Effects of the Hormone Replacement Therapy and of Soy Isoflavones on Bone Resorption in Postmenopause
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Design
2.2. Clinical Investigations
2.3. Statistical Analysis
3. Results
3.1. The Occurrence of Risk Factors for Osteoporosis
3.2. Determination of T-Score (DXA) and Correlation with Risk Factors
3.3. Evolution of the Bone Mineral Density
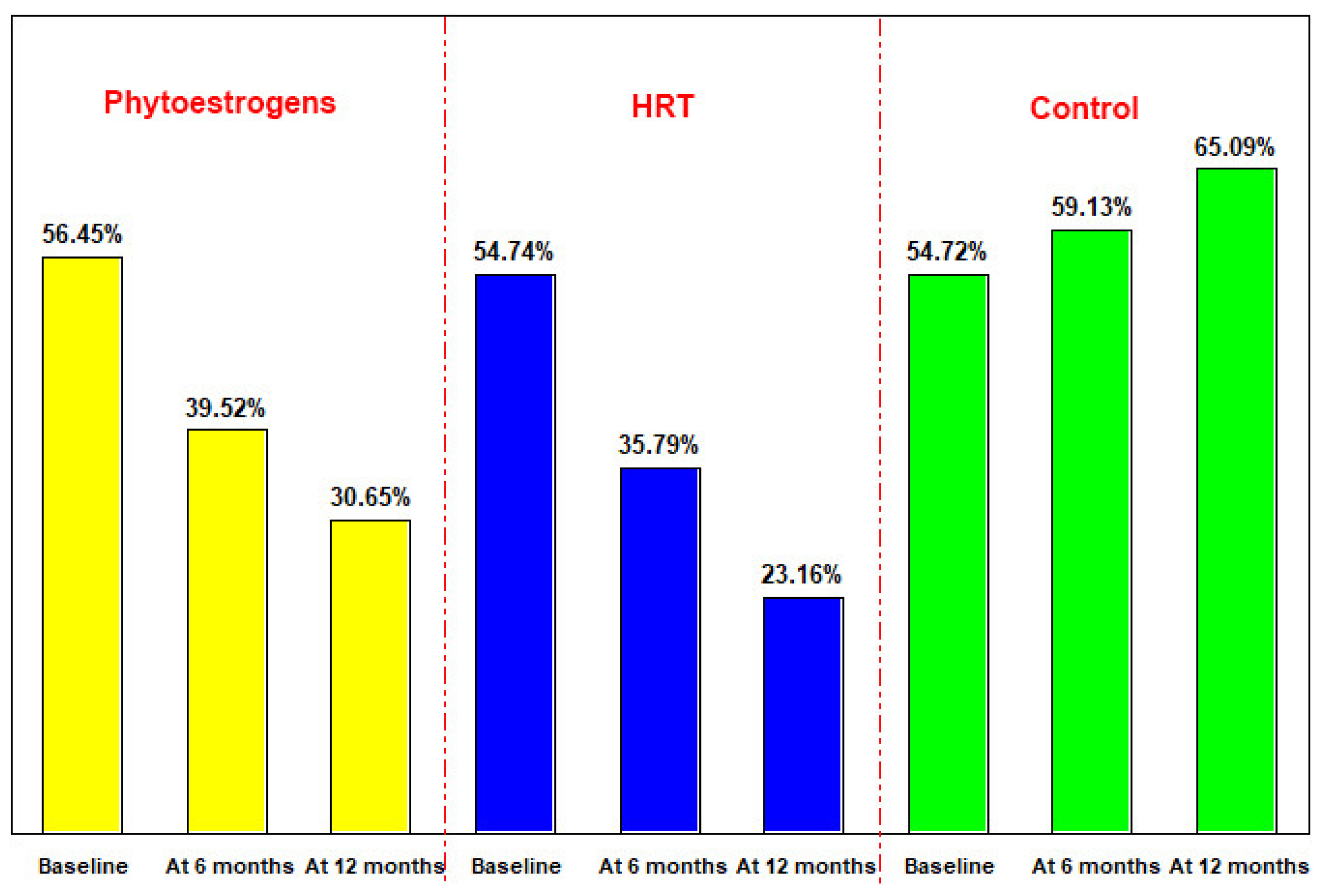
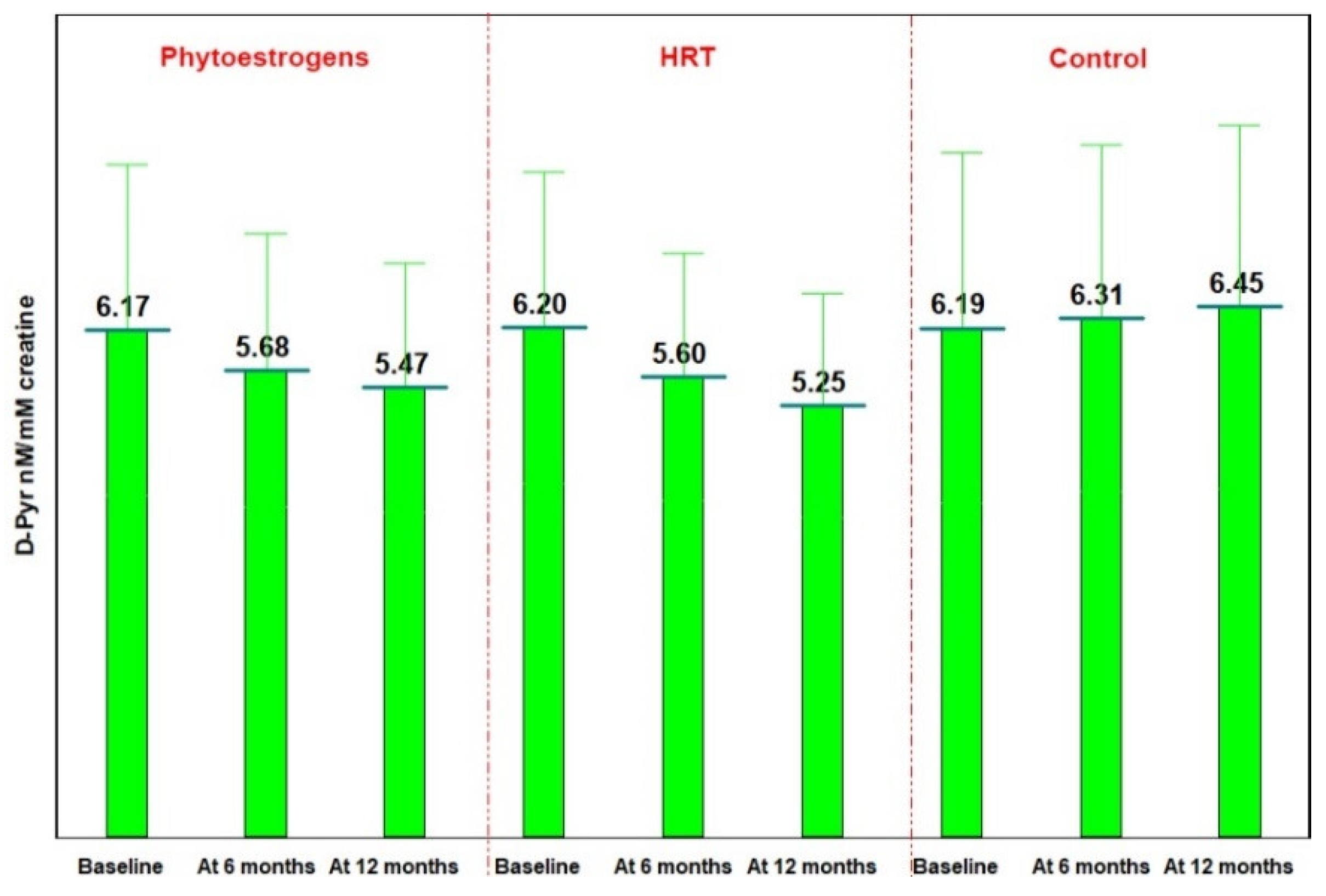
3.4. Bone Resorption Evaluation—DPD Determination in Urine
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Finkelstein, J.S.; Brockwell, S.E.; Vinay, M.; Greendale, G.A.; Sowers, M.R.; Ettinger, B.; Lo, J.C.; Johnston, J.M.; Cauley, J.A.; Danielson, M.E.; et al. Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J. Clin. Endocrinol. Metab. 2008, 93, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, P.; Cooper, C. Osteoporosis. Lancet 2006, 367, 2010–2018. [Google Scholar] [CrossRef]
- Klotzbuecher, C.M.; Ross, P.D.; Landsman, P.B.; Abbott, T.A., III; Berger, M.L. Patients with prior fractures have an increased risk of future fractures: A summary of the literature and statistical synthesis. J. Bone Miner. Res. 2000, 15, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Van der Voort, D.J.M.; Geusens, P.P.; Dinant, G.J. Risk factors for osteoporosis related to their outcome: Fractures. Osteoporos. Int. 2001, 1, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Melton, L.J., 3rd; Chrischilles, E.A.; Cooper, C.; Lane, A.W.; Riggs, B.L. Perspective how many women have osteoporosis? J. Bone Min. Res. 1992, 7, 1005–1010. [Google Scholar] [CrossRef] [PubMed]
- Forddham, J.N. Osteoporosis: Your Questions Answered; Churchill Livingstone: Toronto, ON, Canada, 2004; pp. 154–162. ISBN1 044307366X. ISBN2 9780443073663. [Google Scholar]
- Harvey, N.; Dennison, E.; Cooper, C. Osteoporosis: Impact on healt and economics. Nat. Rev. Rheumatol. 2010, 6, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Palacios, S. Advances in hormone replacement therapy: Making the menopause manageable. BMC Women’s Health 2008, 6874, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Palacios, S.; Calaf, J.; Cano, A.; Parrilla, J.J. Spanish Menopause Society (AEEM), relevant results of the WHI study for the management of the menopause in Spain. Maturitas 2003, 4, 83–86. [Google Scholar] [CrossRef]
- Wells, G.; Tugwell, P.; Shea, B.; Guyatt, G.; Peterson, J.; Zytaruk, N.; Robinson, V.; Henry, D.; O’Connell, D.; Cranney, A.; et al. Meta-analyses of therapies for postmenopausal osteoporosis. V. Meta-analysis of the efficacy of hormone replacement therapy in treating and preventing osteoporosis in postmenopausal women. Endocr. Rev. 2002, 23, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principle results from the Women’s Health Initiative randomized controlled trial. JAMA 2002, 288, 321–333. [Google Scholar] [CrossRef]
- Hsia, J.; Simon, J.A.; Lin, F.; Applegate, W.B.; Vogt, M.T.; Hunninghake, D.; Carr, M. Peripheral arterial disease in randomized trial of estrogen with progestin in women with coronary heart disease: The Heart and Estrogen/Progestin Replacement Study. Circulation 2000, 102, 2228–2232. [Google Scholar] [CrossRef] [PubMed]
- Sowers, M.R.; Huiyong, Z.; Jannausch, M.L.; McConnell, D.; Bin, N.; Harlow, S.; Randolph, J.F. Amount of bone loss in relation to time around the final menstrual period and follicle-stimulating hormone staging of the transmenopause. J. Clin. Endocrinol. Metab. 2010, 95, 2155–2162. [Google Scholar] [CrossRef] [PubMed]
- Sunita, P.; Pattanayak, S.P. Phytoestrogens in postmenopausal indications: A theoretical perspective. Pharmacogn. Rev. 2011, 5, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Bumbu, A.; Pasca, B.; Tit, D.M.; Bungau, S.; Bumbu, G. The effects of soy isoflavones and hormonal replacing therapy on the incidence and evolution of postmenopausal female urinary incontinence. Farmacia 2016, 64, 419–422. [Google Scholar]
- Adlercreutz, H.; Mazur, W. Phyto-oestrogens and western diseases. Ann. Med. 1997, 29, 95–120. [Google Scholar] [CrossRef] [PubMed]
- Somekawa, Y.; Chiguchi, M.; Ishibashi, T.; Aso, T. Soy intake related to menopausal symptoms, serum lipids, and bone mineral density in postmenopausal Japanese women. Obstet. Gynecol. 2001, 97, 109–115. [Google Scholar] [PubMed]
- Zhang, X.; Shu, X.O.; Li, H.; Yang, G.; Li, Q.; Gao, Y.T.; Zheng, W. Prospective cohort study of soy food consumption and risk of bone fracture among postmenopausal women. Arch. Intern. Med. 2005, 165, 1890–1895. [Google Scholar] [CrossRef] [PubMed]
- Messina, M.J.; Wood, C.E. Soy isoflavones, estrogen therapy, and breast cancer risk: Analysis and commentary. Nutr. J. 2008, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Brezinski, A.; Debi, A. Phytoestrogens: The “natural” selective estrogen receptor modulators. Eur. J. Obstet. Gynecol. Rep. Biol. 1999, 85, 47–55. [Google Scholar] [CrossRef]
- da Graça, R.; Campos, M.; Matos, M.P. Bioactivity of Isoflavones: Assessment through a Theoretical Model as a Way to Obtain a “Theoretical Efficacy Related to Estradiol (TERE)”. Int. J. Mol. Sci. 2010, 11, 480–491. [Google Scholar] [CrossRef] [Green Version]
- Szymczak, G.; Wojciak-Kosior, M.; Sowa, I.; Zapala, K.; Strzernski, M.; Kocjan, R. Evaluation of isoflavone content and antioxidant activity of selected soy taxa. J. Food Compost. Anal. 2017, 57, 40–48. [Google Scholar] [CrossRef]
- Fizpatrick, L.A. Soy isoflavones: Hope or Hype. Maturitas 2003, 44, S21–S29. [Google Scholar] [CrossRef]
- Stewart, A.; Kumar, V.; Reid, D.M. Prediction by DXA and QUS: A 10-Year Prospective Study. J. Bone Min. Res. 2006, 21, 413. [Google Scholar] [CrossRef] [PubMed]
- Overgaard, K.; Hansen, M.A.; Riis, B.J.; Christiansen, C. Discriminatory ability of bone mass measurements (SPA and DEXA) for fractures in elderly postmenopausal women. Calcif. Tissue Int. 1992, 50, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Randell, A.G.; Nguyen, T.V.; Bhalerao, N.; Silverman, S.L.; Sam-brook, P.N.; Eisman, J.A. Deterioration of quality of life following hip fracture: A prospective study. Osteoporos. Int. 2000, 11, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; WHO Study Group. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: Synopsis of a WHO report. Osteoporos. Int. 1994, 4, 368–381. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A. Diagnosis of osteoporosis and assessment of fracture risk. Lancet 2002, 359, 1929–1936. [Google Scholar] [CrossRef]
- Boyce, B.F.; Xing, L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch. Biochem. Biophys. 2008, 473, 139–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delmas, P.D.; Ginezts, E.; Bertholin, A.; Garnero, P.; Marchand, F. Immunoassay of pyridinoline crosslink excretion in normal adults and in Paget’s disease. J. Bone Miner. Res. 1993, 8, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.L.; Ho, L.M.; Lim, B.C.; Sim, M.L. Urinary Deoxypyridinoline is a useful biochemical bone marker for the management of postmenopausal osteoporosis. Ann. Acad. Med. Singap. 1998, 27, 527–529. [Google Scholar] [PubMed]
- Tit, D.M.; Bungaau, S.; Cioara, F.; Suciu, N.R. Comparative study on the effects of hormone replacement therapy and phytoestrogens in the prevention of the postmenopausal osteoporosis. In Proceedings of the World Congress on Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (WCO-IOF-ESCEO), Milan, Italy, 26–29 March 2015; p. S245. [Google Scholar]
- Prezelj, J.; Ostanek, B.; Logar, D.B.; Marc, J.; Hawa, G.; Kocjan, T.; Cathepsin, K. predicts femoral neck bone mineral density change in nonosteoporotic peri- and early postmenopausal women. Menopause 2008, 15, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Rubinacci, A.; Melzi, R.; Zampino, M.; Soldarini, A.; Villa, I. Total and free deoxypyridinoline after acute osteoclast activity inhibition. Clin. Chem. 1999, 45, 1510–1516. [Google Scholar] [PubMed]
- Delmas, P.D.; Eastell, R.; Garnero, P.; Seibel, M.J.; Stepan, J. The use of biochemical markers of bone turnover in osteoporosis. Osteoporos Int. 2000, 11, S2–S17. [Google Scholar] [CrossRef] [PubMed]
- Tit, D.M. Comparative Study on the Effects of Hormone Replacement Therapy (HRT) and Phytoestrogens in the Prevention of Postmenopausal Osteoporosis. Ph.D. Thesis, University of Oradea, Oradea, Romania, 2014. [Google Scholar]
- Kanis, J.A.; Johnell, O.; Oden, A.; Sernbo, I.; Redlund-Johnell, I.; Dawson, A.; De Laet, C.; Jonsson, B. Long-term risk of osteoporotic fracture in Malmo. Osteoporos. Int. 2000, 11, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Porter, R.W.; Miller, C.G.; Grainger, D.; Palmer, S.B. Prediction of hip fracture in elderly women: A prospective study. J. Br. Med. 1990, 301, 638–641. [Google Scholar] [CrossRef]
- Lee, S.H.; Dargent-Molina, P.; Breart, G. Risk factors for fractures of the proximal humerus: Results from the EPIDOS prospective study. J. Bone Miner. Res. 2002, 17, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Hassager, C.; Colwell, A.; Assiri, A.M.; Eastell, R.; Russell, R.G.; Christiansen, C. Effect of menopause and HRT on urinary excretion of pyridinium crosslinks: A longitudinal and cross-sectional study. Clin. Endocrinol. 1992, 37, 45–50. [Google Scholar] [CrossRef]
- Marini, H.; Minutoli, L.; Polito, F.; Bitto, A.; Altavilla, D.; Atteritano, M.; Gaudio, A.; Mazzaferro, S.; Frisina, A.; Frisina, N.; et al. Effects of the phytoestrogen genistein on bone metabolism in osteopenia postmenopausal women: A randomized trial. Ann. Intern. Med. 2007, 146, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Gambacciani, M.; Cappagli, B.; Ciaponi, M.; Pepe, A.; Vacca, F.; Genazzani, A.R. Ultra low-dose hormone replacement therapy and bone protection in postmenopausal women. Maturitas 2008, 59, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Tit, D.M.; Pallag, A.; Iovan, C.; Furau, G.; Furau, C.; Bungau, S. Somatic-vegetative symptoms evolution in postmenopausal women treated with phytoestrogens and hormone replacement therapy. Iran. J. Pub. Health 2017, 46, 1128–1134. [Google Scholar]
- Morabito, N.; Crisafulli, A.; Vergara, C.; Gaudio, A.; Lasco, A.; Frisina, N.; D’Anna, R.; Corrado, F.; Pizzoleo, M.A.; Cincotta, M.; et al. Effects of genistein and hormone-replacement therapy on bone loss in early postmenopausal women: A randomized double-blind placebo-controlled study. J. Bone Min. Res. 2002, 17, 1904–1912. [Google Scholar] [CrossRef] [PubMed]
- Espeland, M.A.; Rapp, S.R.; Shumaker, S.A.; Brunner, R.; Manson, J.F.; Sherwin, B.B.; Hsia, J.; Margolis, K.L.; Hogan, P.E.; Wallace, R.; et al. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. JAMA 2004, 291, 2959–2968. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, J.C.; Panay, N.; Pexman-Fieth, C. Oral estradiol and dydrogesterone combination therapy in postmenopausal women: Rewiew of efficacy and safety. Maturitas 2013, 76, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Gennari, L.; Merlotti, D.; Valleggi, F.; Martini, G.; Nuti, R. Selective estrogen receptor modulators for postmenopausal osteoporosis. Drugs Aging 2007, 24, 361. [Google Scholar] [CrossRef] [PubMed]
- Oseni, T.; Patel, R.; Pyle, J.; Jordan, V.C. Selective estrogen receptor modulators and phytoestrogens. Planta. Med. 2008, 74, 1656–1665. [Google Scholar] [CrossRef] [PubMed]
- Moutsatsou, P. The spectrum of phytoestrogens in nature: Our knowledge is expanding. Hormones 2007, 6, 173–193. [Google Scholar] [PubMed]
- Howell, A.; Anderson, A.S.; Clarke, R.B.; Duffy, S.W.; Evans, D.G.; Garcia-Closas, M.; Gescher, A.J.; Key, T.J.; Saxton, J.M.; Harvie, M.N. Risk determination and prevention of breast cancer. Breast Cancer Res. 2014, 16, 446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andres, S.; Abraham, K.; Appel, K.E.; Lampen, A. Risks and benefits of dietary isoflavones for cancer. Crit. Rev. Toxicol. 2011, 41, 463–506. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.Y.; Qin, L.Q. Soy isoflavones consumption and risk of breast cancer incidence or recurrence: A meta-analysis of prospective studies. Breast Cancer Res. Treat. 2011, 125, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.; Madhavan, G.; Tice, J.A.; Leinster, S.J.; Cassidy, A. Effects of isoflavones on breast density in pre- and post-menopausal women: A systematic review and meta-analysis of randomized controlled trials. Hum. Reprod. Update 2010, 16, 745–760. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.H.; Ursin, G.; Koh, W.P.; Wang, R.; Yuan, J.M.; Khoo, K.S.; Yu, M.C. Green tea, soy, and mammographic density in Singapore Chinese women. Cancer Epidemiol. Biomark. Prev. 2008, 17, 3358–3365. [Google Scholar] [CrossRef] [PubMed]
| Risk Factors | Groups | |||||
|---|---|---|---|---|---|---|
| Phytoestrogens | HRT | Control | ||||
| No. | % | No. | % | No. | % | |
| With risk factors | 61 | 49.19 | 44 | 46.32 | 51 | 48.11 |
| Smoking | 36 | 29.03 | 25 | 26.32 | 31 | 29.25 |
| Alcohol | 13 | 10.48 | 7 | 7.37 | 9 | 8.49 |
| Sedentariness | 55 | 44.35 | 40 | 42.11 | 46 | 43.40 |
| Corticosteroids | 5 | 4.03 | 3 | 3.16 | 3 | 2.83 |
| Fracture in history | 8 | 6.45 | 7 | 7.37 | 7 | 6.60 |
| Rheumatoid arthritis | 3 | 2.42 | 1 | 1.05 | 3 | 2.83 |
| FHH * osteoporosis/fractures | 10 | 8.06 | 11 | 11.58 | 12 | 11.32 |
| Without risk factors | 63 | 50.81 | 51 | 53.68 | 55 | 51.89 |
| BMD | Groups | |||||
|---|---|---|---|---|---|---|
| Phytoestrogens | HRT | Control | ||||
| No. | % | No. | % | No. | % | |
| Normal BMD (T-score > −1) | 86 | 69.35 | 65 | 68.42 | 71 | 66.98 |
| Osteopenia (−1 < T-score < −2.5) | 38 | 30.65 | 30 | 31.58 | 35 | 33.02 |
| BMD | Phytoestrogens | HRT | Control | |||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| With risk factors | ||||||
| Normal BMD (T-score > −1) | 34 | 55.74 | 24 | 54.55 | 26 | 50.98 |
| Osteopenia (−1 < T-score < −2.5) | 27 | 44.26 | 20 | 45.45 | 25 | 49.02 |
| Without risk factors | ||||||
| Normal BMD (T-score > −1) | 52 | 82.54 | 41 | 80.39 | 45 | 81.82 |
| Osteopenia (−1 < T-score < −2.5) | 11 | 17.46 | 10 | 19.61 | 10 | 18.18 |
| BMD | Phytoestrogens | HRT | Control | |||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Baseline evaluation | ||||||
| Normal | 86 | 69.35 | 65 | 68.42 | 71 | 66.98 |
| Osteopenia | 38 | 30.65 | 30 | 31.58 | 35 | 33.02 |
| Osteoporosis | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Evaluation at 12 months | ||||||
| Normal | 76 | 61.29 | 62 | 73.68 | 54 | 50.94 |
| Osteopenia | 44 | 35.48 | 32 | 33.68 | 38 | 35.85 |
| Osteoporosis | 4 | 3.23 | 1 | 1.05 | 14 | 13.21 |
| BMD | With Risk Factors | Without Risk Factors | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | At 12 Months | Baseline | At 12 Months | |||||
| No. | % | No. | % | No. | % | No. | % | |
| Phytoestrogens group | ||||||||
| Normal | 34 | 55.74 | 25 | 40.98 | 52 | 82.54 | 51 | 80.95 |
| Osteopenia | 27 | 44.26 | 33 | 54.10 | 11 | 17.46 | 11 | 17.46 |
| Osteoporosis | 0 | 0.00 | 3 | 4.92 | 0 | 0.00 | 1 | 1.59 |
| HRT group | ||||||||
| Normal | 24 | 54.55 | 18 | 40.91 | 41 | 80.39 | 44 | 86.27 |
| Osteopenia | 20 | 45.45 | 25 | 56.82 | 10 | 19.61 | 7 | 13.73 |
| Osteoporosis | 0 | 0.00 | 1 | 2.27 | 0 | 0.00 | 0 | 0.00 |
| Control group | ||||||||
| Normal | 26 | 50.98 | 15 | 29.41 | 45 | 81.82 | 39 | 70.91 |
| Osteopenia | 25 | 49.02 | 28 | 54.90 | 10 | 18.18 | 10 | 18.18 |
| Osteoporosis | 0 | 0.00 | 8 | 15.69 | 0 | 0.00 | 6 | 10.91 |
| D-Pyr Values (nM/mM Creatine) | Groups | |||||
|---|---|---|---|---|---|---|
| Phytoestrogens | HRT | Control | ||||
| No. | % | No. | % | No. | % | |
| 3–3.9 | 8 | 6.45 | 7 | 7.37 | 9 | 8.49 |
| 4–4.9 | 18 | 14.52 | 16 | 16.84 | 13 | 12.26 |
| 5–5.9 | 28 | 22.58 | 20 | 21.05 | 26 | 24.53 |
| 6–6.9 | 38 | 30.65 | 23 | 24.21 | 27 | 25.47 |
| 7–7.9 | 23 | 18.55 | 20 | 21.05 | 21 | 19.81 |
| ≥8 | 9 | 7.26 | 9 | 9.47 | 10 | 9.43 |
| M ± SD | 6.16 ± 2.01 | 6.20 ± 1.89 | 6.19 ± 2.13 | |||
| D-Pyr (nM/mM Creatine) | Groups | |||||
|---|---|---|---|---|---|---|
| Phytoestrogens | HRT | Control | ||||
| No. | % | No. | % | No. | % | |
| With osteopenia | ||||||
| 3–3.9 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| 4–4.9 | 2 | 5.26 | 2 | 6.67 | 3 | 8.57 |
| 5–5.9 | 5 | 13.16 | 4 | 13.33 | 3 | 8.57 |
| 6–6.9 | 9 | 23.68 | 5 | 16.67 | 8 | 22.86 |
| 7–7.9 | 14 | 36.84 | 12 | 40.00 | 12 | 34.29 |
| ≥8 | 8 | 21.05 | 7 | 23.33 | 9 | 25.71 |
| M ± SD | 7.16 ± 2.32 | 7.22 ± 2.41 | 7.23 ± 2.33 | |||
| Without osteopenia | ||||||
| 3–3.9 | 8 | 9.30 | 7 | 10.77 | 9 | 12.68 |
| 4–4.9 | 16 | 18.60 | 14 | 21.54 | 10 | 14.08 |
| 5–5.9 | 23 | 26.74 | 16 | 24.62 | 23 | 32.39 |
| 6–6.9 | 29 | 33.72 | 18 | 27.69 | 19 | 26.76 |
| 7–7.9 | 9 | 10.47 | 8 | 12.31 | 9 | 12.68 |
| ≥8 | 1 | 1.16 | 2 | 3.08 | 1 | 1.41 |
| M ± SD | 5.72 ± 1.64 | 5.70 ± 1.52 | 5.68 ± 1.81 | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tit, D.M.; Bungau, S.; Iovan, C.; Nistor Cseppento, D.C.; Endres, L.; Sava, C.; Sabau, A.M.; Furau, G.; Furau, C. Effects of the Hormone Replacement Therapy and of Soy Isoflavones on Bone Resorption in Postmenopause. J. Clin. Med. 2018, 7, 297. https://doi.org/10.3390/jcm7100297
Tit DM, Bungau S, Iovan C, Nistor Cseppento DC, Endres L, Sava C, Sabau AM, Furau G, Furau C. Effects of the Hormone Replacement Therapy and of Soy Isoflavones on Bone Resorption in Postmenopause. Journal of Clinical Medicine. 2018; 7(10):297. https://doi.org/10.3390/jcm7100297
Chicago/Turabian StyleTit, Delia Mirela, Simona Bungau, Ciprian Iovan, Delia Carmen Nistor Cseppento, Laura Endres, Cristian Sava, Anca Maria Sabau, Gheorghe Furau, and Cristian Furau. 2018. "Effects of the Hormone Replacement Therapy and of Soy Isoflavones on Bone Resorption in Postmenopause" Journal of Clinical Medicine 7, no. 10: 297. https://doi.org/10.3390/jcm7100297
APA StyleTit, D. M., Bungau, S., Iovan, C., Nistor Cseppento, D. C., Endres, L., Sava, C., Sabau, A. M., Furau, G., & Furau, C. (2018). Effects of the Hormone Replacement Therapy and of Soy Isoflavones on Bone Resorption in Postmenopause. Journal of Clinical Medicine, 7(10), 297. https://doi.org/10.3390/jcm7100297









