Association of Pelvic Inflammatory Disease with Risk of Endometriosis: A Nationwide Cohort Study Involving 141,460 Individuals
Abstract
:1. Introduction
2. Methods
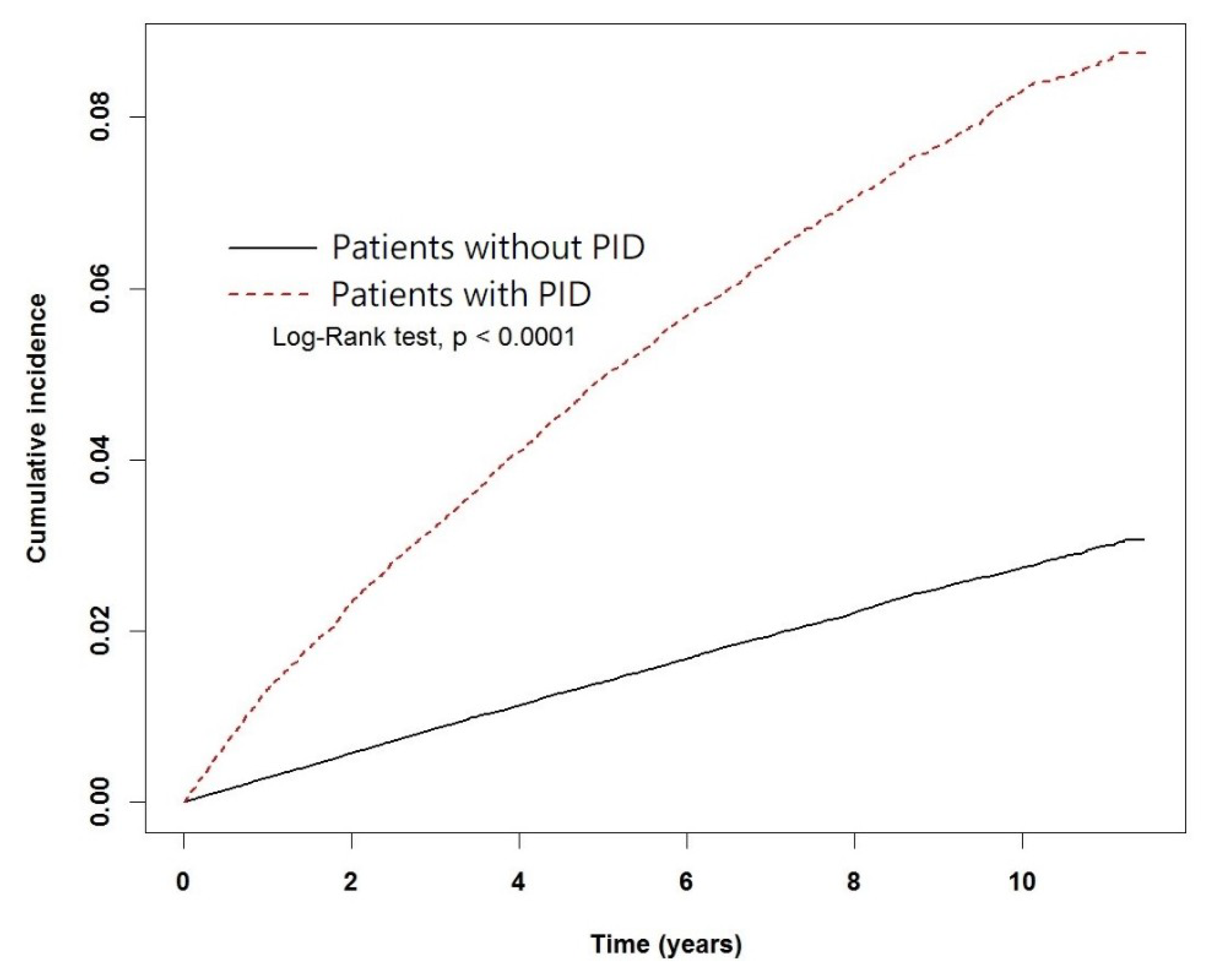
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bulun, S.E. Endometriosis. N. Engl. J. Med. 2009, 360, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Nnoaham, K.E.; Hummelshoj, L.; Webster, P.; d’Hooghe, T.; de Cicco Nardone, F.; de Cicco Nardone, C.; Jenkinson, C.; Kennedy, S.H.; Zondervan, K.T. World Endometriosis Research Foundation Global Study of Women’s Health Consortium. Impact of endometriosis on quality of life and work productivity: A multicenter study across ten countries. Fertil. Steril. 2011, 96, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Sampson, J.A. Metastatic or embolic endometriosis, due to the menstrual dissemination of endometrial tissue into the venous circulation. Am. J. Pathol. 1927, 3, 93–110. [Google Scholar] [PubMed]
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet 2004, 364, 1789–1799. [Google Scholar] [CrossRef]
- Wu, Y.; Kajdacsy-Balla, A.; Strawn, E.; Basir, Z.; Halverson, G.; Jailwala, P.; Wang, Y.; Wang, X.; Ghosh, S.; Guo, S.W. Transcriptional characterizations of differences between eutopic and ectopic endometrium. Endocrinology 2006, 147, 232–246. [Google Scholar] [CrossRef] [PubMed]
- Tseng, J.F.; Ryan, I.P.; Milam, T.D.; Murai, J.T.; Schriock, E.D.; Landers, D.V.; Taylor, R.N. Interleukin-6 secretion in vitro is up-regulated in ectopic and eutopic endometrial stromal cells from women with endometriosis. J. Clin. Endocrinol. Metab. 1996, 81, 1118–1122. [Google Scholar] [PubMed]
- Lucidi, R.S.; Witz, C.A.; Chrisco, M.; Binkley, P.A.; Shain, S.A.; Schenken, R.S. A novel in vitro model of the early endometriotic lesion demonstrates that attachment of endometrial cells to mesothelial cells is dependent on the source of endometrial cells. Fertil. Steril. 2005, 84, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Kao, L.C.; Germeyer, A.; Tulac, S.; Lobo, S.; Yang, J.P.; Taylor, R.N.; Osteen, K.; Lessey, B.A.; Giudice, L.C. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology 2003, 144, 2870–2881. [Google Scholar] [CrossRef] [PubMed]
- Hornung, D.; Ryan, I.P.; Chao, V.A.; Vigne, J.L.; Schriock, E.D.; Taylor, R.N. Immunolocalization and regulation of the chemokine rantes in human endometrial and endometriosis tissues and cells. J. Clin. Endocrinol. Metab. 1997, 82, 1621–1628. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.N.; Lebovic, D.I.; Mueller, M.D. Angiogenic factors in endometriosis. Ann. N. Y. Acad. Sci. 2002, 955, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Cramer, D.W.; Missmer, S.A. The epidemiology of endometriosis. Ann. N. Y. Acad. Sci. 2002, 955, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Moreno, I.; Franasiak, J.M. Endometrial microbiota-new player in town. Fertil. Steril. 2017, 108, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Brunham, R.C.; Gottlieb, S.L.; Paavonen, J. Pelvic inflammatory disease. N. Engl. J. Med. 2015, 372, 2039–2048. [Google Scholar] [CrossRef] [PubMed]
- Kiviat, N.B.; Wolner-Hanssen, P.; Eschenbach, D.A.; Wasserheit, J.N.; Paavonen, J.A.; Bell, T.A.; Critchlow, C.W.; Stamm, W.E.; Moore, D.E.; Holmes, K.K. Endometrial histopathology in patients with culture-proved upper genital tract infection and laparoscopically diagnosed acute salpingitis. Am. J. Surg. Pathol. 1990, 14, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Green, K.A.; Zarek, S.M.; Catherino, W.H. Gynecologic health and disease in relation to the microbiome of the female reproductive tract. Fertil. Steril. 2015, 104, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Braundmeier, A.G.; Lenz, K.M.; Inman, K.S.; Chia, N.; Jeraldo, P.; Walther-Antonio, M.R.; Berg Miller, M.E.; Yang, F.; Creedon, D.J.; Nelson, H.; et al. Individualized medicine and the microbiome in reproductive tract. Front. Physiol. 2015, 6, 97. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Tal, R.; Clark, N.A.; Segars, J.H. Microbiota and pelvic inflammatory disease. Semin. Reprod. Med. 2014, 32, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.D.; Darville, T.; Haggerty, C.L. Does bacterial vaginosis cause pelvic inflammatory disease? Sex. Transm. Dis. 2013, 40, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Brotman, R.M. Vaginal microbiome and sexually transmitted infections: An epidemiologic perspective. J. Clin. Investig. 2011, 121, 4610–4617. [Google Scholar] [CrossRef] [PubMed]
- Nothnick, W.B. Treating endometriosis as an autoimmune disease. Fertil. Steril. 2001, 76, 223–231. [Google Scholar] [CrossRef]
- Bungum, H.F.; Vestergaard, C.; Knudsen, U.B. Endometriosis and type 1 allergies/immediate type hypersensitivity: A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 179, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Kvaskoff, M.; Mu, F.; Terry, K.L.; Harris, H.R.; Poole, E.M.; Farland, L.; Missmer, S.A. Endometriosis: A high-risk population for major chronic diseases? Hum. Reprod. Update 2015, 21, 500–516. [Google Scholar] [CrossRef] [PubMed]
- Gemmill, J.A.; Stratton, P.; Cleary, S.D.; Ballweg, M.L.; Sinaii, N. Cancers, infections, and endocrine diseases in women with endometriosis. Fertil. Steril. 2010, 94, 1627–1631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearce, C.L.; Templeman, C.; Rossing, M.A.; Lee, A.; Near, A.M.; Webb, P.M.; Nagle, C.M.; Doherty, J.A.; Cushing-Haugen, K.L.; Wicklund, K.G.; et al. Association between endometriosis and risk of histological subtypes of ovarian cancer: A pooled analysis of case-control studies. Lancet Oncol. 2012, 13, 385–394. [Google Scholar] [CrossRef]
- Buis, C.C.; van Leeuwen, F.E.; Mooij, T.M.; Burger, C.W.; Group, O.P. Increased risk for ovarian cancer and borderline ovarian tumours in subfertile women with endometriosis. Hum. Reprod. 2013, 28, 3358–3369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, W.C.; Chang, C.Y.; Hsu, Y.A.; Chiang, J.H.; Wan, L. Increased risk of endometriosis in patients with lower genital tract infection: A nationwide cohort study. Medicine 2016, 95, e2773. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.N.; Kitajima, M.; Hiraki, K.; Yamaguchi, N.; Katamine, S.; Matsuyama, T.; Nakashima, M.; Fujishita, A.; Ishimaru, T.; Masuzaki, H. Escherichia coli contamination of menstrual blood and effect of bacterial endotoxin on endometriosis. Fertil. Steril. 2010, 94, 2860–2863. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.N.; Fujishita, A.; Hiraki, K.; Kitajima, M.; Nakashima, M.; Fushiki, S.; Kitawaki, J. Bacterial contamination hypothesis: A new concept in endometriosis. Reprod. Med. Biol. 2018, 17, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.N.; Fujishita, A.; Masumoto, H.; Muto, H.; Kitajima, M.; Masuzaki, H.; Kitawaki, J. Molecular detection of intrauterine microbial colonization in women with endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 199, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Song, X.; Wei, W.; Zhong, H.; Dai, J.; Lan, Z.; Li, F.; Yu, X.; Feng, Q.; Wang, Z.; et al. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat. Commun. 2017, 8, 875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, C.M.; Haick, A.; Nkwopara, E.; Garcia, R.; Rendi, M.; Agnew, K.; Fredricks, D.N.; Eschenbach, D. Colonization of the upper genital tract by vaginal bacterial species in nonpregnant women. Am. J. Obstet. Gynecol. 2015, 212, e611–e619. [Google Scholar] [CrossRef] [PubMed]
- Patton, D.L.; Moore, D.E.; Spadoni, L.R.; Soules, M.R.; Halbert, S.A.; Wang, S.P. A comparison of the fallopian tube’s response to overt and silent salpingitis. Obstet. Gynecol. 1989, 73, 622–630. [Google Scholar] [PubMed]
- Herington, J.L.; Bruner-Tran, K.L.; Lucas, J.A.; Osteen, K.G. Immune interactions in endometriosis. Expert Rev. Clin. Immunol. 2011, 7, 611–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lebovic, D.I.; Mueller, M.D.; Taylor, R.N. Immunobiology of endometriosis. Fertil. Steril. 2001, 75, 1–10. [Google Scholar] [CrossRef]

| Variables | Pelvic Inflammatory Disease | p-Value * | |||
|---|---|---|---|---|---|
| No (n = 113,168) | Yes (n = 28,292) | ||||
| n | % | n | % | ||
| Age, years (mean ± SD) | 34.50 (9.46) | 34.62 (8.91) | |||
| 20–29 | 40,532 | 35.82 | 10,133 | 35.82 | |
| 30–39 | 38,972 | 34.44 | 9743 | 34.44 | |
| 40–49 | 27,868 | 24.63 | 6967 | 24.63 | |
| 50–55 | 5796 | 5.12 | 1449 | 5.12 | |
| Comorbidity | |||||
| Infertility | 1163 | 1.03 | 720 | 2.54 | <0.0001 |
| Uterine Leiomyoma | 2483 | 2.19 | 1442 | 5.1 | <0.0001 |
| Autoimmune diseases | 622 | 0.55 | 212 | 0.75 | <0.0001 |
| Allergic diseases | 11,015 | 9.73 | 3694 | 13.06 | <0.0001 |
| Cancer | 426 | 0.38 | 74 | 0.26 | 0.0036 |
| Breast cancer | 312 | 0.28 | 55 | 0.19 | 0.0162 |
| Cervical cancer | 106 | 0.09 | 19 | 0.07 | 0.1795 |
| Ovarian cancer | 8 | 0.01 | 1 | 0.00 | 0.5050 |
| Follow-up period, years (mean, median) | 8.38 (8.94) | 8.41 (8.99) | |||
| Characteristics | No. of Patients with Endometriosis | Crude | Adjusted | ||||
|---|---|---|---|---|---|---|---|
| n = 4746 | HR | (95% CI) | p-Value | HR | (95% CI) | p-Value | |
| Pelvic inflammatory disease | |||||||
| No | 2646 | 1.00 | reference | 1.00 | reference | ||
| Yes | 2100 | 3.17 | (2.99–3.36) | <0.0001 | 3.02 | (2.85–3.2) | <0.0001 |
| Age, years (mean ± SD) | |||||||
| 20–29 | 1334 | 1.00 | reference | 1.00 | reference | ||
| 30–39 | 2180 | 1.65 | (1.54–1.77) | <0.0001 | 1.61 | (1.5–1.72) | <0.0001 |
| 40–49 | 1185 | 1.25 | (1.15–1.35) | <0.0001 | 1.14 | (1.05–1.24) | 0.0012 |
| 50–55 | 47 | 0.24 | (0.18–0.32) | <0.0001 | 0.22 | (0.16–0.29) | <0.0001 |
| Comorbidity | |||||||
| Infertility | |||||||
| No | 4636 | 1.00 | reference | 1.00 | reference | ||
| Yes | 110 | 1.87 | (1.55–2.26) | <0.0001 | 1.28 | (1.06–1.55) | 0.0107 |
| Uterine Leiomyoma | |||||||
| No | 4388 | 1.00 | reference | 1.00 | reference | ||
| Yes | 358 | 3.07 | (2.76–3.42) | <0.0001 | 2.58 | (2.31–2.88) | <0.0001 |
| Autoimmune diseases | |||||||
| No | 4711 | 1.00 | reference | 1.00 | reference | ||
| Yes | 35 | 1.29 | (0.93–1.8) | 0.1305 | 1.17 | (0.84–1.63) | 0.3538 |
| Allergic diseases | |||||||
| No | 4144 | 1.00 | reference | 1.00 | reference | ||
| Yes | 602 | 1.35 | (1.24–1.47) | <0.0001 | 1.24 | (1.14–1.35) | <0.0001 |
| Cancer | |||||||
| No | 4739 | 1.00 | reference | 1.00 | reference | ||
| Yes | 7 | 0.45 | (0.21–0.94) | 0.0332 | 0.54 | (0.26–1.14) | 0.1058 |
| Variables | Pelvic Inflammatory Disease | Crude HR | Adjusted HR | |||||
|---|---|---|---|---|---|---|---|---|
| No (n = 113168) | Yes (n = 28292) | |||||||
| No. of Patients with Endometriosis | Person-Years | IR | No. of Patients with Endometriosis | Person-Years | IR | (95% CI) | (95% CI) | |
| Total | 2646 | 948,862 | 2.79 | 2100 | 237,882 | 8.83 | 3.17 (2.99–3.36) *** | 3.02 (2.85–3.20) *** |
| Age, years (mean ± SD) | ||||||||
| 20–29 | 714 | 330,919 | 2.16 | 620 | 84,693 | 7.32 | 3.39 (3.05–3.78) *** | 3.26 (2.93–3.64) *** |
| 30–39 | 1254 | 330,618 | 3.79 | 926 | 82,392 | 11.24 | 2.96 (2.72–3.23) *** | 2.81 (2.58–3.07) *** |
| 40–49 | 654 | 238,856 | 2.74 | 531 | 58,540 | 9.07 | 3.32 (2.96–3.72) *** | 3.1 (2.76–3.48) *** |
| 50–55 | 24 | 48,469 | 0.5 | 23 | 12,257 | 1.88 | 3.82 (2.16–6.77) *** | 3.36 (1.88–6.01) *** |
| Comorbidity | ||||||||
| Infertility | ||||||||
| No | 2596 | 939,708 | 2.76 | 2040 | 232,298 | 8.78 | 3.18 (3–3.37) *** | 3.04 (2.87–3.22) *** |
| Yes | 50 | 9154 | 5.46 | 60 | 5584 | 10.75 | 1.96 (1.35–2.85) *** | 1.91 (1.31–2.81) *** |
| Uterine Leiomyoma | ||||||||
| No | 2467 | 929,351 | 2.65 | 1921 | 226,941 | 8.46 | 3.19 (3.01–3.39) *** | 3.14 (2.96–3.34) *** |
| Yes | 179 | 19,511 | 9.17 | 179 | 10,940 | 16.36 | 1.77 (1.44–2.18) *** | 1.67 (1.36–2.06) *** |
| Autoimmune diseases | ||||||||
| No | 2631 | 943,764 | 2.79 | 2080 | 236,230 | 8.8 | 3.16 (2.98–3.35) *** | 3 (2.83–3.18) *** |
| Yes | 15 | 5097 | 2.94 | 20 | 1652 | 12.11 | 4.1 (2.1–8) *** | 4.22 (2.15–8.25) *** |
| Allergic diseases | ||||||||
| No | 2336 | 862,918 | 2.71 | 1808 | 209,416 | 8.63 | 3.19 (3–3.4) *** | 3.05 (2.87–3.25) *** |
| Yes | 310 | 85,943 | 3.61 | 292 | 28,465 | 10.26 | 2.84 (2.42–3.34) *** | 2.75 (2.34–3.23) *** |
| Cancer | ||||||||
| No | 2640 | 945,576 | 2.79 | 2099 | 237,278 | 8.85 | 3.17 (2.99–3.36) *** | 3.02 (2.85–3.2) *** |
| Yes | 6 | 3285 | 1.83 | 1 | 604 | 1.66 | 0.93 (0.11–7.71) | 0.82 (0.1-7) |
| Variable | Adjusted HR (95% CI) | |
|---|---|---|
| Pelvic inflammatory disease | Infertility | |
| No | No | 1 (reference) |
| No | Yes | 1.89 (1.43–2.50) *** |
| Yes | No | 3.05 (2.87–3.23) *** |
| Yes | Yes | 3.52 (2.72–4.55) *** |
| Pelvic inflammatory disease | Uterine Leiomyoma | |
| No | No | 1 (reference) |
| No | Yes | 3.56 (3.05–4.16) *** |
| Yes | No | 3.15 (2.96–3.34) *** |
| Yes | Yes | 6.24 (5.35–7.28) *** |
| Pelvic inflammatory disease | Autoimmune diseases | |
| No | No | 1 (reference) |
| No | Yes | 1.01 (0.61–1.68) |
| Yes | No | 3.01 (2.84–3.19) *** |
| Yes | Yes | 3.80 (2.45–5.91) *** |
| Pelvic inflammatory disease | Allergic diseases | |
| No | No | 1 (reference) |
| No | Yes | 1.29 (1.15–1.45) *** |
| Yes | No | 3.06 (2.87–3.25) *** |
| Yes | Yes | 3.53(3.12–3.99) *** |
| Pelvic inflammatory disease | Cancer | |
| No | No | 1 (reference) |
| No | Yes | 0.65 (0.29–1.44) |
| Yes | No | 3.02 (2.85–3.20) *** |
| Yes | Yes | 0.59 (0.08–4.16) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tai, F.-W.; Chang, C.Y.-Y.; Chiang, J.-H.; Lin, W.-C.; Wan, L. Association of Pelvic Inflammatory Disease with Risk of Endometriosis: A Nationwide Cohort Study Involving 141,460 Individuals. J. Clin. Med. 2018, 7, 379. https://doi.org/10.3390/jcm7110379
Tai F-W, Chang CY-Y, Chiang J-H, Lin W-C, Wan L. Association of Pelvic Inflammatory Disease with Risk of Endometriosis: A Nationwide Cohort Study Involving 141,460 Individuals. Journal of Clinical Medicine. 2018; 7(11):379. https://doi.org/10.3390/jcm7110379
Chicago/Turabian StyleTai, Fei-Wu, Cherry Yin-Yi Chang, Jen-Huai Chiang, Wu-Chou Lin, and Lei Wan. 2018. "Association of Pelvic Inflammatory Disease with Risk of Endometriosis: A Nationwide Cohort Study Involving 141,460 Individuals" Journal of Clinical Medicine 7, no. 11: 379. https://doi.org/10.3390/jcm7110379
APA StyleTai, F.-W., Chang, C. Y.-Y., Chiang, J.-H., Lin, W.-C., & Wan, L. (2018). Association of Pelvic Inflammatory Disease with Risk of Endometriosis: A Nationwide Cohort Study Involving 141,460 Individuals. Journal of Clinical Medicine, 7(11), 379. https://doi.org/10.3390/jcm7110379






