Abstract
Intestinal microbiota play an important role in the pathogenesis of surgical site infections (SSIs) and other surgery-related complications (SRCs). Probiotics and synbiotics were found to lower the risk of surgical infections and other surgery-related adverse events. We systematically reviewed the approach based on the administration of probiotics and synbiotics to diminish SSIs/SRCs rates in patients undergoing various surgical treatments and to determine the mechanisms responsible for their effectiveness. A systematic literature search in PubMed/MEDLINE/Cochrane Central Register of Controlled Trials from the inception of databases to June 2018 for trials in patients undergoing surgery supplemented with pre/pro/synbiotics and randomized to the intervention versus placebo/no treatment and reporting on primarily: (i) putative mechanisms of probiotic/symbiotic action, and secondarily (ii) SSIs and SRCs outcomes. Random-effect model meta-analysis and meta-regression analysis of outcomes was done. Thirty-five trials comprising 3028 adult patients were included; interventions were probiotics (n = 16) and synbiotics (n = 19 trials). We found that C-reactive protein (CRP) and Interleukin-6 (IL-6) were significantly decreased (SMD: −0.40, 95% CI [−0.79, −0.02], p = 0.041; SMD: −0.41, 95% CI [−0.70, −0.02], p = 0.006, respectively) while concentration of acetic, butyric, and propionic acids were elevated in patients supplemented with probiotics (SMD: 1.78, 95% CI [0.80, 2.76], p = 0.0004; SMD: 0.67, 95% CI [0.37, −0.97], p = 0.00001; SMD: 0.46, 95% CI [0.18, 0.73], p = 0.001, respectively). Meta-analysis confirmed that pro- and synbiotics supplementation was associated with significant reduction in the incidence of SRCs including abdominal distention, diarrhea, pneumonia, sepsis, surgery site infection (including superficial incisional), and urinary tract infection, as well as the duration of antibiotic therapy, duration of postoperative pyrexia, time of fluid introduction, solid diet, and duration of hospital stay (p < 0.05). Probiotics and synbiotics administration counteract SSIs/SRCs via modulating gut-immune response and production of short chain fatty acids.
1. Introduction
One of the most challenging health care issues worldwide are surgical site infections (SSIs) [1,2]. Timely administration of effective preoperative antibiotics along with other perioperative quality control interventions recommended by various guidelines [3,4,5] have resulted in a significant reduction of the rate of SSIs. Despite these efforts, globally SSIs occur in 9–22% of procedures, with a direct correlation with the human developmental index [1]. SSIs result in prolonged hospitalizations, unscheduled re-admissions, extended duration of antibiotic therapy, increase mortality rate, and pose high costs to healthcare systems. Therefore, it is of priority to look for other effective, evidence-based interventions capable of reducing the incidence of life-threatening SSIs [6,7,8].
There is increasing evidence that human intestinal microbiota play an important role in the pathogenesis of SSIs. Although historically, gut flora has been considered as a pathogen in human infections [9], recent studies show that alteration of the human microbiome (dysbiosis) may play a role in the pathogenesis of SSIs and other surgery-related complications (SRCs) [10,11,12]. Human gut microbiota composition fluctuates on a daily basis depending predominantly on diet, but also exercise, medications, and exposure to stressful events [13,14,15,16]. The general health status of a patient scheduled for surgery is of particular interest, and the make-up of the microbiota could be of particular interest, because it is believed that the majority of hospital infections originate from the patient’s own microbiota, in part due to noxious and stressful surgical preparatory procedures [2]. Supporting the role of microbiota, it has been shown that mechanic bowel preparation (MBP) before gut resection, accompanied by oral antibiotic therapy, reduces the number of infectious complications, including anastomotic leakages by almost half [17]. However, multiple studies have reported vast disturbances in microbial counts and diversity following these procedures that may itself create microbiota disturbances with health consequences [18,19].
The surgical procedure itself and other pathology not even related to the gastrointestinal tract may be a major cause of alterations in the intestinal microbiota. There are numerous examples in the literature. Dysbiosis has been described in the excluded colon after small bowel stoma [20]. Major burn injury was described to reduce two major phyla within the human gut and to increase Gammaproteobacteria class involved in SSIs [21]. Significant changes of gut flora with increased virulent Escherichia coli, Pseudomonas aeruginosa, and Enterococcus faecalis counts have been described with surgical procedures [21,22,23]. Surgical reconstructions of the gastrointestinal (GI) tract may delay the microbiota refaunation [24,25], and result in enhanced virulent phenotype expression [26]. In severe injuries, more virulent pathogens may predominate in the intestinal ecosystem [27], disrupt the intestinal barrier structure and function, which facilitates the bacterial translocation, and may result in SSIs.
It thus appears that manipulating gut microbiota composition to a healthier variety could be promising. Administration of beneficial microbes (probiotics), fiber (prebiotics), or both (synbiotics) could be an attractive strategy to diminish the incidence of SSIs [28]. There are randomized, double-blind, placebo-controlled trials and meta-analyses that support the efficacy of this strategy [28,29,30,31,32,33]. A recently published meta-analysis aimed to find evidence on prebiotics, probiotics, and synbiotics supplementation on postoperative complications (mostly infective) in surgical patients [28,29,32,34]. Additionally, Wu et al. [29] estimated the efficacy of probiotics and antibiotics combination in the prevention of SSIs and the decrease of antibiotics usage in colorectal surgery, and Kasatpibal et al. [28] conducted a network meta-analysis (NMA) to evaluate the efficacy of probiotics, prebiotics, and synbiotics in reducing SSIs as well as other postoperative complications. Although probiotics have already been used as prophylaxis against SSIs, to the best of our knowledge, none of the guidelines recommend their use. Among the reasons could be lack of data on the precise mechanisms of such interventions in lowering the risk of SSIs and the fact that studies aimed at elucidating the effect of probiotic action on mucosal and stool microbiota lack correlation with clinical outcomes [35].
Therefore, this systematic review was performed to study the role of probiotics and synbiotics in the prevention of SSIs and SRCs. In particular, our study aimed to evaluate:
- The mechanism of action of probiotics and synbiotics in prevention of SSIs;
- The influence of probiotics on gut microbiota alterations related to the surgery;
- A possibility to establish recommendations concerning strain(s), dose, and mode of administration of probiotic in the prevention of SSI and SRCs.
A random-effect model meta-analysis to determine putative mechanisms associated with such intervention was also performed. The meta-analysis (MA) evaluated all available data on the usefulness of probiotics in the prevention of SSIs/SRCs in patients undergoing abdominal surgery. The findings could result in a call to determine the appropriateness of implementation probiotics into clinical practice and consideration for inclusion in guidelines as a potentially cost-effective and life-saving therapy. Finally, a meta-regression was performed in order to try to identify a particular probiotic strain of formula, dose, and duration of the probiotic supplementation, which could be recommended as treatment to prevent SSIs.
2. Materials and Methods
2.1. Search Strategy and Inclusion Criteria
Two independent authors (K.S.-Z., M.K.) searched PubMed/MEDLINE/Cochrane Central Register of Controlled Trials from the inception of databases until 1 June 2018 in English for human trials assessing the efficacy of pre/pro/synbiotic administration in reducing the incidence of SSIs and SRCs. The following search terms with medical subject headings (MeSH–bold font) Supplementary Concept Record terms (SCR italic font) and free text terms were used: (“probiotics” OR probiotic * OR “prebiotics” OR symbiotic * OR fiber OR “dietary fiber” OR microbiota *) AND (operation OR “surgical procedure” OR “surgical procedures, operative” OR “general surgery” OR surgery OR transplantation OR “surgical operation” OR surgery OR “abdominal surgery” OR “colorectal surgery” OR “colectomy” OR “small bowel surgery” OR hepatectomy OR “biliary surgery” OR “pancreas surgery” OR proctology * OR proctocolonic surgery * OR intestine surgery *) AND (readmission OR “readmission rate” OR mortality OR morbidity OR sepsis OR procalcitonin OR calcitonin OR leakage OR “surgical infection” OR “surgery site infection” OR leakage OR “anastomotic leakage” OR SSI OR post-operative wound infection * OR postoperative wound infection * OR complication OR peritonitis OR abscess OR translocation OR lactulose OR zonulin OR calprotectin OR ileus OR “postoperative ileus”). Apart from the electronic search, a manual review of reference lists from existing meta-analysies and relevant reviews was performed.
We used the following inclusion criteria:
- treatment with pro-/pre-/synbiotics;
- randomisation to pre/pro/synbiotic versus placebo/monotherapy/standard care; and
- available meta-analyzable endpoint/change score data on outcomes placed below.
- if a study contained more than two arms, the data were abstracted separately for each comparator.
2.2. Data Abstraction
Two authors (K.S.-Z., M.K.) independently, in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [36], abstracted information from each study, including details of the study (e.g., study design, treatment protocol, duration, number of subjects, gut barrier and SRCs parameters, and risk of bias), intervention (e.g., pre/pro/symbiotic, agent name, dosage, and duration of treatment), and primary patient characteristics (e.g., age, sex, and reason for the surgery). In case of missing data, a request letter for additional information was sent to authors. Any inconsistencies were referred by the senior author (W.M.).
2.3. Outcomes
The primary outcomes that were extracted from each study were the gut-related parameters associated with the putative mechanism of pre/pro/symbiotic action: bacterial translocation, lactulose/mannitol ratio, short chain fatty acids production, zonulin, calprotectin, gut microbiota composition, diamine oxidase (DAO) activity, as well as non-specific indices of inflammation such as C-reactive protein (CRP), interleukin-6 (IL-6) plasma concentration and white blood cells (WBC) count. To update the data reported by other authors on the effectiveness of pre/pro/synbiotics evaluating such interventions in the prevention of SSIs/SRCs the following secondary outcomes were evaluated: abdominal distention, anastomotic leakage, diarrhea, intraabdominal abscess, mortality, methicilin resistant staphylococcus aureus infection, peritonitis, pneumonia, re-operation, sepsis, SSIs, superficial incisional SSIs, deep organ/space SSIs, urinary tract infections, blood loss, duration of antibiotic therapy, duration of postoperative pyrexia, the time of implementation of fluid and solid diet, hospital and intensive care unit stay duration, and operating time.
2.4. Data Synthesis and Statistical Analysis
A random effects meta-analysis [37] of outcomes for which at least three studies contributed data was conducted using software (Comprehensive Meta-Analysis, version 3.3.070; http://www.meta-analysis.com). The between-study variance (τ2) was estimated using the method of moments (DerSimonian and Laird) and the assumption of homogeneity in effects was tested using the Q statistic with a k-1 degree of freedom (k—the number of studies). Pooled standardized mean difference (SMD) in change score/endpoint scores was used to analyze group differences in case of continuous variables. For nominal outcomes the summary risk ratio (RR) was calculated. A two-tailed Z test was used to test the null hypothesis that the summary effect is zero. In addition to classical meta-analysis, a meta-regression was performed under the random-effects model for both continuous and nominal study level covariates. The regression models with single covariates were fit. Funnel plots were inspected to quantify whether publication bias could have influenced the results. The Egger’s regression intercept test for asymmetry of the funnel plots was used. The statistical significance was adopted at two-side p value < 0.05.
2.5. Risk of Bias
Two authors (K.S.-Z. and M.K.) independently assessed the risk of bias using the Cochrane Collaboration’s tool for assessing risk of bias [38]. When a discrepancy occurred, a third author (I.Ł.) was involved. The quality of a study was reported as high when there were more than three low risk of bias assessments.
3. Results
3.1. Search Results
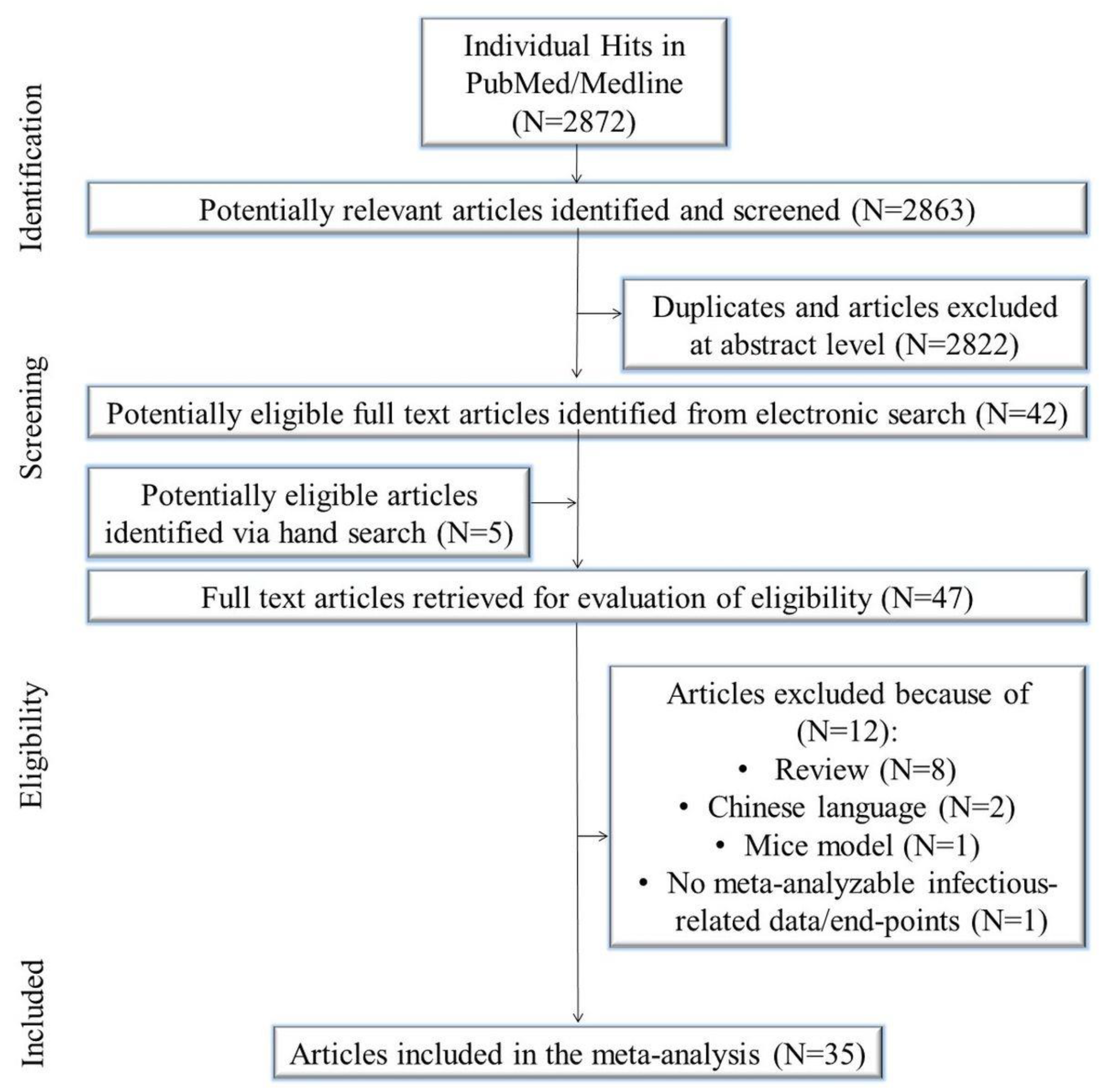
The initial search yielded 2872 citations. Of these, 2822 were duplicates and/or removed after title/abstract evaluation. Five manuscripts were identified using a manual search. Forty-seven articles underwent a full-text review, and some were excluded because they were reviews/meta-analysis/systematic review (N = 8), in the Chinese language (N = 2), mice model (N = 1), and contained no meta-analyzable infectious related data/end-points (N = 1). Eventually, 35 studies were included in the meta-analysis [39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73] (Figure 1).
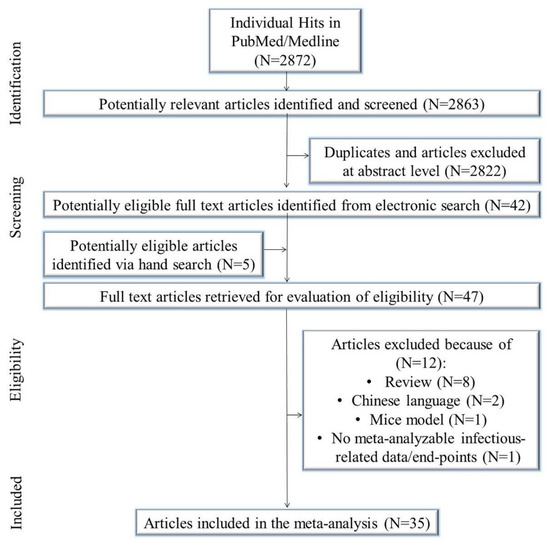
Figure 1.
Study flow chart.
3.2. Study, Patient and Treatment Characteristics
Of the 35 studies included, the majority were double-blind trials (N = 17) [39,42,45,46,47,49,50,51,52,56,60,61,64,71,72,73,74]. The mean study duration was 14.5 ± 5.58 (range: 3–28) days. In 16 studies [39,41,42,46,49,50,51,52,53,54,56,63,64,65,69,70], probiotic intervention was used, while synbiotics were administered in 19 trials [40,43,44,45,47,48,55,57,58,59,60,61,62,66,67,68,71,72,73]. There were two major groups per surgery performed: hepatopancreatobillary (N = 15) [40,43,46,51,54,58,63,64,66,67,68,70,71,72,73] and colorectal (N = 11) [31,41,47,49,50,51,52,56,61,62,65]. In seven studies [42,44,45,48,53,55,60] the procedure was not specified. Two trials involved oesophagectomy [57,59]. The most commonly utilized comparator was placebo (N = 15) [31,42,43,45,47,49,50,51,52,56,60,63,64,69,70]. There were 3028 patients included, with a male predominance (n = 1748, 57.73%). Details are given in Table 1.

Table 1.
Study characteristics.
3.3. Microbiota and Putative Mechanism of Probiotic/Synbiotics’ Action in SSIs/SRCs Prevention—Primary Outcomes
Gut microbiota analyses were present in 14 studies [40,41,42,43,44,52,55,56,57,58,59,64,65,67]. The results confirmed postoperative microbiome alterations in study groups compared to controls. Most studies identified Lactobacillus (phylum Firmicutes) and Bifidobacterium (phylum Actinobacteria) as beneficial for the outcomes. Nine studies [40,41,42,55,56,57,59,67] reported elevations in Bifidobacterium genus (or its particular species) including patients supplemented with microbial agents, but did not reach statistical significance for a benefit. Lactobacillus concentrations were elevated post-surgery in six studies [40,57,59,64,67,75]. In contrast, decreased numbers of beneficial microbes and increased abundance of harmful species (Enterobacteriaceae, Pseudomonas, Staphylococcus, and Candida) were reported in a few no-intervention groups [40,42,44,57]. One study [56] reported a Bifidobacterium/E. coli ratio. In two studies [43,58], there were no significant differences in bacterial species abundance between the groups. For example, Usami et al. [58] concluded that two weeks after the surgery microbiota composition resembled that of before the surgery regardless of the intervention. However, changes of fecal microbiota composition observed by Usami et al. [58] were not consistent with results reported by other authors [67]. Reasons for this discrepancy might be associated with the difference in intestinal microbiota between liver cirrhosis and biliary surgery patients and/or no administration of enteral nutrition in their study [40,67]. Details are given in Table 2.

Table 2.
Gut microbiota alterations following probiotic treatment.
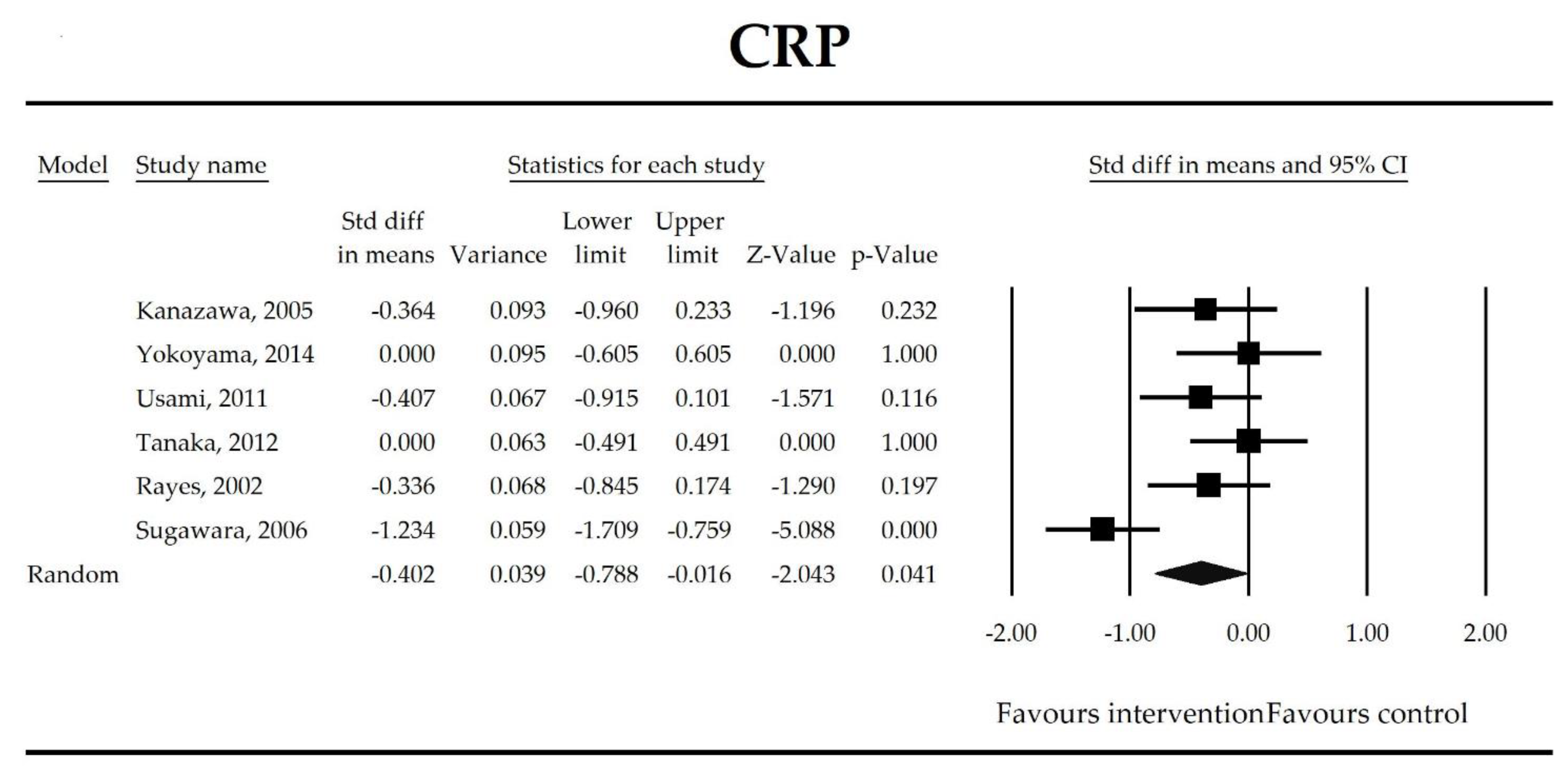
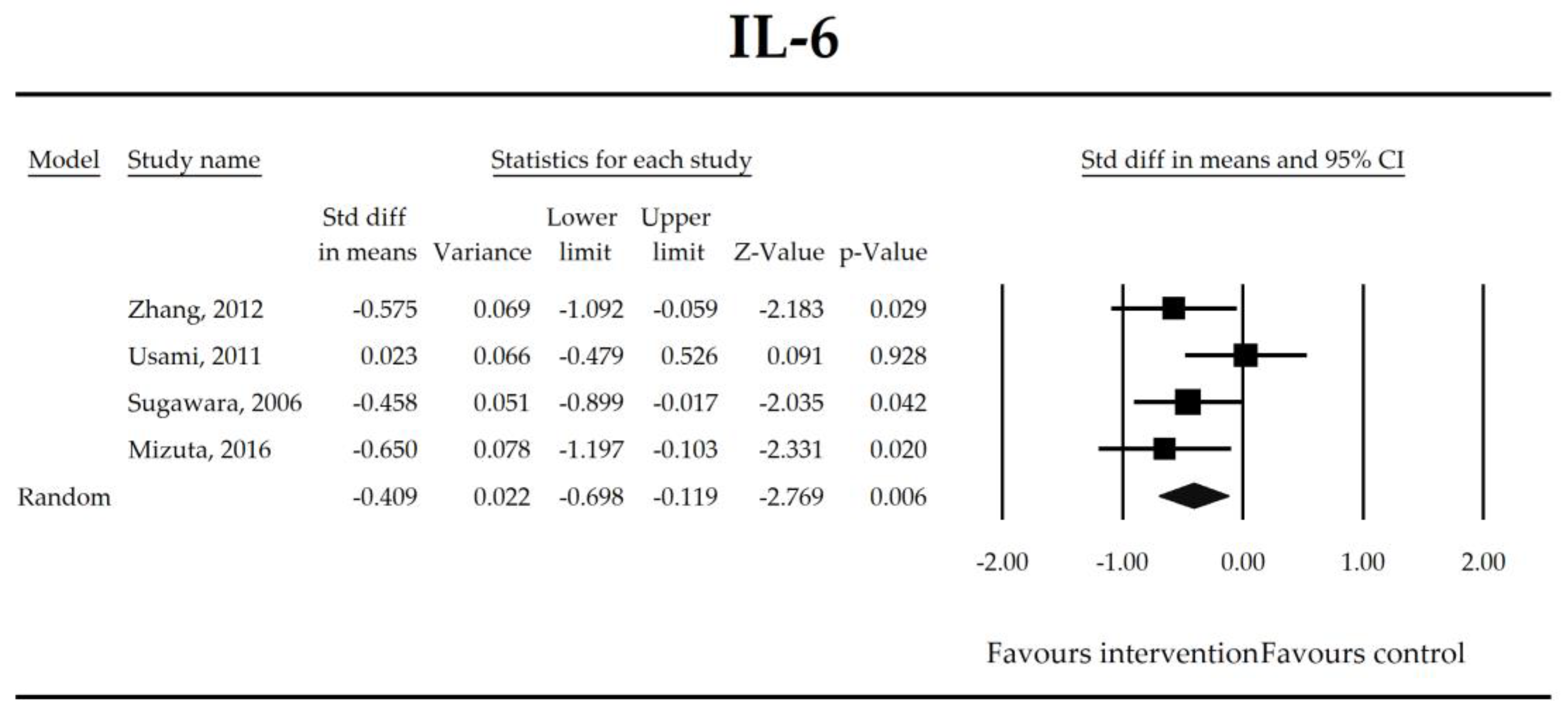
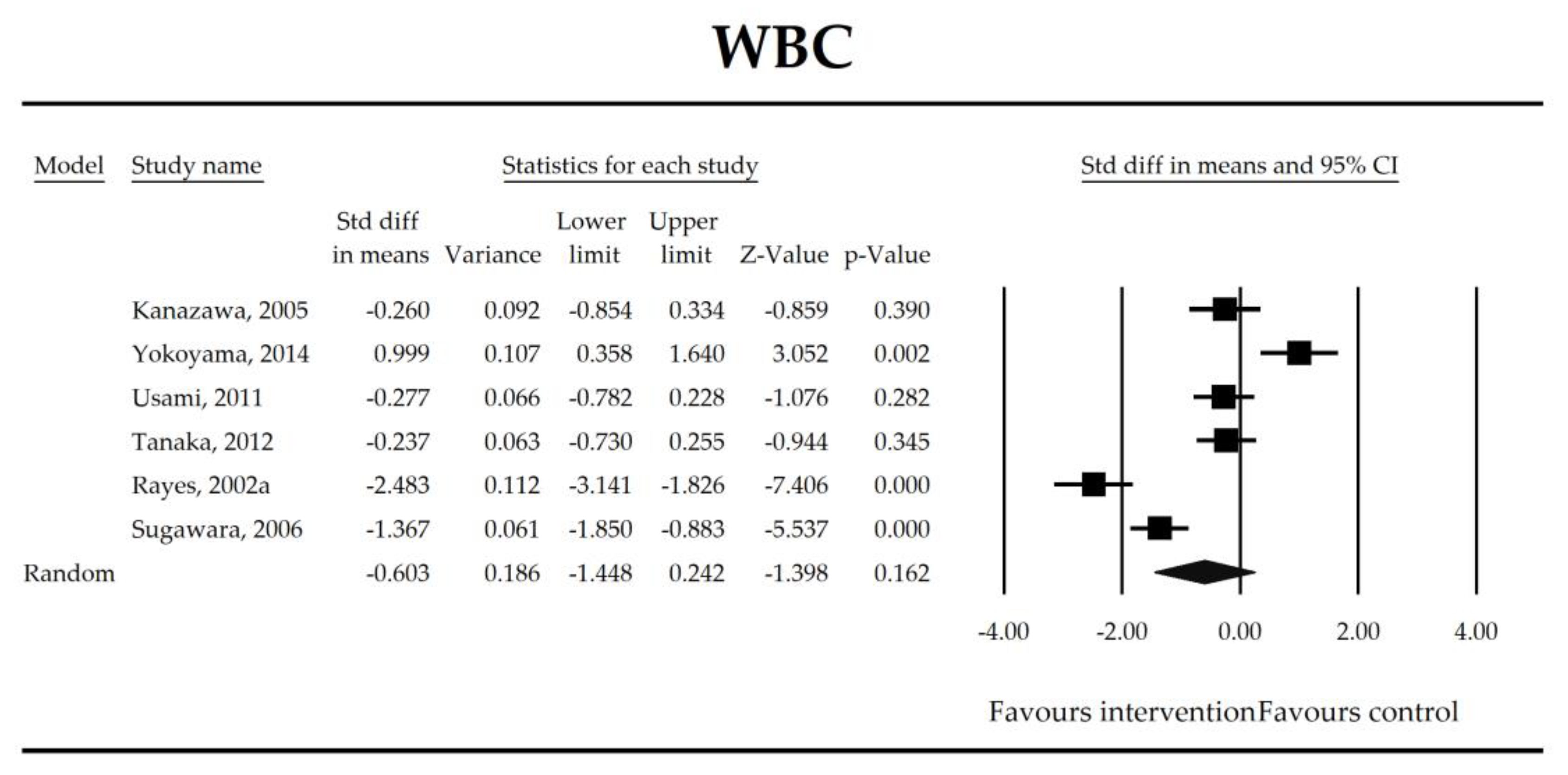
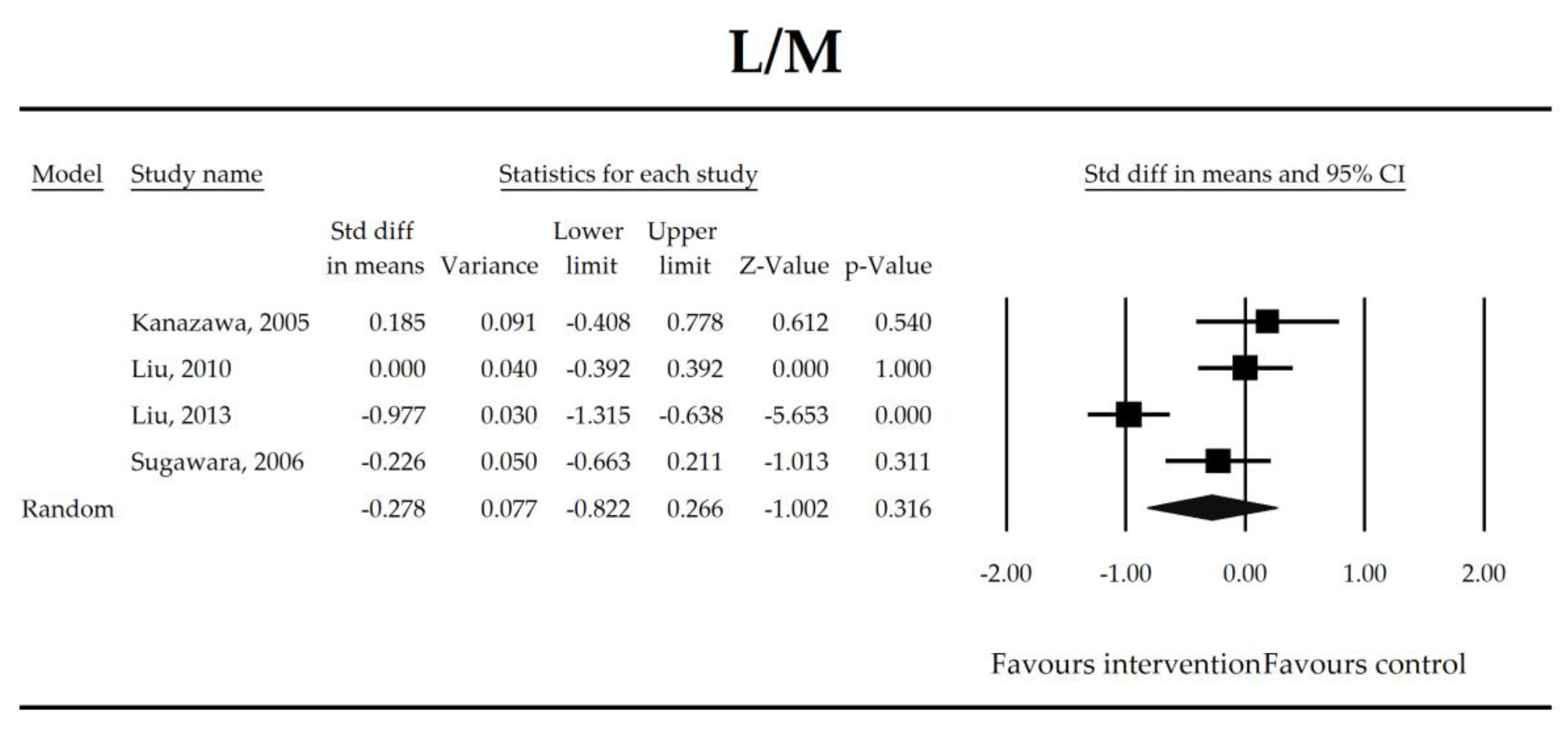
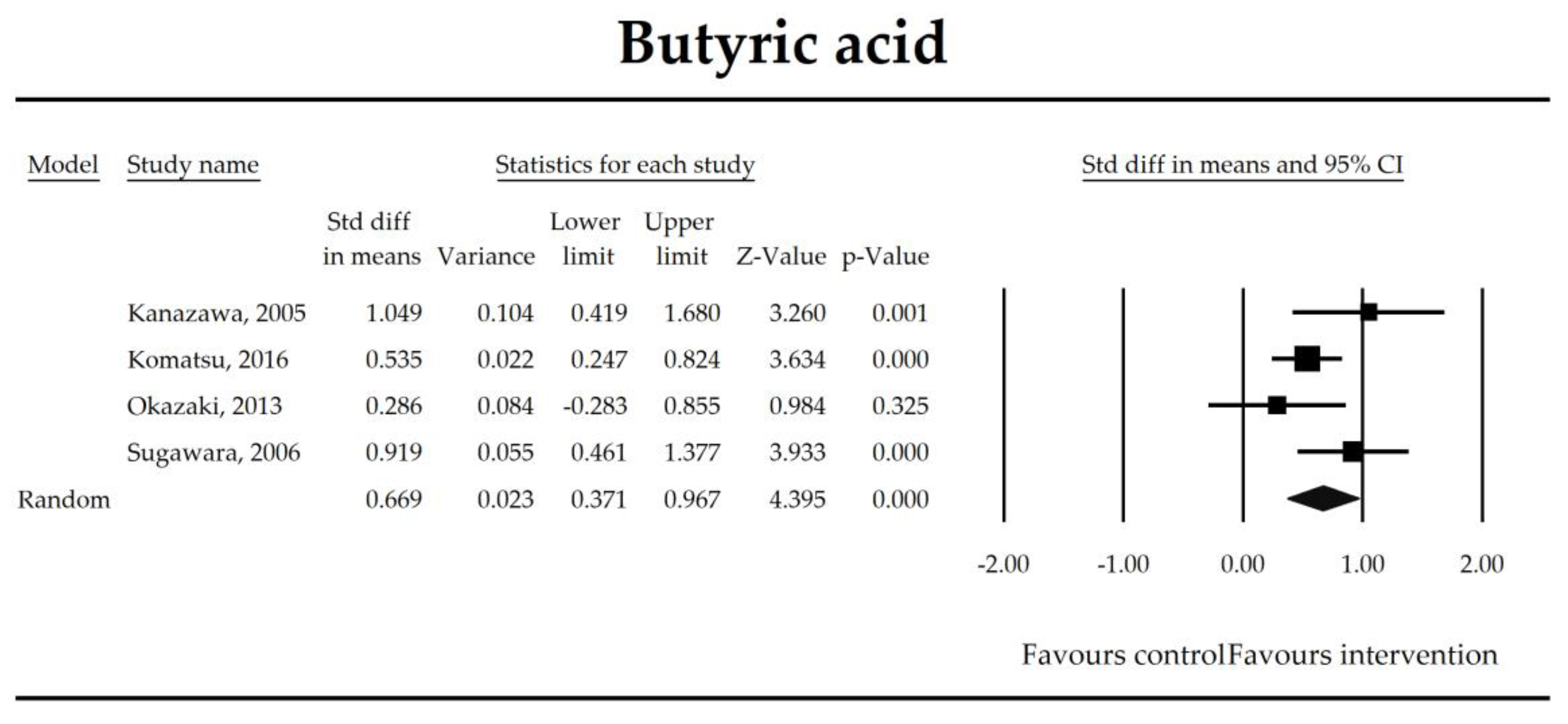
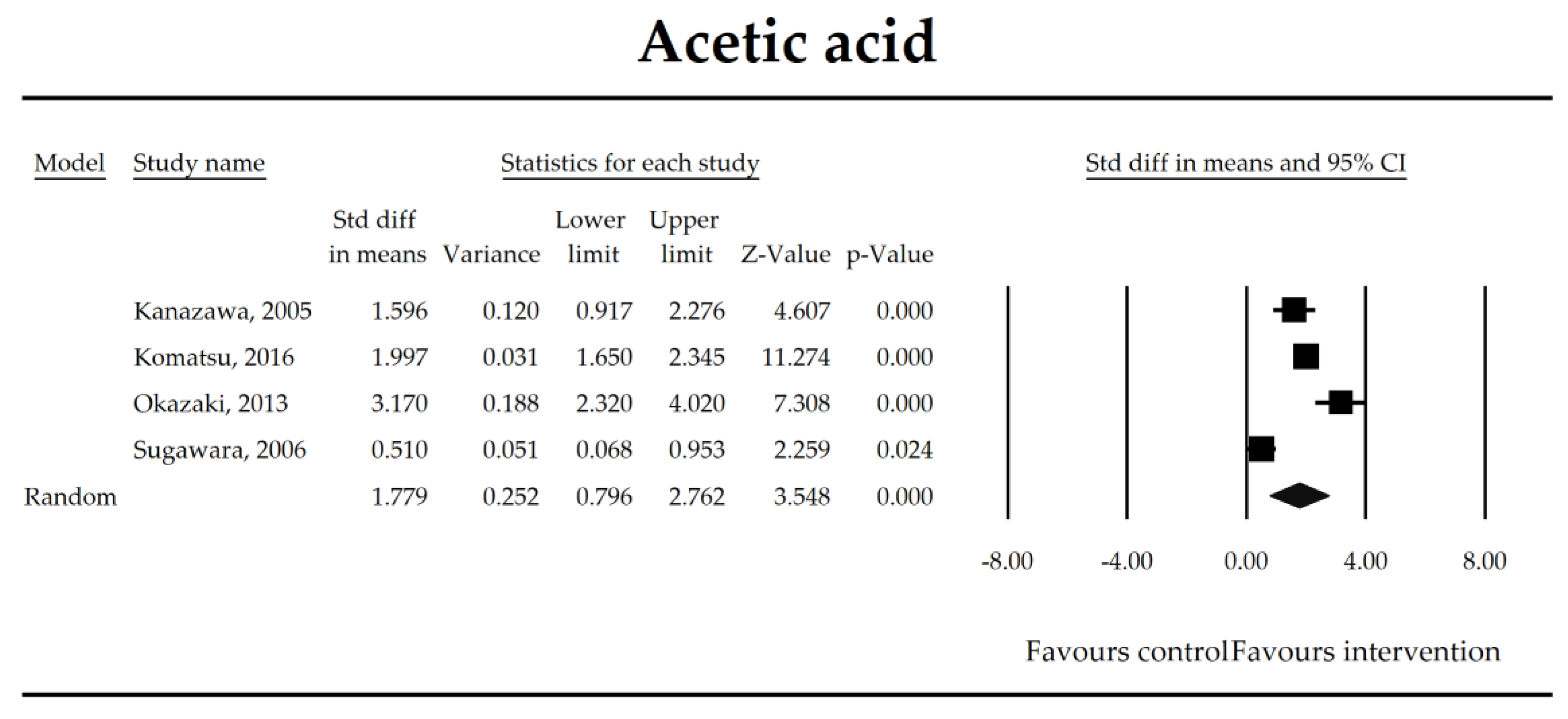
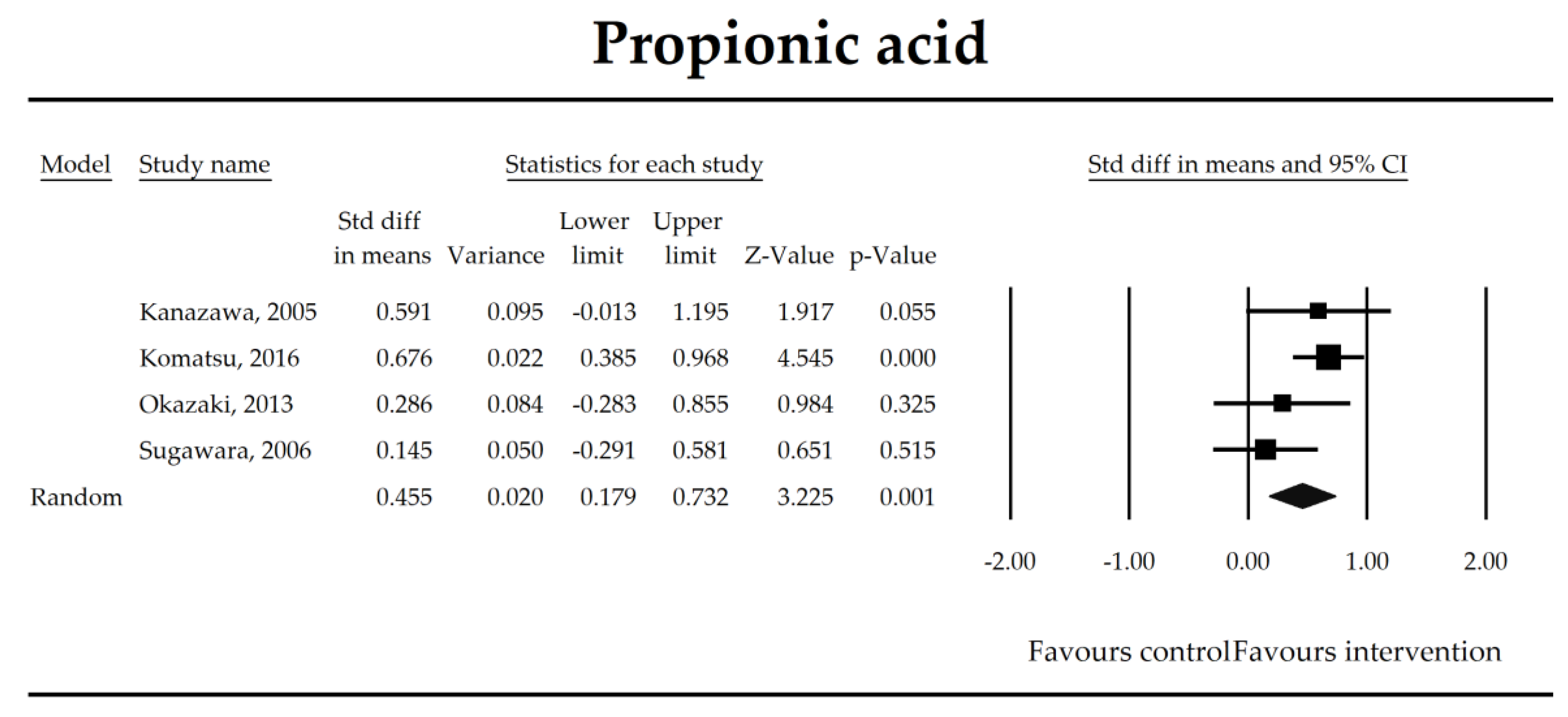
Putatively factors associated with the mechanism of pro/synbiotic action were searched with a focus on gut barrier integrity. These included: (i) bacterial translocation, (ii) lactulose/mannitol permeability test, and (iii) short chain fatty acids (butyrate, acetate, propionate) concentration, as well as non-specific markers of inflammation: (iv) C-reactive protein, (v) IL-6, and (vi) WBC counts. Diamine oxidase (DAO) activity was analyzed in two studies only [40,58], therefore excluded from metanalysis. CRP and IL-6 were significantly decreased (SMD: −0.40, 95% CI [−0.79, −0.02], p = 0.041; SMD: −0.41, 95% CI [−0.70, −0.12], p = 0.006, respectively) and short chain fatty acids (SCFAs)–acetic, butyric and propionic acids–were elevated (SMD: 1.78, 95% CI [0.80, 2.76], p = 0.0004; SMD: 0.67, 95% CI [0.37, 0.97], p = 0.00001; SMD: 0.46, 95% CI [0.18, 0.73], p = 0.001, respectively) in patients supplemented with probiotics. No other statistically significant results were found. Results are presented in Table 3 and Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8 and Figure 9.

Table 3.
Primary outcomes associated with gut barrier implicated in potential mechanisms of probiotic/synbiotic action.
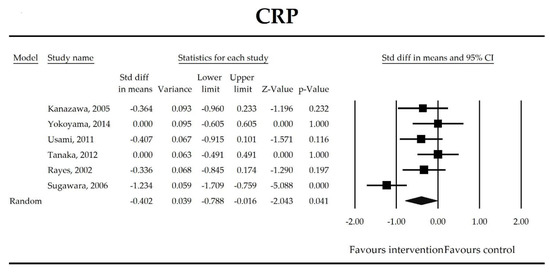
Figure 2.
The effect size (standardized mean difference) for the concentration of CRP in patients taking probiotics (intervention) vs. no probiotics (control).
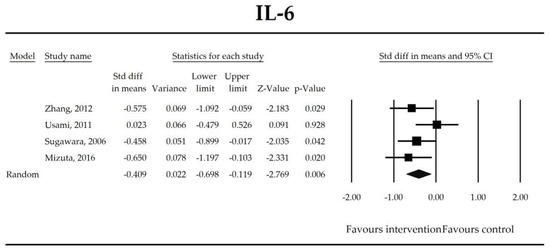
Figure 3.
The effect size (standardized mean difference) for the concentration of IL-6 in patients taking probiotics (intervention) vs. no probiotics (control).
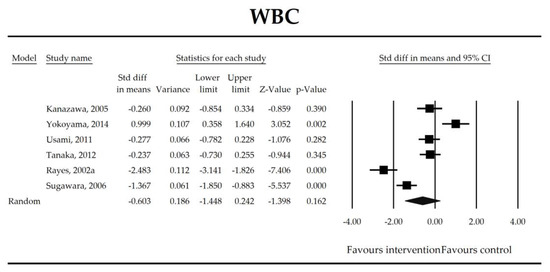
Figure 4.
The effect size (standardized mean difference) for the concentration of WBC in patients taking probiotics (intervention) vs. No probiotics (control).
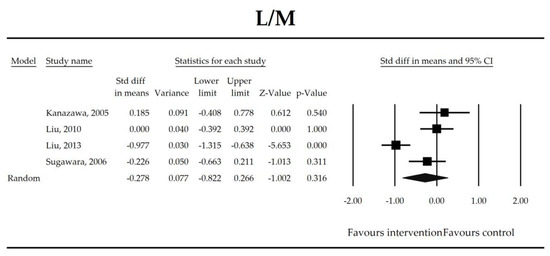
Figure 5.
The effect size (standardized mean difference) for the lactulose/mannitol (L/M) ratio in patients taking probiotics (intervention) vs. no probiotics (control).
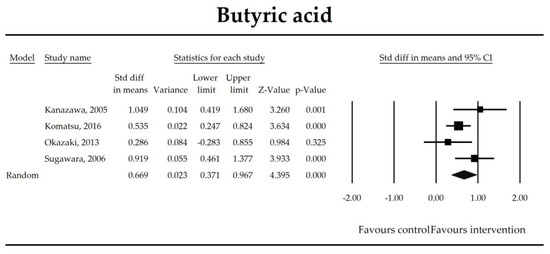
Figure 6.
The effect size (standardized mean difference) for the concentration of butyrate ratio in patients taking probiotics (intervention) vs. no probiotics (control).
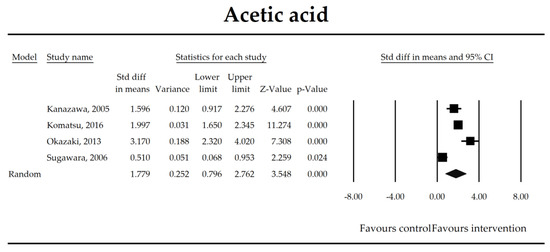
Figure 7.
The effect size (standardized mean difference) for the concentration of acetic ratio in patients taking probiotics (intervention) vs. no probiotics (control).
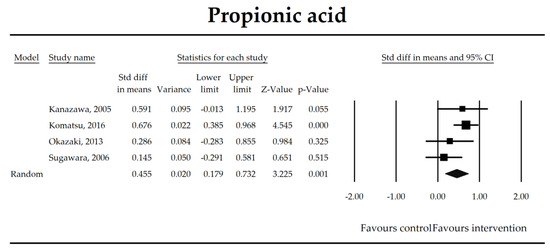
Figure 8.
The effect size (standardized mean difference) for the concentration of propionic ratio in patients taking probiotics (intervention) vs. no probiotics (control).
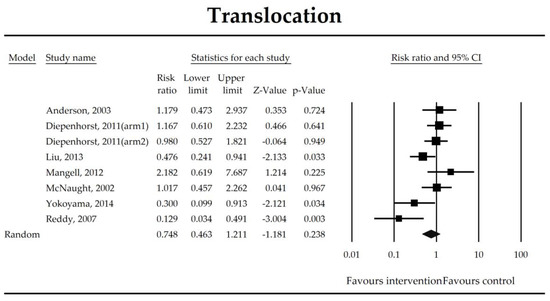
Figure 9.
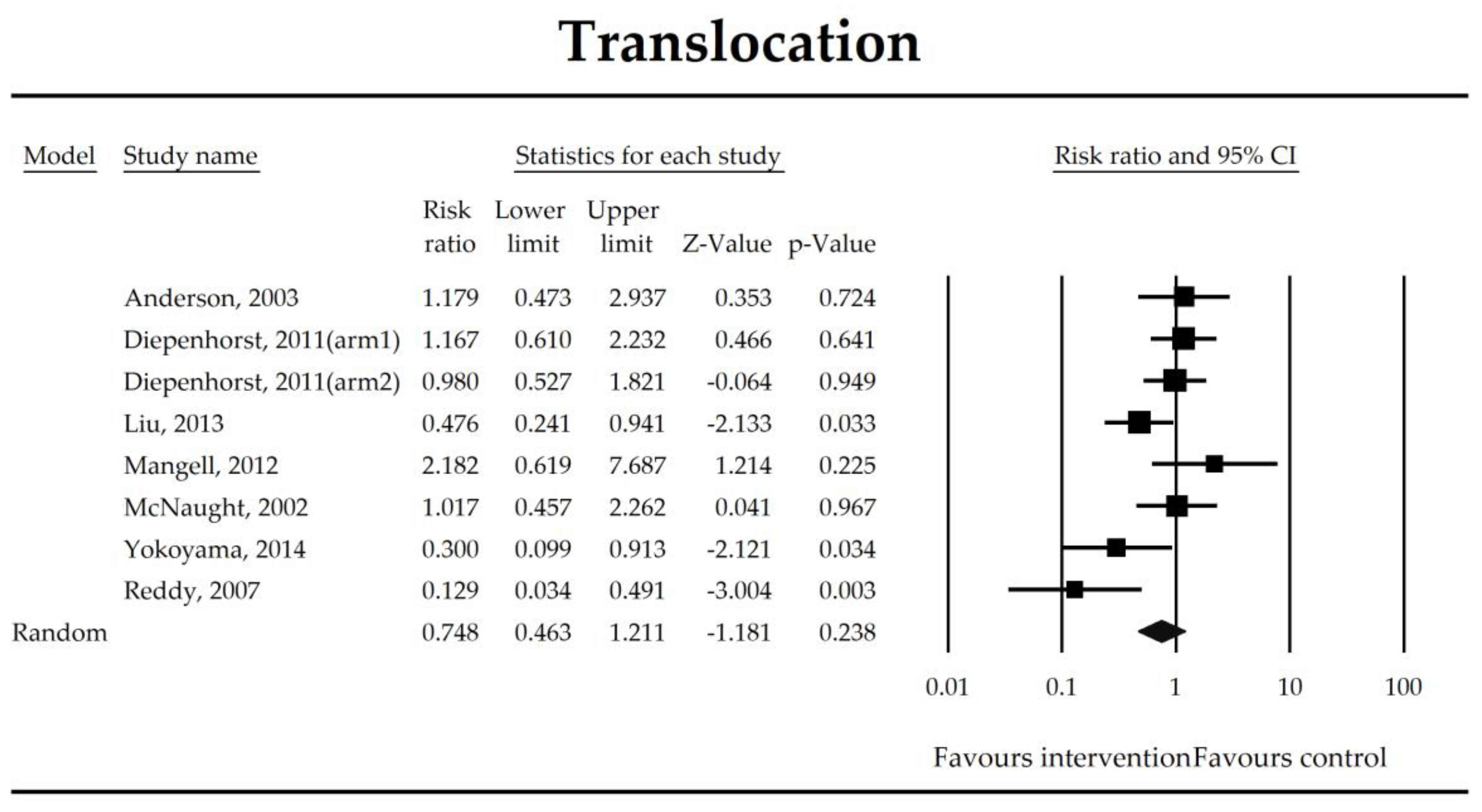
The effect size (risk ratio) for the overall effects of probiotics in the prevention of bacterial translocation.
3.4. Surgery Related Complications (SRCs) and Secondary Outcomes
To evaluate the effectiveness of pro/synbiotic interventions in reducing the incidence of SSIs/SRCs, data was extracted from common surgery-related clinical outcomes. Consequently, meta-analyses were conducted on parameters reported in at least three studies and the data confirmed that microbial supplementation was associated with a significant reduction in the incidence of SSIs and SRCs including: (i) abdominal distention, (ii) diarrhea, (iii) pneumonia, (iv) sepsis, (v) superficial incisional infection, (vi) urinary tract infection, (vii) duration of antibiotic therapy, (viii) duration of postoperative pyrexia, (ix) time of fluid introduction and (x) solid diet, and (xi) duration of hospital stay. Data are given in Supplementary Table S1. Representative forest plots of secondary outcomes are presented in Supplementary Figures S1 and S2. Other forest plots are available upon request.
To obtain data useful for drawing clinical recommendations and new guidelines a meta-regression was conducted (Table 3). Based on the analysis of the selected studies, it was not possible to find a particular probiotic formula or strain, its dose or duration of the probiotic supplementation that could be recommended to manage either primary or secondary outcomes analyzed in this study (p > 0.05). An inverse correlation was only found for propionic acid concentration. For every increase of one unit (day) in treatment duration, the SDM for propionate decreased by 0.0355 (p = 0.049). Also effect sizes were found to be independent of the timing of the intervention (pre + post vs. only post-surgery). It was not possible to show whether the quality of the trial could have influenced its results (p > 0.05).
3.5. Risk of Bias
An analysis of the overall risk of bias from the studies included in the meta-analysis was limited by restricted information being provided. For example, random sequence generation bias could not be determined in 15 studies and allocation concealment bias could not be studied in 13 papers. The unclear risk of bias in performance, detection, short-term outcomes, and reporting sections were reported in 9, 11, 3, and 12 studies, respectively. It was not possible to determine other risks of bias in 24 papers. Overall, 14 studies were of high quality and 21 of low quality. One study achieved maximum points of low risk assessments (i.e., 7 points) and only two studies achieved no low risk of bias assessments points (i.e., 0 points). The results are in Table S2 (Supplementary Material).
4. Discussion
To the best of our knowledge this meta-analysis of 35 trials and 3028 patients is the first one to exclusively investigate the effect and possible mechanism of action of pro-/synbiotics to lower the risk of SSIs and SRCs. The study shows that microbial agents administered perioperatively have the potential to increase the abundance of beneficial bacteria within the gut, elevate the synthesis of short chain fatty acids and thus reduce the immune response. Consequently, it appears to indicate that pro-/synbiotics may serve as preventive strategy toward SSIs and SRCs.
The data are mounting that the host complex of bacteria, fungi, viruses, and Archaea contribute to human biology [76]. In patients scheduled for elective abdominal surgery, the gut microbiota might undergo alterations that have an impact on surgery outcomes. In this study in patients not treated with any microbial agents perioperatively, the predominance of beneficial microbes was decreased, but the counts of potentially harmful ones were elevated. Eubiosis and a proper abundance of protective bacteria in the gut may protect the host against pathogens [75]. In this meta-analysis, the majority of the studies showed that pro-/synbiotic treatment reduced the number of Enterobacteriaceae. However, Mangel et al. [52] showed opposing results and observed increased abundance of Enterobacteriaceae in patients undergoing colon resection who received a probiotic. The explanation of this phenomenon is not clear. One reason might be too short of a probiotic administration to reduce potential pathogen counts, while another could be associated with oatmeal used as a prebiotic, which could act as a substrate for intestinal bacteria, and the third one is that lactobacilli given orally did not survive the passage through the gastrointestinal tract. Another explanation is a different response of Enterobacteriaceae genera to probiotic administration (reduction in the numbers of one genera by the probiotic may result in an expansion of another). This is also of interest as lipopolysaccharide (LPS) attached to the membrane surface of Gram-negative microbes [77,78] may result in enhanced virulence phenotype expression [26]. In severe injuries, more virulent pathogens may predominate in the intestinal ecosystem [27], disrupt the intestinal barrier structure, and function and facilitate bacterial translocation resulting in SSIs and SRCs.
The steady state composition of gut microbiota is crucial in maintaining gut homeostasis [79]. The mechanisms that are implicated in the pathogenesis of complications in patients in the perioperative period are complex. Initially, a healthy microbiota produces lactic acid, which is metabolized to short chain fatty acids (SCFAs), the latter ones are directly related to fecal Bifidobacterium count [66]. SCFAs, predominantly butyrate, are crucial for proper gut barrier structure and function [80,81]. After abdominal surgeries and in the course of multiple nonsurgical diseases, beneficial butyrate, acetate, and propionate concentration diminish as a consequence of the deterioration of lactic acid metabolism, as well as fasting [82]. Butyrate, apart from being an energy source for colonocytes, stimulates mucus production and tight junction proteins synthesis [75]. It has been found to inhibit the expression of virulence genes [83] and restrict the growth of Pseudomonas aueroginosa, a collagenase producer, implicated in the pathogenesis of anastomotic leakage [84,85]. Butyrate controls the function of regulatory T cells in a microbe-associated context [86] and suppresses inflammation via nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) signaling [87]. It also stabilizes the hypoxia inducible factor involved in the augmentation of the barrier function [88]. This meta-analysis shows that the concentrations of acetic, butyric, and propionic acids were elevated in patients supplemented with probiotics. Surprisingly, a meta-regression indicated that the longer duration of probiotic intervention, the smaller the effect size for propionic acid. This seems to be in contrast with mechanistic studies in which propionic acid was discovered to act as an immunosuppressant [89]. This metabolite possesses anti-fungal and anti-bacterial effects [90] responsible for the inhibition of invasion genes in Salmonella typhimurium. Propionic acid is able to diminish the synthesis of eicosanoids via lowering the activity of cyclooxygenase [91,92]. Although the acid may inhibit mitogen-activating lymphocytes proliferation, different studies found that the inhibitory effects may be positively correlated with its concentration [93,94,95]. The discrepancies between concentrations inside and outside the visceral compartment may at least partly explain the observed results. It should be pointed out that this data was extracted from four studies, so the results need to be interpreted with caution [40,44,55,67]. More studies evaluating SCFAs concentration in surgical patients are needed to confirm this finding.
It was also found that in patients supplemented with pro-/synbiotics, the concentration of CRP and IL-6 were significantly decreased in comparison to non-treated patients. As antigens flow through the disrupted intestinal barrier, the activation of the immune response in lamina propria and the production of inflammatory mediators take place. IL-6 and CRP were found to be at higher serum concentrations in patients with low DAO activity following the surgery [58]. This is crucial as DAO being produced at the tip of the villi reflects the integrity of the small intestine barrier. The enzyme serum concentration is of small bowel origin [96,97,98] and its activity was found to be diminished following major hepatectomy [40,58,67]. This study shows that pro-/synbiotic intervention significantly lowered the concentration of IL-6 and CRP. The body of evidence states that IL-6 signaling plays a pivotal role in epithelial stem cells and intraepithelial lymphocytes proliferation and may be involved in wound healing [99]. Recently, Kuhn et al. [100] discovered that intraepithelial lymphocyte-derived IL-6 served positively toward barrier function via claudin-1 protein expression and increased mucus thickness [100]. Although CRP production in hepatocytes was found not to be influenced by medical therapies [101], the most recent meta-analysis by Mazidi et al. proved that probiotic administration may significantly reduce serum CRP with a weighted mean difference (WMD) of −1.35 mg/L; however, that study was not limited to surgical patients only [102].
Gut-derived bacteremia is a result of elevated intestinal permeability which further makes antigens flow through the epithelium, elevate serum inflammatory mediators [58], and enhance bacterial translocation to mesenteric lymph nodes after interventions such as a hepatectomy [103] and an esophagectomy [104]. In this study, it was not possible to demonstrate that microbial intervention diminished the risk of bacterial translocation. However, studies evaluating the bacterial translocation were based on culture-based methods and such methodology was valid to evaluate the presence of well-cultured bacteria only [66]. Culture-independent molecular techniques and sophisticated bioinformatic analyses should therefore be implemented in future trials to evaluate bacterial translocation and assess the functionality of translocated microorganisms in patients in perioperative periods.
This updated systematic review found that patients treated perioperatively with pro-/synbiotics had lower relative risk toward (i) abdominal distention, (ii) diarrhea, (iii) pneumonia, (iv) sepsis, (v) superficial incisional infection, (vi) urinary tract infection, (vii) duration of antibiotic therapy, (viii) duration of postoperative pyrexia, (ix) time of fluid introduction and (x) solid diet, and (xi) duration of hospital stay, and supports other observations [28,29,32].
This study also shows that biochemical parameters associated with the gut barrier were improved in patients treated with pro-/synbiotics, supporting the hypothesis that SSIs and SRCs are actually in large part sourced from the patient’s own gut flora. This is in line with a recent SR by Lederer et al. [105] who reported that the gut microbiome was responsible for postoperative complications including anastomotic leakage and wound infection. The data was not robust enough to establish recommendations for the use of beneficial bacteria in SSIs/SRCs prevention. The limitations of the available data did not allow us to determine which probiotics strain is the optimal choice, particular clinical situations where they could prove beneficial, how long the intervention should last, and the optimal dose of the supplement. The study was unable to establish that synbiotics should be used first-line to reduce specific SSIs and SRCs, which contrasts with the network meta-analysis by Kasatpibal et al. [28]. Apart from different methodological approach, this study included more patients (2952 vs. 3028) but excluded studies in a non-English language that may partly explain the discrepancies. Therefore, on the basis of the results of this study, microbial supplements in general, without strain recommendation in perioperative period, could be advocated. Taking into account the documented stability and safety of probiotics available on the market, the findings could explain the lack of current implementation of probiotics/synbiotics into SSIs/SRCs prevention clinical guidelines. More high-quality studies are needed to draw detailed protocols to evaluate particular probiotic strains, optimal duration of their supplementation, objective outcomes measurements, and maybe even stratify by surgery types to understand the roles. Nevertheless, the evidence is strong to already support dietary supplementation with probiotics in patients undergoing major abdominal surgeries. This topic seems to be of high priority as Berrios-Torres et al. [4] in their recent Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection stated that antimicrobial prophylaxis should be administered only when indicated based on published clinical practice guidelines. The evidence is mounting that the longer post-surgical antibiotic administration, the greater the frequency of SSIs [1]. Antibiotic administration was found to elevate the risk toward inflammatory disorders, predominantly due to commensal bacteria translocation through the gut barrier, thus disturbing the microecological niche within the gut [106]. Also, antibiotic gut decontamination may activate dormant spores, which consequently results in severe infectious complications [107]. Recently, the 6th National Audit Project of the Royal College of Anaesthetists reported antibiotic-induced life-threatening anaphylaxis as well [108]. However, one of the current widely agreed and recommended intervention to decrease the incidence of SSIs/SRCs is perioperative antibiotic administration.
Postsurgical complications (PSCs) are currently one of the most challenging health care issues worldwide [1,2]. Moreover, these unpredictable post-surgical events result in unscheduled readmissions, extended antibiotic therapy, and elevated mortality rate, but importantly generate additional costs of treatment. For example, Tanner et al., evaluated that in the U.K., SSIs secondary to colorectal surgery generated an extra cost of more than £10.000 with only 15% met in primary care [109]. More recently, Straatman et al. [110] pointed that in Netherlands, complications following major abdominal surgery may generate as much as 240% higher costs of treatment, depending on the clinical course of PSC. In the USA, the mean cost for a hospital stay was found to be approximately twice as high in patients with complications compared with those suffering from no PSCs. Consequently, total profit margin was estimated to be about 5.7% lower in patients with complications [111]. On the other hand, as reported by Keenan et al. [112], introducing a preventive strategy, e.g., SSI bundle in colorectal surgery, may significantly diminish the incidence of SSIs, and consequently, health care costs. As our paper provides evidence linking PSCs to host intestinal microenvironment, maintaining healthy microbiota—at least during the hospital stay—to reducing the incidence of these life-threatening events seems to be one of these cost-effective regimens [6,7,8]. Indeed, our study has shown that probiotic intervention significantly decreased the duration of antibiotic therapy (SMD: −0.597, 95% CI: −1.093, −0.10, p = 0.018) and overall length of hospital stay (SMD: −0.479, 95% CI: (−0.660, −0.297, p = 0.0000002). The reduction of these variables, together with the lowest incidence of PSCs reported in our study, extrapolate to a reduction in the cost of a patient’s stay in a hospital. This is in line with the assumptions made recently by Wu et al. [34] who analyzed two studies of Liu et al. [50,51] and reported a lower hospital charge concerning patients receiving probiotics in comparison to the placebo groups. Finally, it was concluded [34] that probiotic prophylaxis in surgery wards may decrease the hospital costs.
Several limitations of this MA require underlining. These include (i) a small number of double-blind clinical trials; (ii) heterogeneous study aims, patient groups, intervention characteristics, and study targets; (iii) a limited number of reported outcomes; and (iv) meta-regression analyses were conducted only for exploratory reasons due to different subsets of patients and treatments. The overall moderate quality of the studies may have significantly influenced the study outcomes. Nevertheless, despite these limitations, this is the first, comprehensive SR/MA that shows a beneficial effect of pro-/synbiotics in reducing the incidence of SSIs/SRCs likely via modulating gut related immune response and production of SCFA.
In conclusion, our MA supports that pro-/synbiotics as a class can have an effect on the outcome, but more granular data on particular types and concentrations cannot be recommended. The effect on SSIs/SRCs is complex, including the modulation of CRP and WBC counts, as well as alteration of SCFAs synthesis and others that need further clarification. More high-quality studies are needed to draw detailed protocols to evaluate particular probiotic strains and optimal duration of their supplementation in patients undergoing surgical procedures. However, the evidence presented in this systematic review strongly supports that dietary supplementation with probiotics in patients undergoing major abdominal surgeries has a beneficial effect.
Supplementary Materials
The following are available online at http://www.mdpi.com/2077-0383/7/12/556/s1. Figure S1: The effect size (risk ratio) for the overall effects of probiotics in the prevention of pneumonia. Figure S2: The effect size (risk ratio) for the overall effects of probiotics in the prevention of surgical site infection. Table S1: The efficacy of probiotics to counteract surgery related complications (SRCs). Table S2: Risk of bias assessment.
Author Contributions
Conceptualization, K.S.-Ż., M.K., I.Ł., and W.M.; Data curation, K.S.-Ż., M.K., L.F.L., A.K., and W.M.; Formal analysis, K.S.-Ż., M.K., I.Ł., A.M., D.M., and W.M.; Investigation, K.S.-Ż., M.K., I.Ł., L.F.L., A.K., A.M., D.M., and W.M.; Methodology, K.S.-Ż.; Project administration, K.S.-Ż.; Software, M.K.; Supervision, I.Ł., L.F.L., A.K., and W.M.; Visualization, M.K.; Writing original draft, K.S.-Ż.; Writing review and editing, K.S.-Ż., M.K., I.Ł., L.F.L., A.K., A.M., D.M., and W.M.
Funding
This research received no external funding.
Conflicts of Interest
Igor Loniewski and Wojciech Marlicz are cofounders and shareholders at Sanprobi company, a producer and distributor of probiotics. Karolina Skonieczna-Żydecka received honoraria from a probiotic company. Other authors have nothing to disclose.
Data Source
Data presented in this manuscript were discussed in the presidential choice session of the WGO-GAT International Conference, Bangkok, 5–8 Dec 2018.
References
- GlobalSurg Collaborative. Surgical site infection after gastrointestinal surgery in high-income, middle-income, and low-income countries: A prospective, international, multicentre cohort study. Lancet Infect. Dis. 2018, 18, 516–525. [Google Scholar] [CrossRef]
- Guyton, K.; Alverdy, J.C. The gut microbiota and gastrointestinal surgery. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 43–54. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Guidelines on the Prevention of Surgical Site Infection. Available online: http://www.who.int/gpsc/ssi-guidelines/en/ (accessed on 7 September 2018).
- Berríos-Torres, S.I.; Umscheid, C.A.; Bratzler, D.W.; Leas, B.; Stone, E.C.; Kelz, R.R.; Reinke, C.E.; Morgan, S.; Solomkin, J.S.; Mazuski, J.E.; et al. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017. JAMA Surg. 2017, 152, 784–791. [Google Scholar] [CrossRef]
- Ban, K.A.; Minei, J.P.; Laronga, C.; Harbrecht, B.G.; Jensen, E.H.; Fry, D.E.; Itani, K.M.F.; Dellinger, E.P.; Ko, C.Y.; Duane, T.M. American College of Surgeons and Surgical Infection Society: Surgical Site Infection Guidelines, 2016 Update. J. Am. Coll. Surg. 2017, 224, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Stone, P.W.; Braccia, D.; Larson, E. Systematic review of economic analyses of health care-associated infections. Am. J. Infect. Control 2005, 33, 501–509. [Google Scholar] [CrossRef] [PubMed]
- WHO Guidelines for Safe Surgery 2009: Safe Surgery Saves Lives; WHO Guidelines Approved by the Guidelines Review Committee; World Health Organization: Geneva, Switzerland, 2009; ISBN 978-92-4-159855-2.
- Nguyen, N.; Yegiyants, S.; Kaloostian, C.; Abbas, M.A.; Difronzo, L.A. The Surgical Care Improvement project (SCIP) initiative to reduce infection in elective colorectal surgery: Which performance measures affect outcome? Am. Surg. 2008, 74, 1012–1016. [Google Scholar]
- Altemeier, W.A.; Culbertson, W.R.; Hummel, R.P. Surgical considerations of endogenous infections—Sources, types, and methods of control. Surg. Clin. N. Am. 1968, 48, 227–240. [Google Scholar] [CrossRef]
- Brial, F.; Le Lay, A.; Dumas, M.-E.; Gauguier, D. Implication of gut microbiota metabolites in cardiovascular and metabolic diseases. Cell. Mol. Life Sci. 2018, 75, 3977–3990. [Google Scholar] [CrossRef]
- Marlicz, W.; Yung, D.E.; Skonieczna-Żydecka, K.; Loniewski, I.; van Hemert, S.; Loniewska, B.; Koulaouzidis, A. From clinical uncertainties to precision medicine: The emerging role of the gut barrier and microbiome in small bowel functional diseases. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 961–978. [Google Scholar] [CrossRef]
- Clemente, J.C.; Manasson, J.; Scher, J.U. The role of the gut microbiome in systemic inflammatory disease. BMJ 2018, 360, j5145. [Google Scholar] [CrossRef]
- Spadoni, I.; Zagato, E.; Bertocchi, A.; Paolinelli, R.; Hot, E.; Sabatino, A.D.; Caprioli, F.; Bottiglieri, L.; Oldani, A.; Viale, G.; et al. A gut-vascular barrier controls the systemic dissemination of bacteria. Science 2015, 350, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.A.; Rinaman, L.; Cryan, J.F. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol. Stress 2017, 7, 124–136. [Google Scholar] [PubMed]
- Sonnenburg, J.L.; Bäckhed, F. Diet-microbiota interactions as moderators of human metabolism. Nature 2016, 535, 56–64. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Materna, A.C.; Friedman, J.; Campos-Baptista, M.I.; Blackburn, M.C.; Perrotta, A.; Erdman, S.E.; Alm, E.J. Host lifestyle affects human microbiota on daily timescales. Genome Biol. 2014, 15, R89. [Google Scholar] [CrossRef] [PubMed]
- Scarborough, J.E.; Mantyh, C.R.; Sun, Z.; Migaly, J. Combined Mechanical and Oral Antibiotic Bowel Preparation Reduces Incisional Surgical Site Infection and Anastomotic Leak Rates after Elective Colorectal Resection: An Analysis of Colectomy-Targeted ACS NSQIP. Ann. Surg. 2015, 262, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Bretagnol, F.; Panis, Y.; Rullier, E.; Rouanet, P.; Berdah, S.; Dousset, B.; Portier, G.; Benoist, S.; Chipponi, J.; Vicaut, E.; et al. Rectal cancer surgery with or without bowel preparation: The French GRECCAR III multicenter single-blinded randomized trial. Ann. Surg. 2010, 252, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, R.; Leonard, D.; Delzenne, N.; Kartheuser, A.; Cani, P.D. Novel insight into the role of microbiota in colorectal surgery. Gut 2017, 66, 738–749. [Google Scholar] [CrossRef]
- Hartman, A.L.; Lough, D.M.; Barupal, D.K.; Fiehn, O.; Fishbein, T.; Zasloff, M.; Eisen, J.A. Human gut microbiome adopts an alternative state following small bowel transplantation. Proc. Natl. Acad. Sci. USA 2009, 106, 17187–17192. [Google Scholar] [CrossRef]
- Shimizu, K.; Ogura, H.; Asahara, T.; Nomoto, K.; Matsushima, A.; Hayakawa, K.; Ikegawa, H.; Tasaki, O.; Kuwagata, Y.; Shimazu, T. Gut microbiota and environment in patients with major burns—A preliminary report. Burns 2015, 41, e28–e33. [Google Scholar] [CrossRef]
- Earley, Z.M.; Akhtar, S.; Green, S.J.; Naqib, A.; Khan, O.; Cannon, A.R.; Hammer, A.M.; Morris, N.L.; Li, X.; Eberhardt, J.M.; et al. Burn Injury Alters the Intestinal Microbiome and Increases Gut Permeability and Bacterial Translocation. PLoS ONE 2015, 10, e0129996. [Google Scholar] [CrossRef]
- Wang, F.; Li, Q.; He, Q.; Geng, Y.; Tang, C.; Wang, C.; Li, J. Temporal variations of the ileal microbiota in intestinal ischemia and reperfusion. Shock 2013, 39, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Gralka, E.; Luchinat, C.; Tenori, L.; Ernst, B.; Thurnheer, M.; Schultes, B. Metabolomic fingerprint of severe obesity is dynamically affected by bariatric surgery in a procedure-dependent manner. Am. J. Clin. Nutr. 2015, 102, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Li, L.; Rezaei, A.; Eslamfam, S.; Che, D.; Ma, X. Metabolites of Dietary Protein and Peptides by Intestinal Microbes and their Impacts on Gut. Curr. Protein Pept. Sci. 2015, 16, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Ojima, M.; Motooka, D.; Shimizu, K.; Gotoh, K.; Shintani, A.; Yoshiya, K.; Nakamura, S.; Ogura, H.; Iida, T.; Shimazu, T. Metagenomic Analysis Reveals Dynamic Changes of Whole Gut Microbiota in the Acute Phase of Intensive Care Unit Patients. Dig. Dis. Sci. 2016, 61, 1628–1634. [Google Scholar] [CrossRef] [PubMed]
- Zaborin, A.; Smith, D.; Garfield, K.; Quensen, J.; Shakhsheer, B.; Kade, M.; Tirrell, M.; Tiedje, J.; Gilbert, J.A.; Zaborina, O.; et al. Membership and Behavior of Ultra-Low-Diversity Pathogen Communities Present in the Gut of Humans during Prolonged Critical Illness. mBio 2014, 5, e01361-14. [Google Scholar] [CrossRef] [PubMed]
- Kasatpibal, N.; Whitney, J.D.; Saokaew, S.; Kengkla, K.; Heitkemper, M.M.; Apisarnthanarak, A. Effectiveness of Probiotic, Prebiotic, and Synbiotic Therapies in Reducing Postoperative Complications: A Systematic Review and Network Meta-analysis. Clin. Infect. Dis. 2017, 64, S153–S160. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-D.; Xu, W.; Liu, M.-M.; Hu, K.-J.; Sun, Y.-Y.; Yang, X.-F.; Zhu, G.-Q.; Wang, Z.-W.; Huang, W. Efficacy of prophylactic probiotics in combination with antibiotics versus antibiotics alone for colorectal surgery: A meta-analysis of randomized controlled trials. J. Surg. Oncol. 2018, 117, 1394–1404. [Google Scholar] [CrossRef]
- Arumugam, S.; Lau, C.S.M.; Chamberlain, R.S. Probiotics and Synbiotics Decrease Postoperative Sepsis in Elective Gastrointestinal Surgical Patients: A Meta-Analysis. J. Gastrointest. Surg. 2016, 20, 1123–1131. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, Q.; Liu, Y.; Fan, D. Effect of Perioperative Probiotics and Synbiotics on Postoperative Infections after Gastrointestinal Surgery: A Systematic Review with Meta-Analysis. JPEN J. Parenter. Enter. Nutr. 2017, 41, 1051–1062. [Google Scholar] [CrossRef]
- Lytvyn, L.; Quach, K.; Banfield, L.; Johnston, B.C.; Mertz, D. Probiotics and synbiotics for the prevention of postoperative infections following abdominal surgery: A systematic review and meta-analysis of randomized controlled trials. J. Hosp. Infect. 2016, 92, 130–139. [Google Scholar] [CrossRef]
- Sawas, T.; Al Halabi, S.; Hernaez, R.; Carey, W.D.; Cho, W.K. Patients Receiving Prebiotics and Probiotics Before Liver Transplantation Develop Fewer Infections Than Controls: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2015, 13, 1567–1574. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-D.; Liu, M.-M.; Liang, X.; Hu, N.; Huang, W. Effects of perioperative supplementation with pro-/synbiotics on clinical outcomes in surgical patients: A meta-analysis with trial sequential analysis of randomized controlled trials. Clin. Nutr. 2018, 37, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Zmora, N.; Zilberman-Schapira, G.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Zur, M.; Regev-Lehavi, D.; Ben-Zeev Brik, R.; Federici, S.; et al. Post-Antibiotic Gut Mucosal Microbiome Reconstitution Is Impaired by Probiotics and Improved by Autologous FMT. Cell 2018, 174, 1406–1423.e16. [Google Scholar] [CrossRef] [PubMed]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 350, g7647. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Yang, Y.; Xia, Y.; Chen, H.; Hong, L.; Feng, J.; Yang, J.; Yang, Z.; Shi, C.; Wu, W.; Gao, R.; et al. The effect of perioperative probiotics treatment for colorectal cancer: Short-term outcomes of a randomized controlled trial. Oncotarget 2016, 7, 8432–8440. [Google Scholar] [CrossRef]
- Kanazawa, H.; Nagino, M.; Kamiya, S.; Komatsu, S.; Mayumi, T.; Takagi, K.; Asahara, T.; Nomoto, K.; Tanaka, R.; Nimura, Y. Synbiotics reduce postoperative infectious complications: A randomized controlled trial in biliary cancer patients undergoing hepatectomy. Langenbeck’s Arch. Surg. 2005, 390, 104–113. [Google Scholar] [CrossRef]
- Aisu, N.; Tanimura, S.; Yamashita, Y.; Yamashita, K.; Maki, K.; Yoshida, Y.; Sasaki, T.; Takeno, S.; Hoshino, S. Impact of perioperative probiotic treatment for surgical site infections in patients with colorectal cancer. Exp. Ther. Med. 2015, 10, 966–972. [Google Scholar] [CrossRef]
- Liu, Z.; Qin, H.; Yang, Z.; Xia, Y.; Liu, W.; Yang, J.; Jiang, Y.; Zhang, H.; Yang, Z.; Wang, Y.; et al. Randomised clinical trial: The effects of perioperative probiotic treatment on barrier function and post-operative infectious complications in colorectal cancer surgery—A double-blind study: Randomised clinical trial: Perioperative probiotics on colon cancer. Aliment. Pharmacol. Ther. 2011, 33, 50–63. [Google Scholar]
- Eguchi, S.; Takatsuki, M.; Hidaka, M.; Soyama, A.; Ichikawa, T.; Kanematsu, T. Perioperative synbiotic treatment to prevent infectious complications in patients after elective living donor liver transplantation: A prospective randomized study. Am. J. Surg. 2011, 201, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, S.; Sakamoto, E.; Norimizu, S.; Shingu, Y.; Asahara, T.; Nomoto, K.; Nagino, M. Efficacy of perioperative synbiotics treatment for the prevention of surgical site infection after laparoscopic colorectal surgery: A randomized controlled trial. Surg. Today 2016, 46, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.D.G. Randomised clinical trial of synbiotic therapy in elective surgical patients. Gut 2004, 53, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Diepenhorst, G.M.P.; van Ruler, O.; Besselink, M.G.H.; van Santvoort, H.C.; Wijnandts, P.R.; Renooij, W.; Gouma, D.J.; Gooszen, H.G.; Boermeester, M.A. Influence of prophylactic probiotics and selective decontamination on bacterial translocation in patients undergoing pancreatic surgery: A randomized controlled trial. Shock 2011, 35, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Flesch, A.T.; Tonial, S.T.; Contu, P.D.C.; Damin, D.C. Perioperative synbiotics administration decreases postoperative infections in patients with colorectal cancer: A randomized, double-blind clinical trial. Rev. Col. Bras. Cir. 2017, 44, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Horvat, M.; Krebs, B.; Potrč, S.; Ivanecz, A.; Kompan, L. Preoperative synbiotic bowel conditioning for elective colorectal surgery. Wien. Klin. Wochenschr. 2010, 122, 26–30. [Google Scholar] [CrossRef]
- Kotzampassi, K.; Stavrou, G.; Damoraki, G.; Georgitsi, M.; Basdanis, G.; Tsaousi, G.; Giamarellos-Bourboulis, E.J. A Four-Probiotics Regimen Reduces Postoperative Complications after Colorectal Surgery: A Randomized, Double-Blind, Placebo-Controlled Study. World J. Surg. 2015, 39, 2776–2783. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-H.; Huang, M.-J.; Zhang, X.-W.; Wang, L.; Huang, N.-Q.; Peng, H.; Lan, P.; Peng, J.-S.; Yang, Z.; Xia, Y.; et al. The effects of perioperative probiotic treatment on serum zonulin concentration and subsequent postoperative infectious complications after colorectal cancer surgery: A double-center and double-blind randomized clinical trial. Am. J. Clin. Nutr. 2013, 97, 117–126. [Google Scholar] [CrossRef]
- Liu, Z.; Li, C.; Huang, M.; Tong, C.; Zhang, X.; Wang, L.; Peng, H.; Lan, P.; Zhang, P.; Huang, N.; et al. Positive regulatory effects of perioperative probiotic treatment on postoperative liver complications after colorectal liver metastases surgery: A double-center and double-blind randomized clinical trial. BMC Gastroenterol. 2015, 15, 34. [Google Scholar] [CrossRef]
- Mangell, P.; Thorlacius, H.; Syk, I.; Ahrné, S.; Molin, G.; Olsson, C.; Jeppsson, B. Lactobacillus plantarum 299v Does Not Reduce Enteric Bacteria or Bacterial Translocation in Patients Undergoing Colon Resection. Dig. Dis. Sci. 2012, 57, 1915–1924. [Google Scholar] [CrossRef]
- McNaught, C.E. A prospective randomised study of the probiotic Lactobacillus plantarum 299V on indices of gut barrier function in elective surgical patients. Gut 2002, 51, 827–831. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T.; Tsuchiya, Y.; Nashimoto, A.; Yabusaki, H.; Takii, Y.; Nakagawa, S.; Sato, N.; Kanbayashi, C.; Tanaka, O. Probiotics reduce infectious complications after pancreaticoduodenectomy. Hepatogastroenterology 2007, 54, 661–663. [Google Scholar] [PubMed]
- Okazaki, M.; Matsukuma, S.; Suto, R.; Miyazaki, K.; Hidaka, M.; Matsuo, M.; Noshima, S.; Zempo, N.; Asahara, T.; Nomoto, K. Perioperative synbiotic therapy in elderly patients undergoing gastroenterological surgery: A prospective, randomized control trial. Nutrition 2013, 29, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-W.; Du, P.; Yang, B.-R.; Gao, J.; Fang, W.-J.; Ying, C.-M. Preoperative Probiotics Decrease Postoperative Infectious Complications of Colorectal Cancer. Am. J. Med. Sci. 2012, 343, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, Y.; Nishigaki, E.; Abe, T.; Fukaya, M.; Asahara, T.; Nomoto, K.; Nagino, M. Randomized clinical trial of the effect of perioperative synbiotics versus no synbiotics on bacterial translocation after oesophagectomy: Synbiotic treatment for patients who undergo oesophagectomy. Br. J. Surg. 2014, 101, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Usami, M.; Miyoshi, M.; Kanbara, Y.; Aoyama, M.; Sakaki, H.; Shuno, K.; Hirata, K.; Takahashi, M.; Ueno, K.; Tabata, S.; et al. Effects of Perioperative Synbiotic Treatment on Infectious Complications, Intestinal Integrity, and Fecal Flora and Organic Acids in Hepatic Surgery with or without Cirrhosis. J. Parenter. Enter. Nutr. 2011, 35, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Yano, M.; Motoori, M.; Kishi, K.; Miyashiro, I.; Ohue, M.; Ohigashi, H.; Asahara, T.; Nomoto, K.; Ishikawa, O. Impact of perioperative administration of synbiotics in patients with esophageal cancer undergoing esophagectomy: A prospective randomized controlled trial. Surgery 2012, 152, 832–842. [Google Scholar] [CrossRef] [PubMed]
- Sommacal, H.M.; Bersch, V.P.; Vitola, S.P.; Osvaldt, A.B. Perioperative synbiotics decrease postoperative complications in periampullary neoplasms: A randomized, double-blind clinical trial. Nutr. Cancer 2015, 67, 457–462. [Google Scholar] [CrossRef]
- Sadahiro, S.; Suzuki, T.; Tanaka, A.; Okada, K.; Kamata, H.; Ozaki, T.; Koga, Y. Comparison between oral antibiotics and probiotics as bowel preparation for elective colon cancer surgery to prevent infection: Prospective randomized trial. Surgery 2014, 155, 493–503. [Google Scholar] [CrossRef]
- Reddy, B.S.; MacFie, J.; Gatt, M.; Larsen, C.N.; Jensen, S.S.; Leser, T.D. Randomized clinical trial of effect of synbiotics, neomycin and mechanical bowel preparation on intestinal barrier function in patients undergoing colectomy. Br. J. Surgery 2007, 94, 546–554. [Google Scholar] [CrossRef]
- Rammohan, A.; Sathyanesan, J.; Rajendran, K.; Pitchaimuthu, A.; Perumal, S.K.; Balaraman, K.; Ramasamy, R.; Palaniappan, R.; Govindan, M. Synbiotics in Surgery for Chronic Pancreatitis: Are They Truly Effective? A Single-blind Prospective Randomized Control Trial. Ann. Surg. 2015, 262, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Grąt, M.; Wronka, K.M.; Lewandowski, Z.; Grąt, K.; Krasnodębski, M.; Stypułkowski, J.; Hołówko, W.; Masior, Ł.; Kosińska, I.; Wasilewicz, M.; et al. Effects of continuous use of probiotics before liver transplantation: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2017, 36, 1530–1539. [Google Scholar] [CrossRef] [PubMed]
- Mizuta, M.; Endo, I.; Yamamoto, S.; Inokawa, H.; Kubo, M.; Udaka, T.; Sogabe, O.; Maeda, H.; Shirakawa, K.; Okazaki, E.; et al. Perioperative supplementation with bifidobacteria improves postoperative nutritional recovery, inflammatory response, and fecal microbiota in patients undergoing colorectal surgery: A prospective, randomized clinical trial. Biosci. Microbiota Food Health 2016, 35, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, Y.; Asahara, T.; Nomoto, K.; Nagino, M. Effects of Synbiotics to Prevent Postoperative Infectious Complications in Highly Invasive Abdominal Surgery. Ann. Nutr. Metab. 2017, 71, 23–30. [Google Scholar] [CrossRef]
- Sugawara, G.; Nagino, M.; Nishio, H.; Ebata, T.; Takagi, K.; Asahara, T.; Nomoto, K.; Nimura, Y. Perioperative Synbiotic Treatment to Prevent Postoperative Infectious Complications in Biliary Cancer Surgery: A Randomized Controlled Trial. Ann. Surg. 2006, 244, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, J.; Wu, J.; Chalson, H.; Merigan, L.; Mitchell, A. Probiotic use in preventing postoperative infection in liver transplant patients. Hepatobiliary Surg. Nutr. 2013, 2, 6. [Google Scholar]
- Rayes, N.; Hansen, S.; Seehofer, D.; Müller, A.R.; Serke, S.; Bengmark, S.; Neuhaus, P. Early enteral supply of fiber and Lactobacilli versus conventional nutrition: A controlled trial in patients with major abdominal surgery. Nutrition 2002, 18, 609–615. [Google Scholar] [CrossRef]
- Rayes, N.; Seehofer, D.; Hansen, S.; Boucsein, K.; Müller, A.R.; Serke, S.; Bengmark, S.; Neuhaus, P. Early enteral supply of lactobacillus and fiber versus selective bowel decontamination: A controlled trial in liver transplant recipients. Transplantation 2002, 74, 123–128. [Google Scholar] [CrossRef]
- Rayes, N.; Seehofer, D.; Theruvath, T.; Schiller, R.A.; Langrehr, J.M.; Jonas, S.; Bengmark, S.; Neuhaus, P. Supply of Pre- and Probiotics Reduces Bacterial Infection Rates After Liver Transplantation-A Randomized, Double-Blind Trial. Am. J. Transplant. 2005, 5, 125–130. [Google Scholar] [CrossRef]
- Rayes, N.; Seehofer, D.; Theruvath, T.; Mogl, M.; Langrehr, J.M.; Nüssler, N.C.; Bengmark, S.; Neuhaus, P. Effect of Enteral Nutrition and Synbiotics on Bacterial Infection Rates After Pylorus-preserving Pancreatoduodenectomy: A Randomized, Double-blind Trial. Ann. Surg. 2007, 246, 36–41. [Google Scholar] [CrossRef]
- Rayes, N.; Pilarski, T.; Stockmann, M.; Bengmark, S.; Neuhaus, P.; Seehofer, D. Effect of pre- and probiotics on liver regeneration after resection: A randomised, double-blind pilot study. Benef. Microbes 2012, 3, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Horvath, A.; Leber, B.; Schmerboeck, B.; Tawdrous, M.; Zettel, G.; Hartl, A.; Madl, T.; Stryeck, S.; Fuchs, D.; Lemesch, S.; et al. Randomised clinical trial: The effects of a multispecies probiotic vs. placebo on innate immune function, bacterial translocation and gut permeability in patients with cirrhosis. Aliment. Pharmacol. Ther. 2016, 44, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, S.; Yokoyama, Y.; Nagino, M. Gut microbiota and bacterial translocation in digestive surgery: The impact of probiotics. Langenbeck’s Arch. Surg. 2017, 402, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Rizzatti, G.; Lopetuso, L.R.; Gibiino, G.; Binda, C.; Gasbarrini, A. Proteobacteria: A Common Factor in Human Diseases. Available online: https://www.hindawi.com/journals/bmri/2017/9351507/ (accessed on 8 September 2018).
- Lupp, C.; Robertson, M.L.; Wickham, M.E.; Sekirov, I.; Champion, O.L.; Gaynor, E.C.; Finlay, B.B. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe 2007, 2, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Hiippala, K.; Jouhten, H.; Ronkainen, A.; Hartikainen, A.; Kainulainen, V.; Jalanka, J.; Satokari, R. The Potential of Gut Commensals in Reinforcing Intestinal Barrier Function and Alleviating Inflammation. Nutrients 2018, 10, 988. [Google Scholar] [CrossRef]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011, 469, 543–547. [Google Scholar] [CrossRef]
- Canani, R.B.; Costanzo, M.D.; Leone, L.; Pedata, M.; Meli, R.; Calignano, A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. Gastroenterol. 2011, 17, 1519–1528. [Google Scholar] [CrossRef]
- Hayakawa, M.; Asahara, T.; Henzan, N.; Murakami, H.; Yamamoto, H.; Mukai, N.; Minami, Y.; Sugano, M.; Kubota, N.; Uegaki, S.; et al. Dramatic changes of the gut flora immediately after severe and sudden insults. Dig. Dis. Sci. 2011, 56, 2361–2365. [Google Scholar] [CrossRef]
- Gantois, I.; Ducatelle, R.; Pasmans, F.; Haesebrouck, F.; Hautefort, I.; Thompson, A.; Hinton, J.C.; Van Immerseel, F. Butyrate specifically down-regulates salmonella pathogenicity island 1 gene expression. Appl. Environ. Microbiol. 2006, 72, 946–949. [Google Scholar] [CrossRef]
- Seal, J.B.; Morowitz, M.; Zaborina, O.; An, G.; Alverdy, J.C. The molecular Koch’s postulates and surgical infection: A view forward. Surgery 2010, 147, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Olivas, A.D.; Shogan, B.D.; Valuckaite, V.; Zaborin, A.; Belogortseva, N.; Musch, M.; Meyer, F.; Trimble, W.L.; An, G.; Gilbert, J.; et al. Intestinal tissues induce an SNP mutation in Pseudomonas aeruginosa that enhances its virulence: Possible role in anastomotic leak. PLoS ONE 2012, 7, e44326. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Inan, M.S.; Rasoulpour, R.J.; Yin, L.; Hubbard, A.K.; Rosenberg, D.W.; Giardina, C. The luminal short-chain fatty acid butyrate modulates NF-kappaB activity in a human colonic epithelial cell line. Gastroenterology 2000, 118, 724–734. [Google Scholar] [CrossRef]
- Kelly, C.J.; Zheng, L.; Campbell, E.L.; Saeedi, B.; Scholz, C.C.; Bayless, A.J.; Wilson, K.E.; Glover, L.E.; Kominsky, D.J.; Magnuson, A.; et al. Crosstalk between Microbiota-Derived Short-Chain Fatty Acids and Intestinal Epithelial HIF Augments Tissue Barrier Function. Cell Host Microbe 2015, 17, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Al-Lahham, S.H.; Peppelenbosch, M.P.; Roelofsen, H.; Vonk, R.J.; Venema, K. Biological effects of propionic acid in humans; metabolism, potential applications and underlying mechanisms. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2010, 1801, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Lawhon, S.D.; Maurer, R.; Suyemoto, M.; Altier, C. Intestinal short-chain fatty acids alter Salmonella typhimurium invasion gene expression and virulence through BarA/SirA. Mol. Microbiol. 2002, 46, 1451–1464. [Google Scholar] [CrossRef]
- Dannhardt, G.; Lehr, M. Nonsteriodal Antiinflammatory Agents, XVII: Inhibition of Bovine Cyclooxygenase and 5-Lipoxygenase by N-Alkyldiphenyl-pyrrolyl Acetic and Propionic Acid Derivatives. Arch. Pharm. 1993, 326, 157–162. [Google Scholar] [CrossRef]
- Bos, C.L.; Richel, D.J.; Ritsema, T.; Peppelenbosch, M.P.; Versteeg, H.H. Prostanoids and prostanoid receptors in signal transduction. Int. J. Biochem. Cell Biol. 2004, 36, 1187–1205. [Google Scholar] [CrossRef]
- Curi, R.; Bond, J.A.; Calder, P.C.; Newsholme, E.A. Propionate regulates lymphocyte proliferation and metabolism. Gen. Pharmacol. Vasc. Syst. 1993, 24, 591–597. [Google Scholar] [CrossRef]
- Wajner, M.; Santos, K.D.; Schlottfeldt, J.L.; Rocha, M.P.; Wannmacher, C.M. Inhibition of mitogen-activated proliferation of human peripheral lymphocytes in vitro by propionic acid. Clin. Sci. 1999, 96, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Cavaglieri, C.R.; Nishiyama, A.; Fernandes, L.C.; Curi, R.; Miles, E.A.; Calder, P.C. Differential effects of short-chain fatty acids on proliferation and production of pro- and anti-inflammatory cytokines by cultured lymphocytes. Life Sci. 2003, 73, 1683–1690. [Google Scholar] [CrossRef]
- Luk, G.D.; Bayless, T.M.; Baylin, S.B. Diamine oxidase (histaminase). A circulating marker for rat intestinal mucosal maturation and integrity. J. Clin. Investig. 1980, 66, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Buffoni, F. Histaminase and Related Amine Oxidases. Pharmacol. Rev. 1966, 18, 1163–1199. [Google Scholar] [PubMed]
- Honzawa, Y.; Nakase, H.; Matsuura, M.; Chiba, T. Clinical significance of serum diamine oxidase activity in inflammatory bowel disease: Importance of evaluation of small intestinal permeability. Inflamm. Bowel Dis. 2011, 17, E23–25. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, K.A.; Manieri, N.A.; Liu, T.-C.; Stappenbeck, T.S. IL-6 Stimulates Intestinal Epithelial Proliferation and Repair after Injury. PLoS ONE 2014, 9, e114195. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, K.A.; Schulz, H.M.; Regner, E.H.; Severs, E.L.; Hendrickson, J.D.; Mehta, G.; Whitney, A.K.; Ir, D.; Ohri, N.; Robertson, C.E.; et al. Bacteroidales recruit IL-6-producing intraepithelial lymphocytes in the colon to promote barrier integrity. Mucosal Immunol. 2018, 11, 357–368. [Google Scholar] [CrossRef]
- Henriksen, M.; Jahnsen, J.; Lygren, I.; Stray, N.; Sauar, J.; Vatn, M.H.; Moum, B.; IBSEN Study Group. C-reactive protein: A predictive factor and marker of inflammation in inflammatory bowel disease. Results from a prospective population-based study. Gut 2008, 57, 1518–1523. [Google Scholar] [CrossRef]
- Mazidi, M.; Rezaie, P.; Ferns, G.A.; Vatanparast, H. Impact of Probiotic Administration on Serum C-Reactive Protein Concentrations: Systematic Review and Meta-Analysis of Randomized Control Trials. Nutrients 2017, 9, 20. [Google Scholar] [CrossRef]
- Mizuno, T.; Yokoyama, Y.; Nishio, H.; Ebata, T.; Sugawara, G.; Asahara, T.; Nomoto, K.; Nagino, M. Intraoperative bacterial translocation detected by bacterium-specific ribosomal rna-targeted reverse-transcriptase polymerase chain reaction for the mesenteric lymph node strongly predicts postoperative infectious complications after major hepatectomy for biliary malignancies. Ann. Surg. 2010, 252, 1013–1019. [Google Scholar] [PubMed]
- Nishigaki, E.; Abe, T.; Yokoyama, Y.; Fukaya, M.; Asahara, T.; Nomoto, K.; Nagino, M. The detection of intraoperative bacterial translocation in the mesenteric lymph nodes is useful in predicting patients at high risk for postoperative infectious complications after esophagectomy. Ann. Surg. 2014, 259, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Lederer, A.-K.; Pisarski, P.; Kousoulas, L.; Fichtner-Feigl, S.; Hess, C.; Huber, R. Postoperative changes of the microbiome: Are surgical complications related to the gut flora? A systematic review. BMC Surg. 2017, 17, 125. [Google Scholar] [CrossRef] [PubMed]
- Knoop, K.A.; McDonald, K.G.; Kulkarni, D.H.; Newberry, R.D. Antibiotics promote inflammation through the translocation of native commensal colonic bacteria. Gut 2016, 65, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Britton, R.A.; Young, V.B. Role of the Intestinal Microbiota in Resistance to Colonization by Clostridium difficle. Gastroenterology 2014, 146, 1547–1553. [Google Scholar] [CrossRef] [PubMed]
- NAP6 Report—The National Institute of Academic Anaesthesia. Available online: http://www.nationalauditprojects.org.uk/NAP6Report (accessed on 26 May 2018).
- Tanner, J.; Khan, D.; Aplin, C.; Ball, J.; Thomas, M.; Bankart, J. Post-discharge surveillance to identify colorectal surgical site infection rates and related costs. J. Hosp. Infect. 2009, 72, 243–250. [Google Scholar] [CrossRef]
- Straatman, J.; Cuesta, M.A.; De Lange-de Klerk, E.S.; Van Der Peet, D.L. Hospital Cost-Analysis of Complications after Major Abdominal Surgery. DSU 2015, 32, 150–156. [Google Scholar] [CrossRef]
- Healy, M.A.; Mullard, A.J.; Campbell, D.A.; Dimick, J.B. Hospital and Payer Costs Associated with Surgical Complications. JAMA Surg. 2016, 151, 823–830. [Google Scholar] [CrossRef]
- Keenan, J.E.; Speicher, P.J.; Thacker, J.K.M.; Walter, M.; Kuchibhatla, M.; Mantyh, C.R. The preventive surgical site infection bundle in colorectal surgery: An effective approach to surgical site infection reduction and health care cost savings. JAMA Surg. 2014, 149, 1045–1052. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).