The Safety of Selective Use of Splenic Flexure Mobilization in Sigmoid and Rectal Resections—Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Study Selection
2.2. Outcomes of Interest
2.3. Data Extraction and Quality Assessment
2.4. Data Analysis
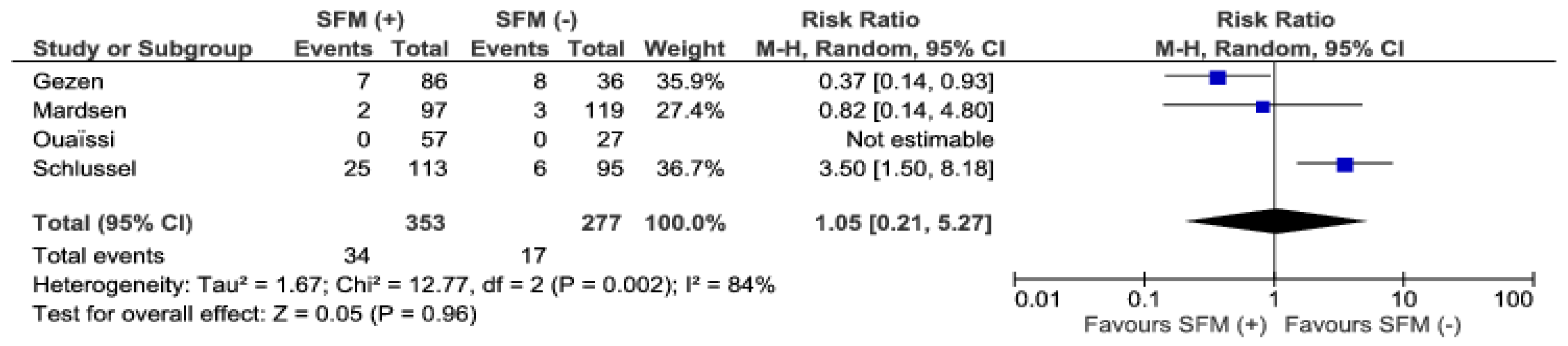
3. Results
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Chand, M.; Moskovic, D.; Parvaiz, A.C. Is splenic flexure mobilization necessary in laparoscopic anterior resection? Dis. Colon. Rectum. 2012, 55, 1195–1197. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y.M.; Lange, M.M.; Buunen, M.; Lange, J.F. Current technique of laparoscopic total mesorectal excision (TME): An international questionnaire among 368 surgeons. Surg. Endosc. 2009, 23, 2796–2801. [Google Scholar] [CrossRef] [PubMed]
- Jamali, F.R.; Soweid, A.M.; Dimassi, H.; Bailey, C.; Leroy, J.; Marescaux, J. Evaluating the degree of difficulty of laparoscopic colorectal surgery. Arch. Surg. 2008, 143, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, R.; Jenkins, I.; Finan, P.J. Splenic flexure mobilization for anterior resection performed for sigmoid and rectal cancer. Ann. R. Coll. Surg. Engl. 2008, 90, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Akiyoshi, T.; Kuroyanagi, H.; Oya, M.; Ueno, M.; Fujimoto, Y.; Konishi, T.; Yamaguchi, T. Factors affecting difficulty of laparoscopic surgery for left-sided colon cancer. Surg. Endosc. 2010, 24, 2749–2754. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.B.; Park, J.S.; Kim, D.W.; Lee, T.G. Intraoperative technical difficulty during laparoscopy-assisted surgery as a prognostic factor for colorectal cancer. Dis. Colon. Rectum. 2010, 53, 1400–1408. [Google Scholar] [CrossRef] [PubMed]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. JAMA 2008, 283. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlson, R.M.; Roberts, P.L.; Hall, J.F.; Marcello, P.W.; Schoetz, D.J.; Read, T.E.; Ricciardi, R. What are 30-day postoperative outcomes following splenic flexure mobilization during anterior resection? Tech. Coloproctol. 2014, 18, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Schlussel, A.T.; Wiseman, J.T.; Kelly, J.F.; Davids, J.S.; Maykel, J.A.; Sturrock, P.R.; Sweeney, W.B.; Alavi, K. Location is everything: The role of splenic flexure mobilization during colon resection for diverticulitis. Int. J. Surg. 2017, 40, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Brennan, D.J.; Moynagh, M.; Brannigan, A.E.; Gleeson, F.; Rowland, M.; O’Connell, P.R. Routine mobilization of the splenic flexure is not necessary during anterior resection for rectal cancer. Dis. Colon. Rectum. 2007, 50, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Chernikovsky, I.L.; Aliev, I.I.; Smirnov, А.А.; Savanovich, N.V.; Gavrilyukov, А.V. Mobilization of splenic flexure during rectal resection. Sib. J. Oncol. 2017, 16, 55–62. [Google Scholar] [CrossRef]
- Gezen, C.; Altuntas, Y.E.; Kement, M.; Vural, S.; Civil, O.; Okkabaz, N.; Aksakal, N.; Oncel, M. Complete versus partial mobilization of splenic flexure during laparoscopic low anterior resection for rectal tumors: A comparative study. J. Laparoendosc. Adv. Surg. Tech. 2012, 22, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Gouvas, N.; Gogos-Pappas, G.; Tsimogiannis, K.; Agalianos, C.; Tsimoyiannis, E.; Dervenis, C.; Xynos, E. Impact of splenic flexure mobilization on short-term outcomes after laparoscopic left colectomy for colorectal cancer. Surg. Laparosc. Endosc. Percutan. Tech. 2014, 24, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Katory, M.; Tang, C.L.; Koh, W.L.; Fook-Chong, S.M.; Loi, T.T.; Ooi, B.S.; Ho, K.S.; Eu, K.W. A 6-year review of surgical morbidity and oncological outcome after high anterior resection for colorectal malignancy with and without splenic flexure mobilization. Color. Dis. 2008, 10, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Marsden, M.R.; Conti, J.A.; Zeidan, S.; Flashman, K.G.; Khan, J.S.; O’Leary, D.P.; Parvaiz, A. The selective use of splenic flexure mobilization is safe in both laparoscopic and open anterior resections. Color. Dis. 2012, 14, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Ouaissi, M.; Mege, D.; Ginger, U.; Iannelli, A.; Lassey, J.; Pirrò, N.; Sielezneff, I.; Sastre, B. Is routine splenic flexure mobilization always mandatory for left colectomy? A comparative study of 80 patients with adenocarcinoma of the sigmoid colon splenic. Am. Surg. 2013, 79, 1305–1308. [Google Scholar] [PubMed]
- Abdollahi, A.; Azizi, R.; Tavasoli, A.; Mashhadi, M.T.R.; Ravan, R.R. The extent of colon mobilization in patients with laparoscopic proctectomy by transanal resection. Color. Dis. 2014, 16, 56–57. [Google Scholar]
- Pędziwiatr, M.; Pisarska, M.; Kisielewski, M.; Major, P.; Mydlowska, A.; Rubinkiewicz, M.; Winiarski, M.; Budzyński, A. ERAS protocol in laparoscopic surgery for colonic versus rectal carcinoma: are there differences in short-term outcomes? Med. Oncol. 2016, 33. [Google Scholar] [CrossRef] [PubMed]
- Pisarska, M.; Pędziwiatr, M.; Małczak, P.; Major, P.; Ochenduszko, S.; Zub-Pokrowiecka, A.; Kulawik, J.; Budzyński, A. Do we really need the full compliance with ERAS protocol in laparoscopic colorectal surgery? A prospective cohort study. Int. J. Surg. 2016, 36, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.; Roulin, D.; Addor, V.; Blanc, C.; Demartines, N.; Hübner, M. Enhanced recovery implementation in colorectal surgery—temporary or persistent improvement? Langenbeck’s Arch. Surg. 2016, 401, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Slieker, J.; Frauche, P.; Jurt, J.; Addor, V.; Blanc, C.; Demartines, N.; Hübner, M. Enhanced recovery ERAS for elderly: A safe and beneficial pathway in colorectal surgery. Int. J. Color. Dis. 2017, 32, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Pędziwiatr, M.; Wierdak, M.; Nowakowski, M.; Pisarska, M.; Stanek, M.; Kisielewski, M.; Matłok, M.; Major, P.; Kłęk, S.; Budzyński, A. Cost minimization analysis of laparoscopic surgery for colorectal cancer within the enhanced recovery after surgery (ERAS) protocol: A single-centre, case-matched study. Videosurg. Other Miniinvasive Tech. 2016, 1, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Wolthuis, A.M.; Bislenghi, G.; Lambrecht, M.; Fieuws, S.; de Buck van Overstraeten, A.; Boeckxstaens, G.; D’Hoore, A. Preoperative risk factors for prolonged postoperative ileus after colorectal resection. Int. J. Colorectal Dis. 2017, 32, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Pędziwiatr, M.; Małczak, P.; Mizera, M.; Witowski, J.; Torbicz, G.; Major, P.; Pisarska, M.; Wysocki, M.; Budzyński, A. There is no difference in outcome between laparoscopic and open surgery for rectal cancer: A systematic review and meta-analysis on short- and long-term oncologic outcomes. Tech. Coloproctol. 2017, 21, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Yi, D.; Monson, J.; Stankiewicz, C.C.; Atallah, S.; Finkler, N.J. Impact of colorectal surgeon case volume on outcomes and applications to quality improvement. Int. J. Colorectal Dis. 2018, 33, 635–644. [Google Scholar] [CrossRef] [PubMed]








| First Author | Year | Country | Study Design | Multi/ Single Centre | Number of Patients SFM (+) | Number of Patients SFM (−) | Female/ Male SFM (+) | Female/ Male SFM (−) | Mean Age SFM + | Mean Age SFM − | Indication Malignant/ Benign SFM (+) | Indication: Malignant/ Benign SFM (−) | (n) Type of ProcedureSFM (+) | (n) Type of Procedure SFM (−) | LAP/OPEN SFM (+) | LAP/OPEN SFM (−) | Study Quality in NOS Scale |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Brennan [12] | 2007 | Ireland | CS | Single | 26 | 74 | 10/16 | 28/46 | 62.0 | 54.0 | 26/0 | 74/0 | 16 LAR, 10 AR | 42 LAR, 32 AR | 0/100 | 0/100 | 7 |
| Carlson [10] | 2014 | USA | CS | Multi | 3890 | 7222 | 2014/1876 | 3694/3528 | 60.0 | 61.3 | 1209/2681 | 2776/4446 | 3890 LAR | 7222 LAR | 1939/1951 | 2849/4373 | 5 |
| Chernikovsky [13] | 2016 | Russia | RC | Single | 32 | 94 | 22/10 | 56/38 | N/A | N/A | 32/0 | 94/0 | 32 LAR | 94 LAR | 32/0 | 94/0 | 5 |
| Gezen [14] | 2012 | Turkey | CS | Multi | 86 | 36 | 29/57 | 16/20 | 59.3 | 55.5 | 86 | 36 | 86 LAR | 36 LAR | 86/0 | 36/0 | 6 |
| Gouvas [15] | 2014 | Greece | CS | Single | 160 | 69 | 77/83 | 36/33 | 64.5 | 64.0 | 160/0 | 69/0 | 160 sigmoid | 69 sigmoid | 160/0 | 69/0 | 5 |
| Katory [16] | 2008 | Singapore | CS | Single | 176 | 531 | 94/82 | 259/272 | 66.0 | 66.0 | 176/0 | 531/0 | 176 HAR | 531 HAR | 0/176 | 0/531 | 7 |
| Mardsen [17] | 2012 | UK | CS | Single | 97 | 119 | 37/60 | 43/76 | N/A | N/A | 97/0 | 119/0 | 58 LAR, 39 HAR | 30 LAR, 89 HAR | 44/53 | 94/25 | 6 |
| Ouaïssi [18] | 2013 | France | CS | Single | 57 | 27 | 36/17 | 8/19 | 70.0 | 69.0 | 57/0 | 27/0 | 57 sigmoid | 27 sigmoid | 17/36 | 12/15 | 7 |
| Schlussel [11] | 2017 | USA | CS | Multi | 113 | 95 | 63/50 | 48/47 | 57.0 | 56.0 | 0/113 | 0/95 | N/A | N/A | 88/25 | 59/36 | 5 |
| Abdollahi [19] | 2014 | Iran | Abstract | Single | 20 | 20 | N/A | N/A | N/A | N/A | N/A | N/A | 20 LAR | 20 LAR | N/A | N/A | 3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowakowski, M.; Małczak, P.; Mizera, M.; Rubinkiewicz, M.; Lasek, A.; Wierdak, M.; Major, P.; Budzyński, A.; Pędziwiatr, M. The Safety of Selective Use of Splenic Flexure Mobilization in Sigmoid and Rectal Resections—Systematic Review and Meta-Analysis. J. Clin. Med. 2018, 7, 392. https://doi.org/10.3390/jcm7110392
Nowakowski M, Małczak P, Mizera M, Rubinkiewicz M, Lasek A, Wierdak M, Major P, Budzyński A, Pędziwiatr M. The Safety of Selective Use of Splenic Flexure Mobilization in Sigmoid and Rectal Resections—Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2018; 7(11):392. https://doi.org/10.3390/jcm7110392
Chicago/Turabian StyleNowakowski, Michał, Piotr Małczak, Magdalena Mizera, Mateusz Rubinkiewicz, Anna Lasek, Mateusz Wierdak, Piotr Major, Andrzej Budzyński, and Michał Pędziwiatr. 2018. "The Safety of Selective Use of Splenic Flexure Mobilization in Sigmoid and Rectal Resections—Systematic Review and Meta-Analysis" Journal of Clinical Medicine 7, no. 11: 392. https://doi.org/10.3390/jcm7110392





