Long-Term Effects of Spironolactone on Kidney Function and Hyperkalemia-Associated Hospitalization in Patients with Chronic Kidney Disease
Abstract
:1. Introduction
2. Experimental Section
2.1. Data Source
2.2. Study Design and Participants
2.3. Outcome Measures and Relevant Variables
2.4. Statistical Analyses
3. Results
3.1. Characteristics of Patients
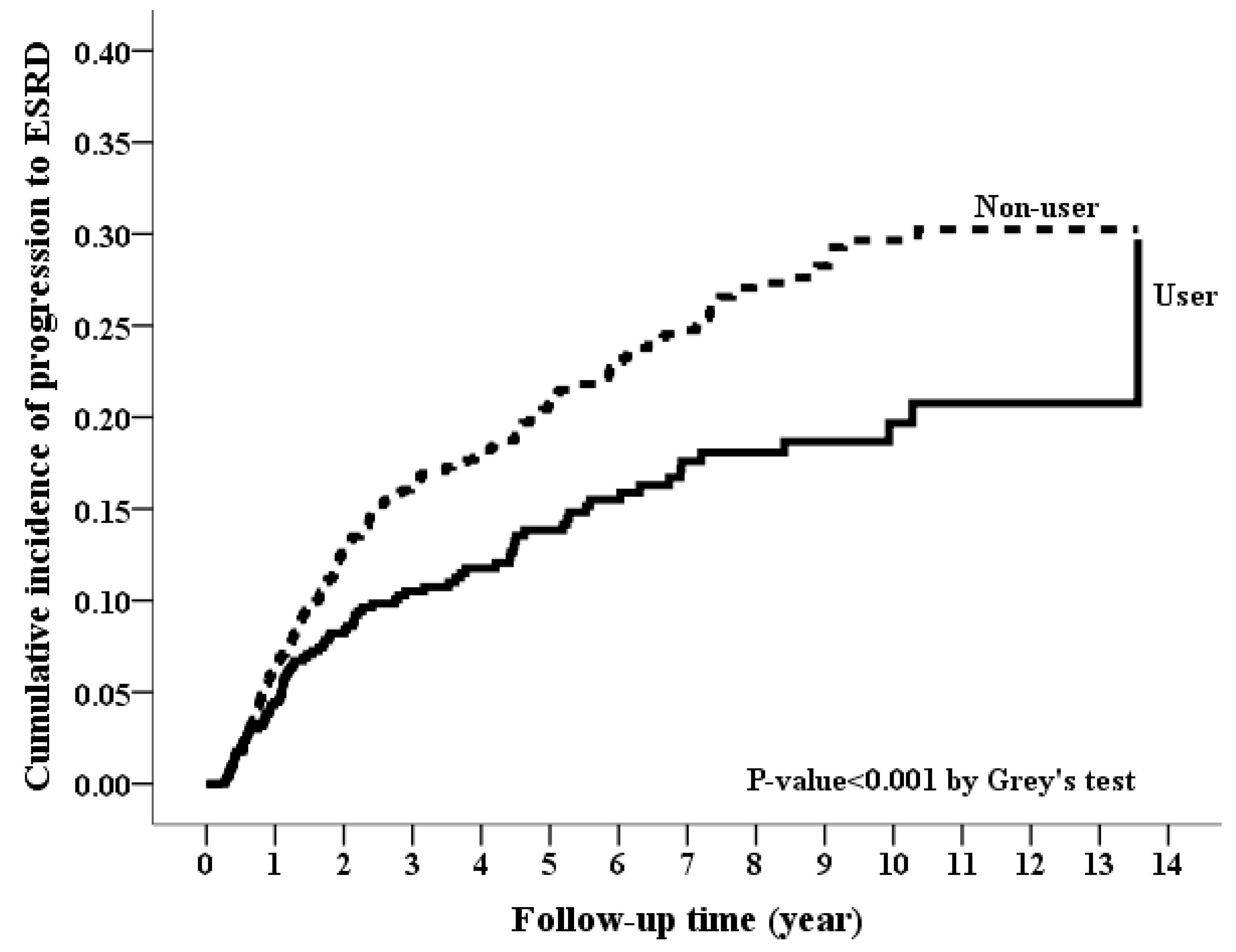
3.2. Long-Term Risk of Incident ESRD
3.3. Long-Term Risk of Hyperkalemia-Associated Hospitalization
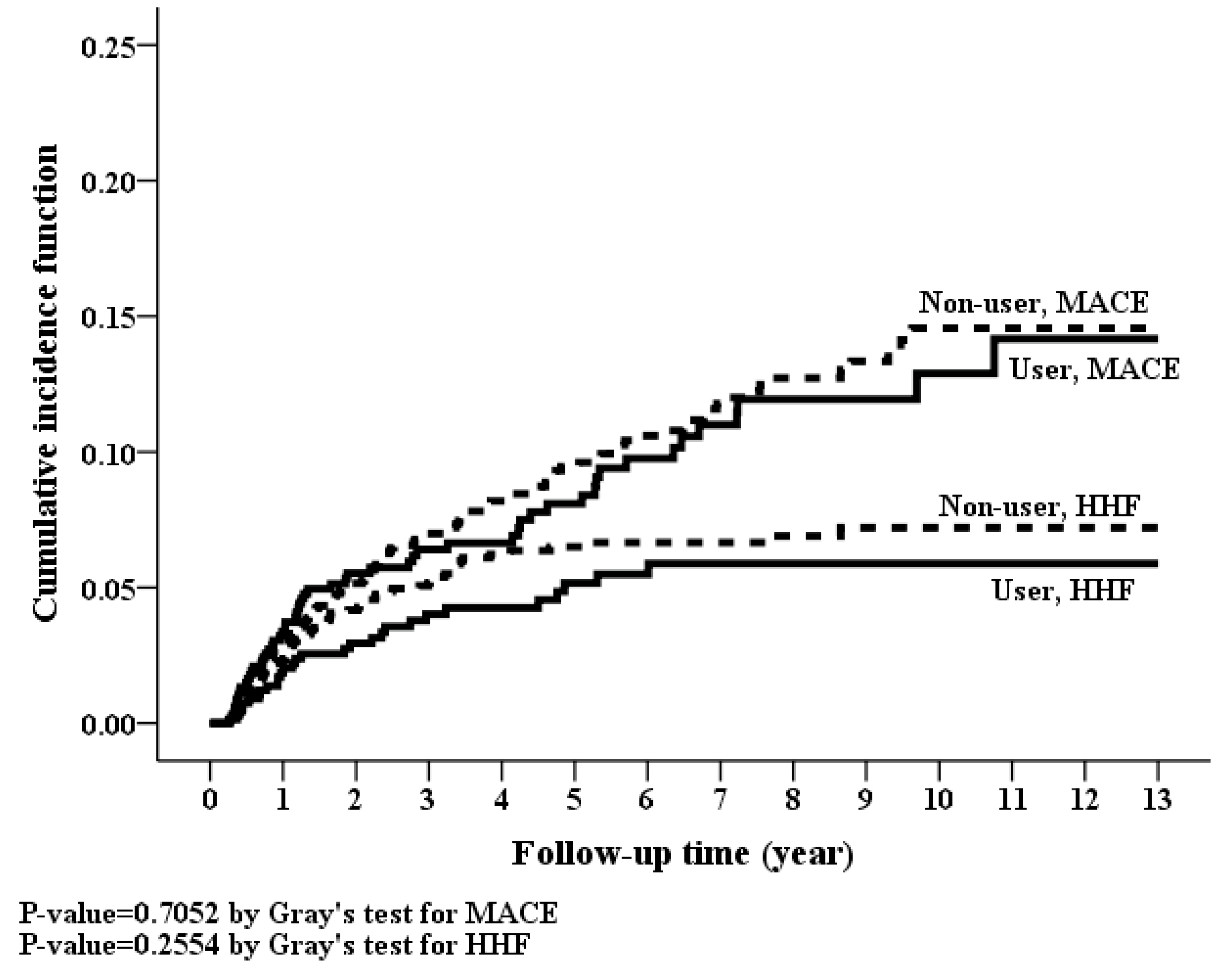
3.4. Long-Term Risk of MACE, Hospitalization for Heart Failure and Mortality
3.5. Subgroup Analysis
3.6. Sensitivity Analyses
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Gansevoort, R.T.; Correa-Rotter, R.; Hemmelgarn, B.R.; Jafar, T.H.; Heerspink, H.J.; Mann, J.F.; Matsushita, K.; Wen, C.P. Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. Lancet 2017, 382, 339–352. [Google Scholar] [CrossRef]
- Tonelli, M.; Wiebe, N.; Culleton, B.; House, A.; Rabbat, C.; Fok, M.; McAlister, F.; Garg, A.X. Chronic kidney disease and mortality risk: A systematic review. J. Am. Soc. Nephrol. 2006, 17, 2034–2047. [Google Scholar] [CrossRef] [PubMed]
- Tomiyama, C.; Higa, A.; Dalboni, M.A.; Cendoroglo, M.; Draibe, S.A.; Cuppari, L.; Carvalho, A.B.; Neto, E.M.; Canziani, M.E. The impact of traditional and nontraditional risk factors on coronary calcification in pre-dialysis patients. Nephrol. Dial. Transpl. 2006, 21, 2464–2471. [Google Scholar] [CrossRef] [PubMed]
- Brenner, B.M.; Cooper, M.E.; de Zeeuw, D.; Keane, W.F.; Mitch, W.E.; Parving, H.H.; Remuzzi, G.; Snapinn, S.M.; Zhang, Z.; Shahinfar, S.; et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N. Engl. J. Med. 2001, 345, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Jafar, T.H.; Schmid, C.H.; Landa, M.; Giatras, I.; Toto, R.; Remuzzi, G.; Maschio, G.; Brenner, B.M.; Kamper, A.; Zucchelli, P.; et al. Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease. A meta-analysis of patient-level data. Ann. Intern. Med. 2001, 135, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.J.; Fogo, A.B. Role of angiotensin II in glomerular injury. Semin. Nephrol. 2001, 21, 544–553. [Google Scholar] [CrossRef] [PubMed]
- McElvie, R.S.; Yusuf, S.; Pericak, D.; Avezum, A.; Burns, R.J.; Probstfield, J.; Tsuyuki, R.T.; White, M.; Rouleau, J.; Latini, R.; et al. Comparison of candesartan, enalapril, and their combination in congestive heart failure: Randomized evaluation of strategies for left ventricular dysfunction (RESOLVD) pilot study. Circulation 1999, 10, 1056–1064. [Google Scholar] [CrossRef]
- Bolignano, D.; Palmer, S.C.; Navaneethan, S.D.; Strippoli, G.F. Aldosterone antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst. Rev. 2014, 4, CD007004. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.P.; Arnold, J.; Sharif, A.; Gill, P.; Townend, J.N.; Ferro, C.J. Cardiovascular actions of mineralocorticoid receptor antagonists in patients with chronic kidney disease: A systematic review and meta-analysis. J. Renin. Angiotensin Aldosterone Syst. 2015, 16, 599–613. [Google Scholar] [CrossRef] [PubMed]
- Epstein, M. Mineralocorticoid receptor antagonists: Part of an emerging treatment paradigm for chronic kidney disease. Lancet Diabetes Endocrinol. 2014, 2, 925–927. [Google Scholar] [CrossRef]
- Alfie, J.; Aparicio, L.S.; Waisman, G.D. Current strategies to achieve further cardiac and renal protection through enhanced renin-angiotensin-aldosterone system inhibition. Rev. Recent Clin. Trials 2011, 6, 134–146. [Google Scholar] [PubMed]
- Schjoedt, K.J.; Andersen, S.; Rossing, P.; Tarnow, L.; Parving, H.H. Aldosterone escape during blockade of the renin-angiotensin-aldosterone system in diabetic nephropathy is associated with enhanced decline in glomerular filtration rate. Diabetologia 2004, 47, 1936–1939. [Google Scholar] [CrossRef] [PubMed]
- Cerezo, C.; Ruilope, L.M.; Segura, J.; Garcia-Donaire, J.A.; de la Cruz, J.J.; Banegas, J.R.; Waeber, B.; Rabelink, T.J.; Messerli, F.H. Microalbuminuria breakthrough under chronic renin-angiotensin-aldosterone system suppression. J. Hypertens. 2012, 30, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Edwards, N.C.; Steeds, R.P.; Stewart, P.M.; Ferro, C.J.; Townend, J.N. Effect of spironolactone on left ventricular mass and aortic stiffness in early-stage chronic kidney disease: A randomized controlled trial. J. Am. Coll. Cardiol. 2009, 54, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Rossing, K.; Schjoedt, K.J.; Smidt, U.M.; Boomsma, F.; Parving, H.H. Beneficial effects of adding spironolactone to recommended antihypertensive treatment in diabetic nephropathy: A randomized, double-masked, cross-over study. Diabetes Care 2005, 28, 2106–2112. [Google Scholar] [CrossRef] [PubMed]
- Tylicki, L.; Rutkowski, P.; Renke, M.; Larczyński, W.; Aleksandrowicz, E.; Lysiak-Szydlowska, W.; Rutkowski, B. Triple pharmacological blockade of the renin-angiotensin-aldosterone system in nondiabetic CKD: An open-label crossover randomized controlled trial. Am. J. Kidney Dis. 2008, 52, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, L.; Zhou, Z.; Gao, J.; Sun, Y. Effect of spironolactone combined with angiotensin converting enzyme inhibitors and/or angiotensin II receptor blockers on chronic glomerular disease. Exp. Ther. Med. 2013, 6, 1527–1531. [Google Scholar] [CrossRef] [PubMed]
- Currie, G.; Taylor, A.H.; Fujita, T.; Ohtsu, H.; Lindhardt, M.; Rossing, P.; Boesby, L.; Edwards, N.C.; Ferro, C.J.; Townend, J.N.; et al. Effect of mineralocorticoid receptor antagonists on proteinuria and progression of chronic kidney disease: A systematic review and meta-analysis. BMC Nephrol. 2016, 17, 127. [Google Scholar] [CrossRef] [PubMed]
- Bakris, G.L.; Agarwal, R.; Chan, J.C.; Cooper, M.E.; Gansevoort, R.T.; Haller, H.; Remuzzi, G.; Rossing, P.; Schmieder, R.E.; Nowack, C.; et al. Effect of finerenone on albuminuria in patients with diabetic nephropathy: A randomized clinical trial. JAMA 2015, 314, 884–894. [Google Scholar] [CrossRef] [PubMed]
- Taira, M.; Toba, H.; Murakami, M.; Iga, I.; Serizawa, R.; Murata, S.; Kobara, M.; Nakata, T. Spironolactone exhibits direct renoprotective effects and inhibits renal renin-angiotensin-aldosterone system in diabetic rats. Eur. J. Pharmacol. 2008, 589, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Rocha, R.; Rudolph, A.E.; Frierdich, G.E.; Nachowiak, D.A.; Kekec, B.K.; Blomme, E.A.; McMahon, E.G.; Delyani, J.A. Aldosterone induces a vascular inflammatory phenotype in the rat heart. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H1802–H1810. [Google Scholar] [CrossRef] [PubMed]
- Terada, Y.; Ueda, S.; Hamada, K.; Shimamura, Y.; Ogata, K.; Inoue, K.; Taniguchi, Y.; Kagawa, T.; Horino, T.; Takao, T. Aldosterone stimulates nuclear factor-kappa b activity and transcription of intercellular adhesion molecule-1 and connective tissue growth factor in rat mesangial cells via serum- and glucocorticoid-inducible protein kinase-1. Clin. Exp. Nephrol. 2012, 16, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Bauersachs, J.; Jaisser, F.; Toto, R. Mineralocorticoid receptor activation and mineralocorticoid receptor antagonist treatment in cardiac and renal diseases. Hypertension 2015, 65, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Queisser, N.; Amann, K.; Hey, V.; Habib, S.L.; Schupp, N. Blood pressure has only minor influence on aldosterone-induced oxidative stress and DNA damage in vivo. Free Radic. Biol. Med. 2013, 54, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Rocha, R.; Stier, C.T., Jr.; Kifor, I.; Ochoa-Maya, M.R.; Rennke, H.G.; Williams, G.H.; Adler, G.K. Aldosterone: A mediator of myocardial necrosis and renal arteriopathy. Endocrinology 2000, 141, 3871–3878. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Horimai, C.; Harada, K.; Wakamatsu, T.; Fukasawa, H.; Muto, S.; Itai, A.; Hayashi, M. Aldosterone-induced kidney injury is mediated by NFκB activation. Clin. Exp. Nephrol. 2011, 15, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Lian, M.; Hewitson, T.D.; Wigg, B.; Samuel, C.S.; Chow, F.; Becker, G.J. Long-term mineralocorticoid receptor blockade ameliorates progression of experimental diabetic renal disease. Nephrol. Dial. Transpl. 2012, 27, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liang, W.; Jia, J.; van Goor, H.; Singhal, P.C.; Ding, G. Aldosterone induces apoptosis in rat podocytes: Role of PI3-K/Akt and p38MAPK signaling pathways. Nephron Exp. Nephrol. 2009, 113, e26–e34. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Hara, K.; Tojo, A.; Onozato, M.L.; Honda, T.; Yoshida, K.; Mita, S.; Nakano, S.; Tsubokou, Y.; Matsuoka, H. Eplerenone shows renoprotective effect by reducing LOX-1-mediated adhesion molecule, PKCepsilon-MAPK-p90RSK, and Rho-kinase pathway. Hypertension 2005, 45, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Perez-Rojas, J.M.; Derive, S.; Blanco, J.A.; Cruz, C.; Martínez de la Maza, L.; Gamba, G.; Bobadilla, N.A. Renocortical mRNA expression of vasoactive factors during spironolactone protective effect in chronic cyclosporine nephrotoxicity. Am. J. Physiol. Ren. Physiol. 2005, 289, F1020–F1030. [Google Scholar] [CrossRef] [PubMed]
- Luther, J.M.; Luo, P.; Wang, Z.; Cohen, S.E.; Kim, H.S.; Fogo, A.B.; Brown, N.J. Aldosterone deficiency and mineralocorticoid receptor antagonism prevent angiotensin ii-induced cardiac, renal, and vascular injury. Kidney Int. 2012, 82, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Nagase, M.; Shibata, S.; Yoshida, S.; Nagase, T.; Gotoda, T.; Fujita, T. Podocyte injury underlies the glomerulopathy of Dahl salt-hypertensive rats and is reversed by aldosterone blocker. Hypertension 2006, 47, 1084–1093. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, Z.; Kokeny, G.; Godo, M.; Mózes, M.; Rosivall, L.; Gross, M.L.; Ritz, E.; Hamar, P. Increased renoprotection with ACE inhibitor plus aldosterone antagonist as compared to monotherapies—The effect on podocytes. Nephrol. Dial. Transpl. 2009, 24, 3640–3651. [Google Scholar] [CrossRef] [PubMed]
- Tseng, W.C.; Liu, J.S.; Hung, S.C.; Kuo, K.L.; Chen, Y.H.; Tarng, D.C.; Hsu, C.C. Effect of spironolactone on the risks of mortality and hospitalization for heart failure in pre-dialysis advanced chronic kidney disease: A nationwide population-based study. Int. J. Cardiol. 2017, 238, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Benson, K.; Hartz, A.J. A comparison of observational studies and randomized, controlled trials. N. Engl. J. Med. 2000, 342, 1878–1886. [Google Scholar] [CrossRef] [PubMed]





| Before Propensity-Score Matching | After Propensity-Score Matching | |||||
|---|---|---|---|---|---|---|
| Non-User | User | p-Value | Non-User | User | p-Value | |
| Patient number | 13,884 | 785 | 1386 | 693 | ||
| Age, years | 63 ± 16 | 65 ± 15 | <0.001 | 65 ± 15 | 65 ± 16 | 0.814 |
| Gender, Male | 7738 (55.73%) | 440 (56.05%) | 0.862 | 767 (55.34%) | 380 (54.83%) | 0.827 |
| Monthly income, New Taiwan Dollars | 14,195 ± 14,667 | 12,283 ± 12,489 | <0.001 | 12,419 ± 12,580 | 12,397 ± 12,662 | 0.971 |
| Geographic location | ||||||
| Northern | 6661 (47.98%) | 317 (40.38%) | <0.001 | 532 (38.38%) | 279 (40.26%) | 0.436 |
| Middle | 2396 (17.26%) | 207 (26.37%) | <0.001 | 393 (28.35%) | 180 (25.97%) | 0.274 |
| Southern | 4452 (32.07%) | 248 (31.59%) | 0.813 | 439 (31.67%) | 223 (32.18%) | 0.855 |
| Eastern | 375 (2.7%) | 13 (1.66%) | 0.0968 | 22 (1.59%) | 11 (1.59%) | 0.852 |
| Comorbidities within 1 year before the index date | ||||||
| Hypertension | 9192 (66.21%) | 584 (74.39%) | <0.001 | 1033 (74.53%) | 514 (74.17%) | 0.859 |
| Diabetes | 5533 (39.85%) | 371 (47.26%) | <0.001 | 666 (48.05%) | 326 (47.04%) | 0.664 |
| Coronary artery disease | 2665 (19.19%) | 231 (29.43%) | <0.001 | 398 (28.72%) | 195 (28.14%) | 0.783 |
| Stroke | 1924 (13.86%) | 135 (17.2%) | 0.009 | 258 (18.61%) | 114 (16.45%) | 0.225 |
| Atrial fibrillation | 300 (2.16%) | 44 (5.61%) | <0.001 | 64 (4.62%) | 34 (4.91%) | 0.770 |
| Cirrhosis | 202 (1.45%) | 72 (9.17%) | <0.001 | 74 (5.34%) | 36 (5.19%) | 0.890 |
| PAOD | 267 (1.92%) | 23 (2.93%) | 0.049 | 38 (2.74%) | 21 (3.03%) | 0.709 |
| Cancer | 801 (5.77%) | 57 (7.26%) | 0.083 | 82 (5.92%) | 46 (6.64%) | 0.519 |
| COPD | 2015 (14.51%) | 166 (21.15%) | <0.001 | 298 (21.5%) | 144 (20.78%) | 0.705 |
| CHF | 1359 (9.79%) | 209 (26.62%) | <0.001 | 359 (25.9%) | 172 (24.82%) | 0.594 |
| Charlson comorbidity index | 3.1 ± 2.4 | 4 ± 2.6 | <0.001 | 3.8 ± 2.5 | 3.8 ± 2.5 | 0.826 |
| Anti-hypertensive drugs | ||||||
| ACEI/ARB | 6030 (43.43%) | 417 (53.12%) | <0.001 | 727 (52.45%) | 358 (51.66%) | 0.733 |
| α-blocker | 1393 (10.03%) | 90 (11.46%) | 0.195 | 158 (11.4%) | 79 (11.4%) | 1.000 |
| β--blocker | 4828 (34.77%) | 325 (41.4%) | <0.001 | 546 (39.39%) | 278 (40.12%) | 0.751 |
| Calcium channel blocker | ||||||
| Non-DHP | 1439 (10.36%) | 118 (15.03%) | <0.001 | 213 (15.37%) | 105 (15.15%) | 0.897 |
| DHP | 5544 (39.93%) | 349 (44.46%) | 0.012 | 628 (45.31%) | 311 (44.88%) | 0.852 |
| Other Diuretics | ||||||
| Thiazide | 2298 (16.55%) | 181 (23.06%) | <0.001 | 313 (22.58%) | 147 (21.21%) | 0.478 |
| Loop diuretics | 1623 (11.69%) | 246 (31.34%) | <0.001 | 343 (24.75%) | 179 (25.83%) | 0.592 |
| Miscellaneous | 670 (4.83%) | 62 (7.9%) | <0.001 | 106 (7.65%) | 50 (7.22%) | 0.724 |
| Antidiabetic medication | ||||||
| Sulfonylurea | 3943 (28.4%) | 277 (35.29%) | <0.001 | 479 (34.56%) | 241 (34.78%) | 0.922 |
| Meglitinide | 685 (4.93%) | 51 (6.5%) | 0.051 | 88 (6.35%) | 41 (5.92%) | 0.700 |
| α-glucosidase inhibitor | 998 (7.19%) | 79 (10.06%) | 0.003 | 147 (10.61%) | 70 (10.1%) | 0.723 |
| Biguanide | 3611 (26.01%) | 249 (31.72%) | <0.001 | 437 (31.53%) | 221 (31.89%) | 0.868 |
| Thiazolidinedione | 1013 (7.3%) | 70 (8.92%) | 0.091 | 111 (8.01%) | 63 (9.09%) | 0.401 |
| Insulin | 937 (6.75%) | 87 (11.08%) | <0.001 | 146 (10.53%) | 69 (9.96%) | 0.684 |
| Statins | 3724 (26.82%) | 245 (31.21%) | 0.007 | 430 (31.02%) | 216 (31.17%) | 0.947 |
| Aspirin | 3753 (27.03%) | 274 (34.9%) | <0.001 | 492 (35.5%) | 236 (34.05%) | 0.516 |
| NSAIDs | 2095 (15.09%) | 149 (18.98%) | 0.003 | 257 (18.54%) | 126 (18.18%) | 0.841 |
| NaHCO3 | 147 (1.06%) | 10 (1.27%) | 0.569 | 13 (0.94%) | 8 (1.15%) | 0.642 |
| Nephrology visit within 1 year before the index date | 1.4 ± 2.1 | 1.3 ± 2.1 | 0.243 | 1.3 ± 2 | 1.3 ± 2.1 | 0.994 |
| Propensity score | 0.05 ± 0.06 | 0.14 ± 0.17 | <0.001 | 0.1 ± 0.11 | 0.1 ± 0.11 | 0.999 |
| Outcome | ||||||
| ESRD | 2399 (17.28%) | 102 (12.99%) | 0.002 | 266 (19.19%) | 88 (12.7%) | <0.001 |
| MACEs | 1084 (7.81%) | 63 (8.03%) | 0.878 | 123 (8.87%) | 56 (8.08%) | 0.599 |
| Hospitalization for heart-failure | 423 (3.05%) | 36 (4.59%) | 0.021 | 76 (5.48%) | 29 (4.18%) | 0.243 |
| Hyperkalemia-associated hospitalization | 724 (5.21%) | 151 (19.24%) | <0.001 | 92 (6.64%) | 123 (17.75%) | <0.001 |
| Mortality | 2857 (20.58%) | 226 (28.79%) | <0.001 | 386 (27.85%) | 192 (27.71%) | 0.9448 |
| CVD death | 413 (3.0%) | 39 (5.0%) | 0.002 | 70 (5.1%) | 34 (4.9%) | 0.972 |
| Users | Non-Users | Users Compared to Non-Users | ||||||
|---|---|---|---|---|---|---|---|---|
| Event | IR (95% CI) | Event | IR (95% CI) | Crude HR (95% CI) | p-Value | Adjusted HR † (95% CI) | p-Value | |
| ESRD | 88 | 39.2 (31.01–47.39) | 266 | 53.69 (47.24–60.14) | 0.65 (0.51–0.83) | <0.001 | 0.66 (0.51–0.84) | <0.001 |
| MACE § | 56 | 24.94 (18.41–31.48) | 123 | 24.83 (20.44–29.21) | 0.93 (0.68–1.27) | 0.647 | 0.93 (0.67–1.28) | 0.647 |
| Hospitalization for heart-failure | 29 | 12.92 (8.22–17.62) | 76 | 15.34 (11.89–18.79) | 0.77 (0.50–1.19) | 0.238 | 0.77 (0.50–1.18) | 0.225 |
| Hyperkalemia-associated hospitalization | 123 | 54.79 (45.1–64.47) | 92 | 18.57 (14.77–22.36) | 2.98 (2.28–3.90) | <0.001 | 3.17 (2.41–4.17) | <0.001 |
| All-cause mortality | 192 | 64.42 (55.31–73.53) | 386 | 60.47 (54.44–66.5) | 1.07 (0.90–1.27) | 0.432 | 1.10 (0.92–1.30) | 0.294 |
| Cardiovascular death | 34 | 11.41 (7.57–15.24) | 70 | 10.97 (8.4–13.53) | 1.02 (0.67–1.53) | 0.941 | 1.14 (0.75–1.74) | 0.533 |
| Outcomes | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ESRD | MACE § | HHF | HKAH | All-Cause Mortality | CVD Mortality | |||||||
| Adj. HR † (95% CI) | p-Value | Adj. HR † (95% CI) | p-Value | Adj. HR † (95% CI) | p-Value | Adj. HR † (95% CI) | p-Value | Adj. HR † (95% CI) | p-Value | Adj. HR † (95% CI) | p-Value | |
| Prescribed daily dose (mg) | ||||||||||||
| Spironolactone (vs. non-use) | ||||||||||||
| <12.5 mg | 0.73 (0.52–1.00) | 0.0523 | 0.97 (0.64–1.45) | 0.8688 | 0.78 (0.43–1.41) | 0.4102 | 2.81 (1.96–4.03) | <0.0001 | 1.15 (0.91–1.45) | 0.2304 | 0.97 (0.54–1.71) | 0.904 |
| 12.5–25 mg | 0.59 (0.39–0.91) | 0.0175 | 0.57 (0.29–1.11) | 0.0965 | 0.90 (0.45–1.81) | 0.7732 | 2.74 (1.8–4.17) | <0.0001 | 1.15 (0.87–1.53) | 0.3302 | 1.34 (0.73–2.46) | 0.3437 |
| ≥25 mg | 0.57 (0.35–0.91) | 0.0193 | 1.34 (0.79–2.25) | 0.2803 | 0.61 (0.26–1.44) | 0.2572 | 4.80 (3.3–6.97) | <0.0001 | 0.95 (0.70–1.29) | 0.744 | 0.87 (0.41–1.84) | 0.7107 |
| p-trend | 0.0057 | 0.2428 | 0.5971 | <0.0001 | 0.4920 | 0.7568 | ||||||
| Cumulative defined daily dose (cDDD) (vs. non-use) | ||||||||||||
| Spironolactone | ||||||||||||
| ≤30 cDDD | 0.68 (0.52–0.89) | 0.005 | 0.82 (0.57–1.19) | 0.2951 | 0.84 (0.53–1.34) | 0.4749 | 2.77 (2.04–3.75) | <0.0001 | 1.16 (0.96–1.4) | 0.1312 | 1.09 (0.69–1.7) | 0.7206 |
| >30 cDDD | 0.60 (0.37–0.97) | 0.0353 | 1.36 (0.80–2.33) | 0.2600 | 0.53 (0.20–1.37) | 0.1903 | 4.70 (3.21–6.87) | <0.0001 | 0.94 (0.68–1.28) | 0.6708 | 0.89 (0.42–1.9) | 0.7695 |
| p-trend | 0.0038 | 0.2588 | 0.3522 | <0.0001 | 0.2535 | 0.9644 | ||||||
| Outcomes | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ESRD | MACE § | HHF | HKAH | All-Cause Mortality | CVD-Mortality | |||||||
| Adj. HR † (95% CI) | p-Value | Adj. HR † (95% CI) | p-Value | Adj. HR † (95% CI) | p-Value | Adj. HR † (95% CI) | p-Value | Adj. HR † (95% CI) | p-Value | Adj. HR † (95% CI) | p-Value | |
| Time intervals for defining Spironolactone users/non-users | ||||||||||||
| Within 60 days | ||||||||||||
| Spironolactone use (vs. non-use) | 0.59 (0.46–0.77) | <0.0001 | 1.08 (0.77–1.50) | 0.6618 | 0.82 (0.53–1.28) | 0.3861 | 2.40 (1.82–3.16) | <0.0001 | 1.09 (0.91–1.30) | 0.3495 | 1.20 (0.80–1.82) | 0.3785 |
| Within 120 days | ||||||||||||
| Spironolactone use (vs. non-use) | 0.73 (0.58–0.92) | 0.0088 | 0.96 (0.70–1.32) | 0.8003 | 1.12 (0.74–1.71) | 0.5816 | 2.52 (1.94–3.26) | <0.0001 | 1.01 (0.85–1.19) | 0.9148 | 1.12 (0.18–7.09) | 0.904 |
| Within 180 days | ||||||||||||
| Spironolactone use (vs. non-use) | 0.69 (0.55–0.87) | 0.0019 | 0.95 (0.68–1.32) | 0.7508 | 0.84 (0.54–1.3) | 0.4367 | 3.08 (2.28–4.16) | <0.0001 | 1.01 (0.85–1.21) | 0.8976 | 1.00 (0.62–1.61) | 0.9847 |
| Exclude control cohort receiving spironolactone during the follow-up period | ||||||||||||
| Spironolactone use (vs. non-use) | 0.63 (0.49–0.81) | 0.0002 | 0.91 (0.66–1.26) | 0.5724 | 0.76 (0.49–1.19) | 0.2263 | 3.65 (2.71–4.91) | <0.0001 | 1.07 (0.89–1.27) | 0.4773 | 1.11 (0.70–1.78) | 0.6565 |
| Consider spironolactone status change as censored (As-treat model) | ||||||||||||
| Spironolactone use (vs. non-use) | 0.37 (0.21–0.64) | 0.0004 | 0.71 (0.44–1.14) | 0.1553 | 1.11 (0.65–1.92) | 0.6967 | 9.18 (6.69–12.6) | <0.0001 | 1.17 (0.83–1.66) | 0.3642 | 1.42 (0.68–2.97) | 0.3571 |
| Cohort in 2000–2006 | ||||||||||||
| Spironolactone use (vs. non-use) | 0.48 (0.28–0.81) | 0.0064 | 1.17 (0.60–2.28) | 0.6515 | 1.02 (0.24–4.27) | 0.9828 | 2.38 (1.3–4.35) | 0.0048 | 1.34 (0.76–2.37) | 0.3125 | 1.34 (0.35–5.07) | 0.6672 |
| Cohort in 2007–2013 | ||||||||||||
| Spironolactone use (vs. non-use) | 0.71 (0.53–0.94) | 0.0184 | 0.88 (0.61–1.27) | 0.5035 | 0.73 (0.43–1.21) | 0.2167 | 3.33 (2.45–4.52) | <0.0001 | 1.18 (0.98–1.43) | 0.0818 | 1.16 (0.74–1.82) | 0.5137 |
| Using raw data (before propensity-score matching) as analyzed data | ||||||||||||
| Spironolactone use (vs. non-use) | 0.65 (0.53–0.80) | <0.0001 | 1.02 (0.78–1.33) | 0.9091 | 0.85 (0.59–1.21) | 0.3649 | 3.00 (2.46–3.67) | <0.0001 | 1.21 (1.04–1.40) | 0.0141 | 1.23 (0.27–5.62) | 0.7899 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.-T.; Kor, C.-T.; Hsieh, Y.-P. Long-Term Effects of Spironolactone on Kidney Function and Hyperkalemia-Associated Hospitalization in Patients with Chronic Kidney Disease. J. Clin. Med. 2018, 7, 459. https://doi.org/10.3390/jcm7110459
Yang C-T, Kor C-T, Hsieh Y-P. Long-Term Effects of Spironolactone on Kidney Function and Hyperkalemia-Associated Hospitalization in Patients with Chronic Kidney Disease. Journal of Clinical Medicine. 2018; 7(11):459. https://doi.org/10.3390/jcm7110459
Chicago/Turabian StyleYang, Chen-Ta, Chew-Teng Kor, and Yao-Peng Hsieh. 2018. "Long-Term Effects of Spironolactone on Kidney Function and Hyperkalemia-Associated Hospitalization in Patients with Chronic Kidney Disease" Journal of Clinical Medicine 7, no. 11: 459. https://doi.org/10.3390/jcm7110459
APA StyleYang, C.-T., Kor, C.-T., & Hsieh, Y.-P. (2018). Long-Term Effects of Spironolactone on Kidney Function and Hyperkalemia-Associated Hospitalization in Patients with Chronic Kidney Disease. Journal of Clinical Medicine, 7(11), 459. https://doi.org/10.3390/jcm7110459




