lan, H.; Shih, N.-C.; Sepehrband, F.; Choupan, J. Imaging perivascular space structure and function using brain MRI. NeuroImage 2022, 257, 119329. [Google Scholar] [CrossRef]
- Klein, A.; Tourville, J. 101 labeled brain images and a consistent human cortical labeling protocol. Front. Neurosci. 2012, 6, 171. [Google Scholar] [CrossRef]
- Schaefer, A.; Kong, R.; Gordon, E.M.; Laumann, T.O.; Zuo, X.-N.; Holmes, A.J.; Eickhoff, S.B.; Yeo, B.T.T. Local-global parcellation of the human cerebral cortex from intrinsic functional connectivity MRI. Cereb. Cortex 2018, 28, 3095–3114. [Google Scholar] [CrossRef]
- Greene, D.J.; Marek, S.; Gordon, E.M.; Siegel, J.S.; Gratton, C.; Laumann, T.O.; Gilmore, A.W.; Berg, J.J.; Nguyen, A.L.; Dierker, D.; et al. Integrative and Network-Specific Connectivity of the Basal Ganglia and Thalamus Defined in Individuals. Neuron 2020, 105, 742–758.e6. [Google Scholar] [CrossRef]
- Waizbard-Bartov, E.; Fein, D.; Lord, C.; Amaral, D.G. Autism severity and its relationship to disability. Autism Res. 2023, 16, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Zhou, X. Executive control in language processing. Neurosci. Biobehav. Rev. 2009, 33, 1168–1177. [Google Scholar] [CrossRef]
- Sherman, L.E.; Rudie, J.D.; Pfeifer, J.H.; Masten, C.L.; McNealy, K.; Dapretto, M. Development of the default mode and central executive networks across early adolescence: A longitudinal study. Dev. Cogn. Neurosci. 2014, 10, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Adolphs, R. The neurobiology of social cognition. Curr. Opin. Neurobiol. 2001, 11, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Gordon, E.M.; Laumann, T.O.; Marek, S.; Raut, R.V.; Gratton, C.; Newbold, D.J.; Greene, D.J.; Coalson, R.S.; Snyder, A.Z.; Schlaggar, B.L.; et al. Default-mode network streams for coupling to language and control systems. Proc. Natl. Acad. Sci. USA 2020, 117, 17308–17319. [Google Scholar] [CrossRef]
- Maximo, J.O.; Cadena, E.J.; Kana, R.K. The Implications of Brain Connectivity in the Neuropsychology of Autism. Neuropsychol. Rev. 2014, 24, 16–31. [Google Scholar] [CrossRef]
- Crinion, J.; Turner, R.; Grogan, A.; Hanakawa, T.; Noppeney, U.; Devlin, J.T.; Aso, T.; Urayama, S.; Fukuyama, H.; Stockton, K.; et al. Language control in the bilingual brain. Science 2006, 312, 1537–1540. [Google Scholar] [CrossRef]
- Baron-Cohen, S.; Ashwin, E.; Ashwin, C.; Tavassoli, T.; Chakrabarti, B. Talent in autism: Hyper-systemizing, hyper-attention to detail and sensory hypersensitivity. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1377–1383. [Google Scholar] [CrossRef]
- Koziol, L.F.; Budding, D.E.; Chidekel, D. Sensory integration, sensory processing, and sensory modulation disorders: Putative functional neuroanatomic underpinnings. Cerebellum 2011, 10, 770–792. [Google Scholar] [CrossRef]
- Menon, V.; Uddin, L.Q. Saliency, switching, attention and control: A network model of insula function. Brain Struct. Funct. 2010, 214, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, L.; Fang, H.; Wang, F.; Gao, T.; Zhu, Q.; Jiao, G.; Ke, X. Social Brain Network of Children with Autism Spectrum Disorder: Characterization of Functional Connectivity and Potential Association with Stereotyped Behavior. Brain Sci. 2023, 13, 280. [Google Scholar] [CrossRef] [PubMed]
- LeMonda, B.C.; Holtzer, R.; Goldman, S. Relationship between executive functions and motor stereotypies in children with Autistic Disorder. Res. Autism Spectr. Disord. 2012, 6, 1099–1106. [Google Scholar] [CrossRef]
- Di Martino, A.; Kelly, C.; Grzadzinski, R.; Zuo, X.-N.; Mennes, M.; Mairena, M.A.; Lord, C.; Castellanos, F.X.; Milham, M.P. Aberrant striatal functional connectivity in children with autism. Biol. Psychiatry 2011, 69, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Kõlves, K.; Fitzgerald, C.; Nordentoft, M.; Wood, S.J.; Erlangsen, A. Assessment of suicidal behaviors among individuals with autism spectrum disorder in Denmark. JAMA Netw. Open 2021, 4, e2033565. [Google Scholar] [CrossRef]
- Eickhoff, S.B.; Yeo, B.T.T.; Genon, S. Imaging-based parcellations of the human brain. Nat. Rev. Neurosci. 2018, 19, 672–686. [Google Scholar] [CrossRef]
- Agam, Y.; Joseph, R.M.; Barton, J.J.; Manoach, D.S. Reduced cognitive control of response inhibition by the anterior cingulate cortex in autism spectrum disorders. NeuroImage 2010, 52, 336–347. [Google Scholar] [CrossRef]
- Ebisch, S.J.; Gallese, V.; Willems, R.M.; Mantini, D.; Groen, W.B.; Romani, G.L.; Buitelaar, J.K.; Bekkering, H. Altered intrinsic functional connectivity of anterior and posterior insula regions in high-functioning participants with autism spectrum disorder. Hum. Brain Mapp. 2010, 32, 1013–1028. [Google Scholar] [CrossRef]
- Monk, C.S.; Weng, S.-J.; Wiggins, J.L.; Kurapati, N.; Louro, H.M.; Carrasco, M.; Maslowsky, J.; Risi, S.; Lord, C. Neural circuitry of emotional face processing in autism spectrum disorders. J. Psychiatry Neurosci. 2010, 35, 105–114. [Google Scholar] [CrossRef]


| Age (n = 36) | Mean = 4 y (SD 2.3) Range = 1–9 y | ≤3 y (n = 21) >3 y (n = 15) | |
|---|---|---|---|
| Sex (n = 36) | M = 22 | F = 14 | |
| Level of rehab needs (n = 36) | Level 1 and 2 (n = 16) | Level 3 (n = 20) | |
| Motor stereotypes (n = 33) | Yes (n = 17) | No (n = 16) | n.k. (n = 3) |
| Sensorial disturbances (n = 33) | Yes (n = 20) | No (n = 13) | n.k. (n = 3) |
| Language impairment (n = 36) | Low–moderate (n = 21) | Absent language (n = 15) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sotgiu, S.; Barisano, G.; Cavassa, V.; Puci, M.V.; Sotgiu, M.A.; Nuvoli, A.; Masala, S.; Carta, A. Cognitive Brain Networks and Enlarged Perivascular Spaces: Implications for Symptom Severity and Support Needs in Children with Autism. J. Clin. Med. 2025, 14, 3029. https://doi.org/10.3390/jcm14093029
Sotgiu S, Barisano G, Cavassa V, Puci MV, Sotgiu MA, Nuvoli A, Masala S, Carta A. Cognitive Brain Networks and Enlarged Perivascular Spaces: Implications for Symptom Severity and Support Needs in Children with Autism. Journal of Clinical Medicine. 2025; 14(9):3029. https://doi.org/10.3390/jcm14093029
Chicago/Turabian StyleSotgiu, Stefano, Giuseppe Barisano, Vanna Cavassa, Mariangela Valentina Puci, Maria Alessandra Sotgiu, Angela Nuvoli, Salvatore Masala, and Alessandra Carta. 2025. "Cognitive Brain Networks and Enlarged Perivascular Spaces: Implications for Symptom Severity and Support Needs in Children with Autism" Journal of Clinical Medicine 14, no. 9: 3029. https://doi.org/10.3390/jcm14093029
APA StyleSotgiu, S., Barisano, G., Cavassa, V., Puci, M. V., Sotgiu, M. A., Nuvoli, A., Masala, S., & Carta, A. (2025). Cognitive Brain Networks and Enlarged Perivascular Spaces: Implications for Symptom Severity and Support Needs in Children with Autism. Journal of Clinical Medicine, 14(9), 3029. https://doi.org/10.3390/jcm14093029









