A Systematic Review, Meta-Analysis, and Meta-Regression Evaluating the Efficacy and Mechanisms of Action of Probiotics and Synbiotics in the Prevention of Surgical Site Infections and Surgery-Related Complications
Abstract
:1. Introduction
- The mechanism of action of probiotics and synbiotics in prevention of SSIs;
- The influence of probiotics on gut microbiota alterations related to the surgery;
- A possibility to establish recommendations concerning strain(s), dose, and mode of administration of probiotic in the prevention of SSI and SRCs.
2. Materials and Methods
2.1. Search Strategy and Inclusion Criteria
- treatment with pro-/pre-/synbiotics;
- randomisation to pre/pro/synbiotic versus placebo/monotherapy/standard care; and
- available meta-analyzable endpoint/change score data on outcomes placed below.
- if a study contained more than two arms, the data were abstracted separately for each comparator.
2.2. Data Abstraction
2.3. Outcomes
2.4. Data Synthesis and Statistical Analysis
2.5. Risk of Bias
3. Results
3.1. Search Results
3.2. Study, Patient and Treatment Characteristics
3.3. Microbiota and Putative Mechanism of Probiotic/Synbiotics’ Action in SSIs/SRCs Prevention—Primary Outcomes
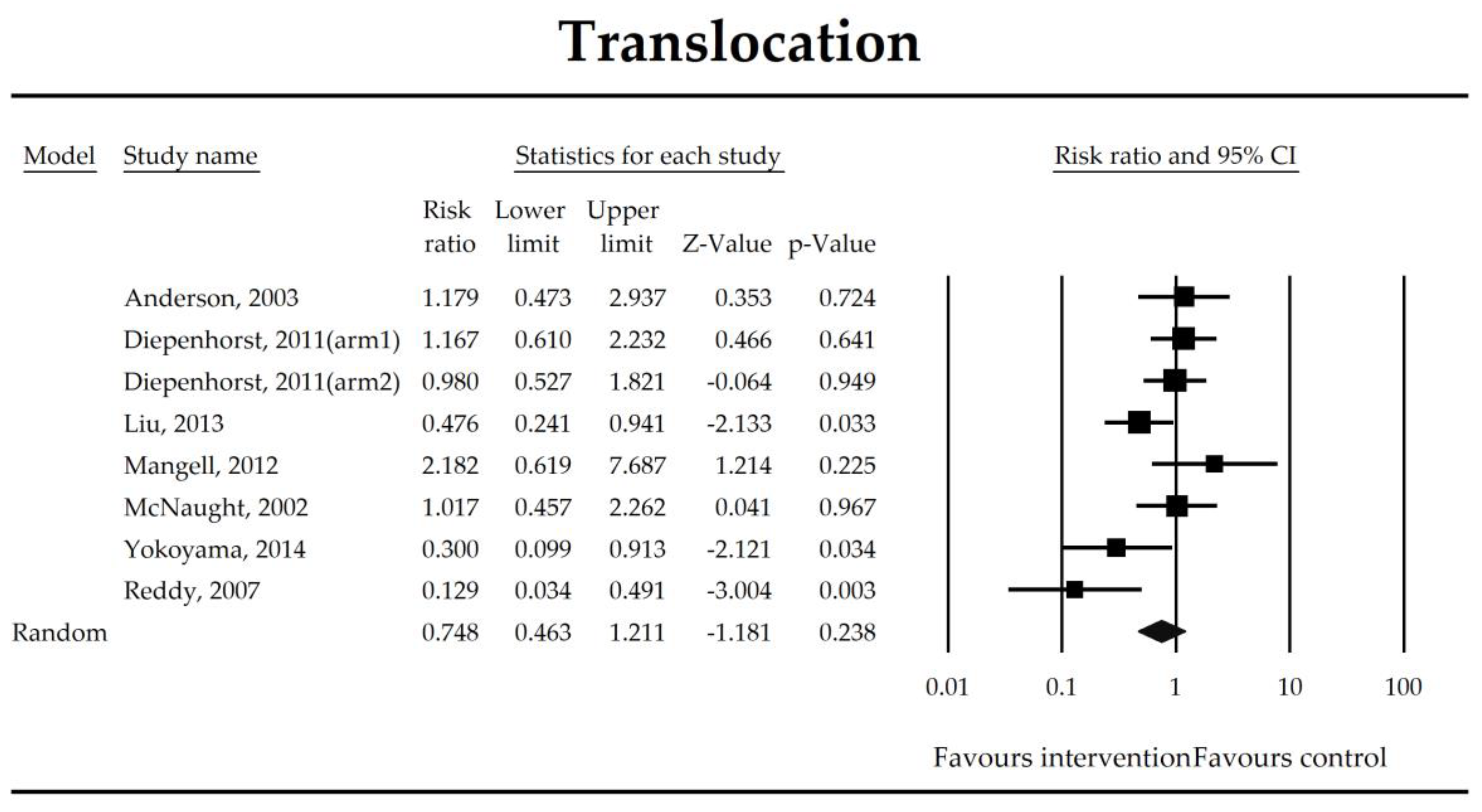
3.4. Surgery Related Complications (SRCs) and Secondary Outcomes
3.5. Risk of Bias
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Data Source
References
- GlobalSurg Collaborative. Surgical site infection after gastrointestinal surgery in high-income, middle-income, and low-income countries: A prospective, international, multicentre cohort study. Lancet Infect. Dis. 2018, 18, 516–525. [Google Scholar] [CrossRef]
- Guyton, K.; Alverdy, J.C. The gut microbiota and gastrointestinal surgery. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 43–54. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Guidelines on the Prevention of Surgical Site Infection. Available online: http://www.who.int/gpsc/ssi-guidelines/en/ (accessed on 7 September 2018).
- Berríos-Torres, S.I.; Umscheid, C.A.; Bratzler, D.W.; Leas, B.; Stone, E.C.; Kelz, R.R.; Reinke, C.E.; Morgan, S.; Solomkin, J.S.; Mazuski, J.E.; et al. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017. JAMA Surg. 2017, 152, 784–791. [Google Scholar] [CrossRef]
- Ban, K.A.; Minei, J.P.; Laronga, C.; Harbrecht, B.G.; Jensen, E.H.; Fry, D.E.; Itani, K.M.F.; Dellinger, E.P.; Ko, C.Y.; Duane, T.M. American College of Surgeons and Surgical Infection Society: Surgical Site Infection Guidelines, 2016 Update. J. Am. Coll. Surg. 2017, 224, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Stone, P.W.; Braccia, D.; Larson, E. Systematic review of economic analyses of health care-associated infections. Am. J. Infect. Control 2005, 33, 501–509. [Google Scholar] [CrossRef] [PubMed]
- WHO Guidelines for Safe Surgery 2009: Safe Surgery Saves Lives; WHO Guidelines Approved by the Guidelines Review Committee; World Health Organization: Geneva, Switzerland, 2009; ISBN 978-92-4-159855-2.
- Nguyen, N.; Yegiyants, S.; Kaloostian, C.; Abbas, M.A.; Difronzo, L.A. The Surgical Care Improvement project (SCIP) initiative to reduce infection in elective colorectal surgery: Which performance measures affect outcome? Am. Surg. 2008, 74, 1012–1016. [Google Scholar]
- Altemeier, W.A.; Culbertson, W.R.; Hummel, R.P. Surgical considerations of endogenous infections—Sources, types, and methods of control. Surg. Clin. N. Am. 1968, 48, 227–240. [Google Scholar] [CrossRef]
- Brial, F.; Le Lay, A.; Dumas, M.-E.; Gauguier, D. Implication of gut microbiota metabolites in cardiovascular and metabolic diseases. Cell. Mol. Life Sci. 2018, 75, 3977–3990. [Google Scholar] [CrossRef]
- Marlicz, W.; Yung, D.E.; Skonieczna-Żydecka, K.; Loniewski, I.; van Hemert, S.; Loniewska, B.; Koulaouzidis, A. From clinical uncertainties to precision medicine: The emerging role of the gut barrier and microbiome in small bowel functional diseases. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 961–978. [Google Scholar] [CrossRef]
- Clemente, J.C.; Manasson, J.; Scher, J.U. The role of the gut microbiome in systemic inflammatory disease. BMJ 2018, 360, j5145. [Google Scholar] [CrossRef] [Green Version]
- Spadoni, I.; Zagato, E.; Bertocchi, A.; Paolinelli, R.; Hot, E.; Sabatino, A.D.; Caprioli, F.; Bottiglieri, L.; Oldani, A.; Viale, G.; et al. A gut-vascular barrier controls the systemic dissemination of bacteria. Science 2015, 350, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.A.; Rinaman, L.; Cryan, J.F. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol. Stress 2017, 7, 124–136. [Google Scholar] [PubMed]
- Sonnenburg, J.L.; Bäckhed, F. Diet-microbiota interactions as moderators of human metabolism. Nature 2016, 535, 56–64. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Materna, A.C.; Friedman, J.; Campos-Baptista, M.I.; Blackburn, M.C.; Perrotta, A.; Erdman, S.E.; Alm, E.J. Host lifestyle affects human microbiota on daily timescales. Genome Biol. 2014, 15, R89. [Google Scholar] [CrossRef] [PubMed]
- Scarborough, J.E.; Mantyh, C.R.; Sun, Z.; Migaly, J. Combined Mechanical and Oral Antibiotic Bowel Preparation Reduces Incisional Surgical Site Infection and Anastomotic Leak Rates after Elective Colorectal Resection: An Analysis of Colectomy-Targeted ACS NSQIP. Ann. Surg. 2015, 262, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Bretagnol, F.; Panis, Y.; Rullier, E.; Rouanet, P.; Berdah, S.; Dousset, B.; Portier, G.; Benoist, S.; Chipponi, J.; Vicaut, E.; et al. Rectal cancer surgery with or without bowel preparation: The French GRECCAR III multicenter single-blinded randomized trial. Ann. Surg. 2010, 252, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, R.; Leonard, D.; Delzenne, N.; Kartheuser, A.; Cani, P.D. Novel insight into the role of microbiota in colorectal surgery. Gut 2017, 66, 738–749. [Google Scholar] [CrossRef] [Green Version]
- Hartman, A.L.; Lough, D.M.; Barupal, D.K.; Fiehn, O.; Fishbein, T.; Zasloff, M.; Eisen, J.A. Human gut microbiome adopts an alternative state following small bowel transplantation. Proc. Natl. Acad. Sci. USA 2009, 106, 17187–17192. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, K.; Ogura, H.; Asahara, T.; Nomoto, K.; Matsushima, A.; Hayakawa, K.; Ikegawa, H.; Tasaki, O.; Kuwagata, Y.; Shimazu, T. Gut microbiota and environment in patients with major burns—A preliminary report. Burns 2015, 41, e28–e33. [Google Scholar] [CrossRef]
- Earley, Z.M.; Akhtar, S.; Green, S.J.; Naqib, A.; Khan, O.; Cannon, A.R.; Hammer, A.M.; Morris, N.L.; Li, X.; Eberhardt, J.M.; et al. Burn Injury Alters the Intestinal Microbiome and Increases Gut Permeability and Bacterial Translocation. PLoS ONE 2015, 10, e0129996. [Google Scholar] [CrossRef]
- Wang, F.; Li, Q.; He, Q.; Geng, Y.; Tang, C.; Wang, C.; Li, J. Temporal variations of the ileal microbiota in intestinal ischemia and reperfusion. Shock 2013, 39, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Gralka, E.; Luchinat, C.; Tenori, L.; Ernst, B.; Thurnheer, M.; Schultes, B. Metabolomic fingerprint of severe obesity is dynamically affected by bariatric surgery in a procedure-dependent manner. Am. J. Clin. Nutr. 2015, 102, 1313–1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, P.; Li, L.; Rezaei, A.; Eslamfam, S.; Che, D.; Ma, X. Metabolites of Dietary Protein and Peptides by Intestinal Microbes and their Impacts on Gut. Curr. Protein Pept. Sci. 2015, 16, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Ojima, M.; Motooka, D.; Shimizu, K.; Gotoh, K.; Shintani, A.; Yoshiya, K.; Nakamura, S.; Ogura, H.; Iida, T.; Shimazu, T. Metagenomic Analysis Reveals Dynamic Changes of Whole Gut Microbiota in the Acute Phase of Intensive Care Unit Patients. Dig. Dis. Sci. 2016, 61, 1628–1634. [Google Scholar] [CrossRef] [PubMed]
- Zaborin, A.; Smith, D.; Garfield, K.; Quensen, J.; Shakhsheer, B.; Kade, M.; Tirrell, M.; Tiedje, J.; Gilbert, J.A.; Zaborina, O.; et al. Membership and Behavior of Ultra-Low-Diversity Pathogen Communities Present in the Gut of Humans during Prolonged Critical Illness. mBio 2014, 5, e01361-14. [Google Scholar] [CrossRef] [PubMed]
- Kasatpibal, N.; Whitney, J.D.; Saokaew, S.; Kengkla, K.; Heitkemper, M.M.; Apisarnthanarak, A. Effectiveness of Probiotic, Prebiotic, and Synbiotic Therapies in Reducing Postoperative Complications: A Systematic Review and Network Meta-analysis. Clin. Infect. Dis. 2017, 64, S153–S160. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-D.; Xu, W.; Liu, M.-M.; Hu, K.-J.; Sun, Y.-Y.; Yang, X.-F.; Zhu, G.-Q.; Wang, Z.-W.; Huang, W. Efficacy of prophylactic probiotics in combination with antibiotics versus antibiotics alone for colorectal surgery: A meta-analysis of randomized controlled trials. J. Surg. Oncol. 2018, 117, 1394–1404. [Google Scholar] [CrossRef]
- Arumugam, S.; Lau, C.S.M.; Chamberlain, R.S. Probiotics and Synbiotics Decrease Postoperative Sepsis in Elective Gastrointestinal Surgical Patients: A Meta-Analysis. J. Gastrointest. Surg. 2016, 20, 1123–1131. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, Q.; Liu, Y.; Fan, D. Effect of Perioperative Probiotics and Synbiotics on Postoperative Infections after Gastrointestinal Surgery: A Systematic Review with Meta-Analysis. JPEN J. Parenter. Enter. Nutr. 2017, 41, 1051–1062. [Google Scholar] [CrossRef]
- Lytvyn, L.; Quach, K.; Banfield, L.; Johnston, B.C.; Mertz, D. Probiotics and synbiotics for the prevention of postoperative infections following abdominal surgery: A systematic review and meta-analysis of randomized controlled trials. J. Hosp. Infect. 2016, 92, 130–139. [Google Scholar] [CrossRef]
- Sawas, T.; Al Halabi, S.; Hernaez, R.; Carey, W.D.; Cho, W.K. Patients Receiving Prebiotics and Probiotics Before Liver Transplantation Develop Fewer Infections Than Controls: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2015, 13, 1567–1574. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-D.; Liu, M.-M.; Liang, X.; Hu, N.; Huang, W. Effects of perioperative supplementation with pro-/synbiotics on clinical outcomes in surgical patients: A meta-analysis with trial sequential analysis of randomized controlled trials. Clin. Nutr. 2018, 37, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Zmora, N.; Zilberman-Schapira, G.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Zur, M.; Regev-Lehavi, D.; Ben-Zeev Brik, R.; Federici, S.; et al. Post-Antibiotic Gut Mucosal Microbiome Reconstitution Is Impaired by Probiotics and Improved by Autologous FMT. Cell 2018, 174, 1406–1423.e16. [Google Scholar] [CrossRef] [PubMed]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 350, g7647. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Yang, Y.; Xia, Y.; Chen, H.; Hong, L.; Feng, J.; Yang, J.; Yang, Z.; Shi, C.; Wu, W.; Gao, R.; et al. The effect of perioperative probiotics treatment for colorectal cancer: Short-term outcomes of a randomized controlled trial. Oncotarget 2016, 7, 8432–8440. [Google Scholar] [CrossRef]
- Kanazawa, H.; Nagino, M.; Kamiya, S.; Komatsu, S.; Mayumi, T.; Takagi, K.; Asahara, T.; Nomoto, K.; Tanaka, R.; Nimura, Y. Synbiotics reduce postoperative infectious complications: A randomized controlled trial in biliary cancer patients undergoing hepatectomy. Langenbeck’s Arch. Surg. 2005, 390, 104–113. [Google Scholar] [CrossRef]
- Aisu, N.; Tanimura, S.; Yamashita, Y.; Yamashita, K.; Maki, K.; Yoshida, Y.; Sasaki, T.; Takeno, S.; Hoshino, S. Impact of perioperative probiotic treatment for surgical site infections in patients with colorectal cancer. Exp. Ther. Med. 2015, 10, 966–972. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Qin, H.; Yang, Z.; Xia, Y.; Liu, W.; Yang, J.; Jiang, Y.; Zhang, H.; Yang, Z.; Wang, Y.; et al. Randomised clinical trial: The effects of perioperative probiotic treatment on barrier function and post-operative infectious complications in colorectal cancer surgery—A double-blind study: Randomised clinical trial: Perioperative probiotics on colon cancer. Aliment. Pharmacol. Ther. 2011, 33, 50–63. [Google Scholar]
- Eguchi, S.; Takatsuki, M.; Hidaka, M.; Soyama, A.; Ichikawa, T.; Kanematsu, T. Perioperative synbiotic treatment to prevent infectious complications in patients after elective living donor liver transplantation: A prospective randomized study. Am. J. Surg. 2011, 201, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, S.; Sakamoto, E.; Norimizu, S.; Shingu, Y.; Asahara, T.; Nomoto, K.; Nagino, M. Efficacy of perioperative synbiotics treatment for the prevention of surgical site infection after laparoscopic colorectal surgery: A randomized controlled trial. Surg. Today 2016, 46, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.D.G. Randomised clinical trial of synbiotic therapy in elective surgical patients. Gut 2004, 53, 241–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diepenhorst, G.M.P.; van Ruler, O.; Besselink, M.G.H.; van Santvoort, H.C.; Wijnandts, P.R.; Renooij, W.; Gouma, D.J.; Gooszen, H.G.; Boermeester, M.A. Influence of prophylactic probiotics and selective decontamination on bacterial translocation in patients undergoing pancreatic surgery: A randomized controlled trial. Shock 2011, 35, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Flesch, A.T.; Tonial, S.T.; Contu, P.D.C.; Damin, D.C. Perioperative synbiotics administration decreases postoperative infections in patients with colorectal cancer: A randomized, double-blind clinical trial. Rev. Col. Bras. Cir. 2017, 44, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Horvat, M.; Krebs, B.; Potrč, S.; Ivanecz, A.; Kompan, L. Preoperative synbiotic bowel conditioning for elective colorectal surgery. Wien. Klin. Wochenschr. 2010, 122, 26–30. [Google Scholar] [CrossRef]
- Kotzampassi, K.; Stavrou, G.; Damoraki, G.; Georgitsi, M.; Basdanis, G.; Tsaousi, G.; Giamarellos-Bourboulis, E.J. A Four-Probiotics Regimen Reduces Postoperative Complications after Colorectal Surgery: A Randomized, Double-Blind, Placebo-Controlled Study. World J. Surg. 2015, 39, 2776–2783. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-H.; Huang, M.-J.; Zhang, X.-W.; Wang, L.; Huang, N.-Q.; Peng, H.; Lan, P.; Peng, J.-S.; Yang, Z.; Xia, Y.; et al. The effects of perioperative probiotic treatment on serum zonulin concentration and subsequent postoperative infectious complications after colorectal cancer surgery: A double-center and double-blind randomized clinical trial. Am. J. Clin. Nutr. 2013, 97, 117–126. [Google Scholar] [CrossRef]
- Liu, Z.; Li, C.; Huang, M.; Tong, C.; Zhang, X.; Wang, L.; Peng, H.; Lan, P.; Zhang, P.; Huang, N.; et al. Positive regulatory effects of perioperative probiotic treatment on postoperative liver complications after colorectal liver metastases surgery: A double-center and double-blind randomized clinical trial. BMC Gastroenterol. 2015, 15, 34. [Google Scholar] [CrossRef]
- Mangell, P.; Thorlacius, H.; Syk, I.; Ahrné, S.; Molin, G.; Olsson, C.; Jeppsson, B. Lactobacillus plantarum 299v Does Not Reduce Enteric Bacteria or Bacterial Translocation in Patients Undergoing Colon Resection. Dig. Dis. Sci. 2012, 57, 1915–1924. [Google Scholar] [CrossRef]
- McNaught, C.E. A prospective randomised study of the probiotic Lactobacillus plantarum 299V on indices of gut barrier function in elective surgical patients. Gut 2002, 51, 827–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nomura, T.; Tsuchiya, Y.; Nashimoto, A.; Yabusaki, H.; Takii, Y.; Nakagawa, S.; Sato, N.; Kanbayashi, C.; Tanaka, O. Probiotics reduce infectious complications after pancreaticoduodenectomy. Hepatogastroenterology 2007, 54, 661–663. [Google Scholar] [PubMed]
- Okazaki, M.; Matsukuma, S.; Suto, R.; Miyazaki, K.; Hidaka, M.; Matsuo, M.; Noshima, S.; Zempo, N.; Asahara, T.; Nomoto, K. Perioperative synbiotic therapy in elderly patients undergoing gastroenterological surgery: A prospective, randomized control trial. Nutrition 2013, 29, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-W.; Du, P.; Yang, B.-R.; Gao, J.; Fang, W.-J.; Ying, C.-M. Preoperative Probiotics Decrease Postoperative Infectious Complications of Colorectal Cancer. Am. J. Med. Sci. 2012, 343, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, Y.; Nishigaki, E.; Abe, T.; Fukaya, M.; Asahara, T.; Nomoto, K.; Nagino, M. Randomized clinical trial of the effect of perioperative synbiotics versus no synbiotics on bacterial translocation after oesophagectomy: Synbiotic treatment for patients who undergo oesophagectomy. Br. J. Surg. 2014, 101, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Usami, M.; Miyoshi, M.; Kanbara, Y.; Aoyama, M.; Sakaki, H.; Shuno, K.; Hirata, K.; Takahashi, M.; Ueno, K.; Tabata, S.; et al. Effects of Perioperative Synbiotic Treatment on Infectious Complications, Intestinal Integrity, and Fecal Flora and Organic Acids in Hepatic Surgery with or without Cirrhosis. J. Parenter. Enter. Nutr. 2011, 35, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Yano, M.; Motoori, M.; Kishi, K.; Miyashiro, I.; Ohue, M.; Ohigashi, H.; Asahara, T.; Nomoto, K.; Ishikawa, O. Impact of perioperative administration of synbiotics in patients with esophageal cancer undergoing esophagectomy: A prospective randomized controlled trial. Surgery 2012, 152, 832–842. [Google Scholar] [CrossRef] [PubMed]
- Sommacal, H.M.; Bersch, V.P.; Vitola, S.P.; Osvaldt, A.B. Perioperative synbiotics decrease postoperative complications in periampullary neoplasms: A randomized, double-blind clinical trial. Nutr. Cancer 2015, 67, 457–462. [Google Scholar] [CrossRef]
- Sadahiro, S.; Suzuki, T.; Tanaka, A.; Okada, K.; Kamata, H.; Ozaki, T.; Koga, Y. Comparison between oral antibiotics and probiotics as bowel preparation for elective colon cancer surgery to prevent infection: Prospective randomized trial. Surgery 2014, 155, 493–503. [Google Scholar] [CrossRef]
- Reddy, B.S.; MacFie, J.; Gatt, M.; Larsen, C.N.; Jensen, S.S.; Leser, T.D. Randomized clinical trial of effect of synbiotics, neomycin and mechanical bowel preparation on intestinal barrier function in patients undergoing colectomy. Br. J. Surgery 2007, 94, 546–554. [Google Scholar] [CrossRef]
- Rammohan, A.; Sathyanesan, J.; Rajendran, K.; Pitchaimuthu, A.; Perumal, S.K.; Balaraman, K.; Ramasamy, R.; Palaniappan, R.; Govindan, M. Synbiotics in Surgery for Chronic Pancreatitis: Are They Truly Effective? A Single-blind Prospective Randomized Control Trial. Ann. Surg. 2015, 262, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Grąt, M.; Wronka, K.M.; Lewandowski, Z.; Grąt, K.; Krasnodębski, M.; Stypułkowski, J.; Hołówko, W.; Masior, Ł.; Kosińska, I.; Wasilewicz, M.; et al. Effects of continuous use of probiotics before liver transplantation: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2017, 36, 1530–1539. [Google Scholar] [CrossRef] [PubMed]
- Mizuta, M.; Endo, I.; Yamamoto, S.; Inokawa, H.; Kubo, M.; Udaka, T.; Sogabe, O.; Maeda, H.; Shirakawa, K.; Okazaki, E.; et al. Perioperative supplementation with bifidobacteria improves postoperative nutritional recovery, inflammatory response, and fecal microbiota in patients undergoing colorectal surgery: A prospective, randomized clinical trial. Biosci. Microbiota Food Health 2016, 35, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, Y.; Asahara, T.; Nomoto, K.; Nagino, M. Effects of Synbiotics to Prevent Postoperative Infectious Complications in Highly Invasive Abdominal Surgery. Ann. Nutr. Metab. 2017, 71, 23–30. [Google Scholar] [CrossRef]
- Sugawara, G.; Nagino, M.; Nishio, H.; Ebata, T.; Takagi, K.; Asahara, T.; Nomoto, K.; Nimura, Y. Perioperative Synbiotic Treatment to Prevent Postoperative Infectious Complications in Biliary Cancer Surgery: A Randomized Controlled Trial. Ann. Surg. 2006, 244, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, J.; Wu, J.; Chalson, H.; Merigan, L.; Mitchell, A. Probiotic use in preventing postoperative infection in liver transplant patients. Hepatobiliary Surg. Nutr. 2013, 2, 6. [Google Scholar]
- Rayes, N.; Hansen, S.; Seehofer, D.; Müller, A.R.; Serke, S.; Bengmark, S.; Neuhaus, P. Early enteral supply of fiber and Lactobacilli versus conventional nutrition: A controlled trial in patients with major abdominal surgery. Nutrition 2002, 18, 609–615. [Google Scholar] [CrossRef]
- Rayes, N.; Seehofer, D.; Hansen, S.; Boucsein, K.; Müller, A.R.; Serke, S.; Bengmark, S.; Neuhaus, P. Early enteral supply of lactobacillus and fiber versus selective bowel decontamination: A controlled trial in liver transplant recipients. Transplantation 2002, 74, 123–128. [Google Scholar] [CrossRef]
- Rayes, N.; Seehofer, D.; Theruvath, T.; Schiller, R.A.; Langrehr, J.M.; Jonas, S.; Bengmark, S.; Neuhaus, P. Supply of Pre- and Probiotics Reduces Bacterial Infection Rates After Liver Transplantation-A Randomized, Double-Blind Trial. Am. J. Transplant. 2005, 5, 125–130. [Google Scholar] [CrossRef] [Green Version]
- Rayes, N.; Seehofer, D.; Theruvath, T.; Mogl, M.; Langrehr, J.M.; Nüssler, N.C.; Bengmark, S.; Neuhaus, P. Effect of Enteral Nutrition and Synbiotics on Bacterial Infection Rates After Pylorus-preserving Pancreatoduodenectomy: A Randomized, Double-blind Trial. Ann. Surg. 2007, 246, 36–41. [Google Scholar] [CrossRef]
- Rayes, N.; Pilarski, T.; Stockmann, M.; Bengmark, S.; Neuhaus, P.; Seehofer, D. Effect of pre- and probiotics on liver regeneration after resection: A randomised, double-blind pilot study. Benef. Microbes 2012, 3, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Horvath, A.; Leber, B.; Schmerboeck, B.; Tawdrous, M.; Zettel, G.; Hartl, A.; Madl, T.; Stryeck, S.; Fuchs, D.; Lemesch, S.; et al. Randomised clinical trial: The effects of a multispecies probiotic vs. placebo on innate immune function, bacterial translocation and gut permeability in patients with cirrhosis. Aliment. Pharmacol. Ther. 2016, 44, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, S.; Yokoyama, Y.; Nagino, M. Gut microbiota and bacterial translocation in digestive surgery: The impact of probiotics. Langenbeck’s Arch. Surg. 2017, 402, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Rizzatti, G.; Lopetuso, L.R.; Gibiino, G.; Binda, C.; Gasbarrini, A. Proteobacteria: A Common Factor in Human Diseases. Available online: https://www.hindawi.com/journals/bmri/2017/9351507/ (accessed on 8 September 2018).
- Lupp, C.; Robertson, M.L.; Wickham, M.E.; Sekirov, I.; Champion, O.L.; Gaynor, E.C.; Finlay, B.B. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe 2007, 2, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Hiippala, K.; Jouhten, H.; Ronkainen, A.; Hartikainen, A.; Kainulainen, V.; Jalanka, J.; Satokari, R. The Potential of Gut Commensals in Reinforcing Intestinal Barrier Function and Alleviating Inflammation. Nutrients 2018, 10, 988. [Google Scholar] [CrossRef]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011, 469, 543–547. [Google Scholar] [CrossRef]
- Canani, R.B.; Costanzo, M.D.; Leone, L.; Pedata, M.; Meli, R.; Calignano, A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. Gastroenterol. 2011, 17, 1519–1528. [Google Scholar] [CrossRef]
- Hayakawa, M.; Asahara, T.; Henzan, N.; Murakami, H.; Yamamoto, H.; Mukai, N.; Minami, Y.; Sugano, M.; Kubota, N.; Uegaki, S.; et al. Dramatic changes of the gut flora immediately after severe and sudden insults. Dig. Dis. Sci. 2011, 56, 2361–2365. [Google Scholar] [CrossRef]
- Gantois, I.; Ducatelle, R.; Pasmans, F.; Haesebrouck, F.; Hautefort, I.; Thompson, A.; Hinton, J.C.; Van Immerseel, F. Butyrate specifically down-regulates salmonella pathogenicity island 1 gene expression. Appl. Environ. Microbiol. 2006, 72, 946–949. [Google Scholar] [CrossRef]
- Seal, J.B.; Morowitz, M.; Zaborina, O.; An, G.; Alverdy, J.C. The molecular Koch’s postulates and surgical infection: A view forward. Surgery 2010, 147, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Olivas, A.D.; Shogan, B.D.; Valuckaite, V.; Zaborin, A.; Belogortseva, N.; Musch, M.; Meyer, F.; Trimble, W.L.; An, G.; Gilbert, J.; et al. Intestinal tissues induce an SNP mutation in Pseudomonas aeruginosa that enhances its virulence: Possible role in anastomotic leak. PLoS ONE 2012, 7, e44326. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Inan, M.S.; Rasoulpour, R.J.; Yin, L.; Hubbard, A.K.; Rosenberg, D.W.; Giardina, C. The luminal short-chain fatty acid butyrate modulates NF-kappaB activity in a human colonic epithelial cell line. Gastroenterology 2000, 118, 724–734. [Google Scholar] [CrossRef]
- Kelly, C.J.; Zheng, L.; Campbell, E.L.; Saeedi, B.; Scholz, C.C.; Bayless, A.J.; Wilson, K.E.; Glover, L.E.; Kominsky, D.J.; Magnuson, A.; et al. Crosstalk between Microbiota-Derived Short-Chain Fatty Acids and Intestinal Epithelial HIF Augments Tissue Barrier Function. Cell Host Microbe 2015, 17, 662–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Lahham, S.H.; Peppelenbosch, M.P.; Roelofsen, H.; Vonk, R.J.; Venema, K. Biological effects of propionic acid in humans; metabolism, potential applications and underlying mechanisms. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2010, 1801, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Lawhon, S.D.; Maurer, R.; Suyemoto, M.; Altier, C. Intestinal short-chain fatty acids alter Salmonella typhimurium invasion gene expression and virulence through BarA/SirA. Mol. Microbiol. 2002, 46, 1451–1464. [Google Scholar] [CrossRef] [Green Version]
- Dannhardt, G.; Lehr, M. Nonsteriodal Antiinflammatory Agents, XVII: Inhibition of Bovine Cyclooxygenase and 5-Lipoxygenase by N-Alkyldiphenyl-pyrrolyl Acetic and Propionic Acid Derivatives. Arch. Pharm. 1993, 326, 157–162. [Google Scholar] [CrossRef]
- Bos, C.L.; Richel, D.J.; Ritsema, T.; Peppelenbosch, M.P.; Versteeg, H.H. Prostanoids and prostanoid receptors in signal transduction. Int. J. Biochem. Cell Biol. 2004, 36, 1187–1205. [Google Scholar] [CrossRef]
- Curi, R.; Bond, J.A.; Calder, P.C.; Newsholme, E.A. Propionate regulates lymphocyte proliferation and metabolism. Gen. Pharmacol. Vasc. Syst. 1993, 24, 591–597. [Google Scholar] [CrossRef]
- Wajner, M.; Santos, K.D.; Schlottfeldt, J.L.; Rocha, M.P.; Wannmacher, C.M. Inhibition of mitogen-activated proliferation of human peripheral lymphocytes in vitro by propionic acid. Clin. Sci. 1999, 96, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Cavaglieri, C.R.; Nishiyama, A.; Fernandes, L.C.; Curi, R.; Miles, E.A.; Calder, P.C. Differential effects of short-chain fatty acids on proliferation and production of pro- and anti-inflammatory cytokines by cultured lymphocytes. Life Sci. 2003, 73, 1683–1690. [Google Scholar] [CrossRef]
- Luk, G.D.; Bayless, T.M.; Baylin, S.B. Diamine oxidase (histaminase). A circulating marker for rat intestinal mucosal maturation and integrity. J. Clin. Investig. 1980, 66, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Buffoni, F. Histaminase and Related Amine Oxidases. Pharmacol. Rev. 1966, 18, 1163–1199. [Google Scholar] [PubMed]
- Honzawa, Y.; Nakase, H.; Matsuura, M.; Chiba, T. Clinical significance of serum diamine oxidase activity in inflammatory bowel disease: Importance of evaluation of small intestinal permeability. Inflamm. Bowel Dis. 2011, 17, E23–25. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, K.A.; Manieri, N.A.; Liu, T.-C.; Stappenbeck, T.S. IL-6 Stimulates Intestinal Epithelial Proliferation and Repair after Injury. PLoS ONE 2014, 9, e114195. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, K.A.; Schulz, H.M.; Regner, E.H.; Severs, E.L.; Hendrickson, J.D.; Mehta, G.; Whitney, A.K.; Ir, D.; Ohri, N.; Robertson, C.E.; et al. Bacteroidales recruit IL-6-producing intraepithelial lymphocytes in the colon to promote barrier integrity. Mucosal Immunol. 2018, 11, 357–368. [Google Scholar] [CrossRef]
- Henriksen, M.; Jahnsen, J.; Lygren, I.; Stray, N.; Sauar, J.; Vatn, M.H.; Moum, B.; IBSEN Study Group. C-reactive protein: A predictive factor and marker of inflammation in inflammatory bowel disease. Results from a prospective population-based study. Gut 2008, 57, 1518–1523. [Google Scholar] [CrossRef]
- Mazidi, M.; Rezaie, P.; Ferns, G.A.; Vatanparast, H. Impact of Probiotic Administration on Serum C-Reactive Protein Concentrations: Systematic Review and Meta-Analysis of Randomized Control Trials. Nutrients 2017, 9, 20. [Google Scholar] [CrossRef]
- Mizuno, T.; Yokoyama, Y.; Nishio, H.; Ebata, T.; Sugawara, G.; Asahara, T.; Nomoto, K.; Nagino, M. Intraoperative bacterial translocation detected by bacterium-specific ribosomal rna-targeted reverse-transcriptase polymerase chain reaction for the mesenteric lymph node strongly predicts postoperative infectious complications after major hepatectomy for biliary malignancies. Ann. Surg. 2010, 252, 1013–1019. [Google Scholar] [PubMed]
- Nishigaki, E.; Abe, T.; Yokoyama, Y.; Fukaya, M.; Asahara, T.; Nomoto, K.; Nagino, M. The detection of intraoperative bacterial translocation in the mesenteric lymph nodes is useful in predicting patients at high risk for postoperative infectious complications after esophagectomy. Ann. Surg. 2014, 259, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Lederer, A.-K.; Pisarski, P.; Kousoulas, L.; Fichtner-Feigl, S.; Hess, C.; Huber, R. Postoperative changes of the microbiome: Are surgical complications related to the gut flora? A systematic review. BMC Surg. 2017, 17, 125. [Google Scholar] [CrossRef] [PubMed]
- Knoop, K.A.; McDonald, K.G.; Kulkarni, D.H.; Newberry, R.D. Antibiotics promote inflammation through the translocation of native commensal colonic bacteria. Gut 2016, 65, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Britton, R.A.; Young, V.B. Role of the Intestinal Microbiota in Resistance to Colonization by Clostridium difficle. Gastroenterology 2014, 146, 1547–1553. [Google Scholar] [CrossRef] [PubMed]
- NAP6 Report—The National Institute of Academic Anaesthesia. Available online: http://www.nationalauditprojects.org.uk/NAP6Report (accessed on 26 May 2018).
- Tanner, J.; Khan, D.; Aplin, C.; Ball, J.; Thomas, M.; Bankart, J. Post-discharge surveillance to identify colorectal surgical site infection rates and related costs. J. Hosp. Infect. 2009, 72, 243–250. [Google Scholar] [CrossRef]
- Straatman, J.; Cuesta, M.A.; De Lange-de Klerk, E.S.; Van Der Peet, D.L. Hospital Cost-Analysis of Complications after Major Abdominal Surgery. DSU 2015, 32, 150–156. [Google Scholar] [CrossRef]
- Healy, M.A.; Mullard, A.J.; Campbell, D.A.; Dimick, J.B. Hospital and Payer Costs Associated with Surgical Complications. JAMA Surg. 2016, 151, 823–830. [Google Scholar] [CrossRef]
- Keenan, J.E.; Speicher, P.J.; Thacker, J.K.M.; Walter, M.; Kuchibhatla, M.; Mantyh, C.R. The preventive surgical site infection bundle in colorectal surgery: An effective approach to surgical site infection reduction and health care cost savings. JAMA Surg. 2014, 149, 1045–1052. [Google Scholar] [CrossRef]



| Study | Reference | Study (Country) | Study Description | Treatment Description | Subjects Description | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study Focus/Primary Study Outcome | Blinding | Trial Duration (Days) | ROB* | Operation Name | Duration of Probiotic Therapy Pre/Postoperatively (Days) | Probiotic/Synbiotic Content | Probiotic Dose | Comparator | N Total Randomized/Analysed | Age (Years) | Male (%) | Primary Disease | |||
| 1 | [41] | Aisu 2015 (Japan) | SSIs and the immune response, intestinal microbiota, and surgical outcome | Psr | ND | 2 | CRC surgery | 3–15/NR | Enterococcus faecalis T110, Clostridium butyricum TO‑A, Bacillus mesentericus TO‑A | 2 mg, 10 mg, 10 mg; 6 tablets/day | No intervention | 156/156 | 68.57 ± 12.49 | 91 (58.33) | CRC |
| 2 | [45] | Anderson 2003 (U.K.) | BT, gastric colonisation, systemic inflammation, and septic morbidity | DB | 12 | 5 | Elective laparotomy | 12/4 | Lactobacillus acidophilus La5, Lactobacillus bulgaricus, Bifidobacterium lactis Bb-12, Streptococcus thermophilus; Prebiotic: oligofructose | 4 × 109 CFU; 16 g; 3 × day | PBO | 137/137 | 71 # | 80 (58.39) | GI malignancy |
| 3 | [46] | Diepenhorst 2011 (The Netherlands) | BT, intestinal barrier function | DB | 14 | 3 | Elective pylorus-preserving pancreaticoduodenectomy | 7/7 | Lactobacillus acidophilus W70, Lactobacillus casei W56, Lactobacillus salivarius W24, Lactococcus lactis W58, Bifidobacterium Bifidum W23, Bifidobacterium infantis W52 | 3 g; 2 × day (an equivalent of 1010 CFU) | Standard care | 20/20 | 64 # | 10 (50) | Periampullary or ampullary pancreatic malignancy |
| Lactobacillus acidophilus W70, Lactobacillus casei W56, Lactobacillus salivarius W24, Lactococcus lactis W58, Bifidobacterium Bifidum W23, Bifidobacterium infantis W52 + SDD | 60 # | 9 (45) | |||||||||||||
| 4 | [43] | Eguchi 2011 (Japan) | Infectious complications | OL | 16 | 1 | Living donor LT | 2/14 | Lactobacillus casei Strain Shirota, Bifidobacterium breve Strain Yakult; Prebiotic: GOS | 20 mg + 15 mg + 15 mg/3 × day | PBO | 50/50 | 56.5 ± NR | 29 (58) | Liver cirrhosis due to HCV |
| 5 | [47] | Flesch 2017 (Brazil) | Surgical wound infection | DB | 19 | 2 | Colorectal resection | 5/14 | Lactobacillus acidophilus NCFM, Lactobacillus rhamnosus HN001, Lactobacillus paracasei Lactobacillus plantarum c-37, Bifidobacterium lactis HN019; Prebiotic: FOS | 109 each, 6 g/2 sachets 2 × day | PBO | 100/91 | 62.93 ± 12.32 | 37 (40.66) | Colorectal adenocarcinoma |
| 6 | [64] | Grąt 2017 (Poland) | Pre- and post-transplant patient outcomes | DB | Varia, depending on the listing for LT | 6 | LT | Varia depending on listing for LT, up to 10 weeks | Lactococcus lactis PB411, Lactobacillus casei PB121, Lactobacillus acidophilus PB111, Bifidobacterium bifidum PB211 | 3 × 109 CFU | PBO | 55/44 | 50.95 | 34 (77.27) | ALD |
| 7 | [48] | Horvat 2010 (Slovenia) | Systemic inflammatory response and clinical outcome | DB | NR | 3 | Abdominal surgery | 3/NR | Pediacoccus pentosaceus 5-33:3, Leuconostoc mesenteroides 32–77:1, Lactobacillus paracasei subsp. Paracasei 19, Lactobacillus plantarum 2362; Prebiotic: 2.5 g betaglucan, 2.5 g inulin, 2.5 g pectin, 2.5 g resistant starch | 40 billion, 10 g of fibers, 2 × day | Bowel cleansing | 76/40 | 62 # | 20 (50) | Colon adenocarcinoma |
| Prebiotic | 76/48 | 63.25 # | 21 (44) | ||||||||||||
| 8 | [40] | Kanazawa 2005 (Japan) | Intestinal integrity, microflora, and surgical outcome | NR | 14 | 1 | Combined liver and extrahepatic bile duct resection with hepaticojejunostomy | 0/14 | Bifidobacterium breve Strain Yakult, Lactobacillus casei Strain Shirota; Prebiotic: GOS ** | 108/g each; 3 g day; 12 g/day | No intervention | 54/44 | 63.75 ± 9.64 | 29 (65.91) | Perihilar cholangiocarcinoma |
| 9 | [44] | Komatsu 2016 (Japan) | Surgical outcome | OL | ≤17 | 5 | Laparoscopy | 7–11/6 | Lactobacillus casei strain Strain Shirota; Prevbiotic: GOS, Bifidobacterium breve Strain Yakult. | 4 × 1010, 2.5 g, 1 × 1010 | No intervention | 370/362 | 67.23 ± 11.11 | 210 (58.01) | Elective laparoscopic colorectal surgery |
| 10 | [49] | Kotzampassi 2015 (Greece) | Prophylaxis for complications after colorectal surgery | DB | 16 | 5 | Colorectal surgery for cancer. | 1/14 | Lacctobacillus acidophilus LA-5, Lactobacillus plantarum, Bifidobacterium lactis BB-12, Saccharomyces boulardii | 1.75 × 109 CFU, 0.5 × 109 CFU, 1.75 × 109, 1.5 × 109 CFU per capsule, 2 × day | PBO | 168/164 | 66.14 ± 11.69 | 115 (70.12) | CRC |
| 11 | [42] | Liu 2010 (China) | Gut barrier function and the surgical outcome | DB | 16 | 4 | Laparotomy | 6/10 | Lactobacillus plantarum CGMCC No. 1258, Lactobacillus acidophilus LA-11, Bifidobacterium longum BL-88 | 2.6 × 1014 CFU, 2 g/day | PBO | 114/100 | 65.5 ± 10.45 | 59 (59) | CRC |
| 12 | [50] | Liu 2013 (China) | Serum zonulin concentrations and postoperative infectious complications | DB | 16 | 5 | Colorectsal carcinoma surgery | 6/10 | Lactobacillus plantarum CGMCC No. 1258, Lactobacillus acidophilus LA-11, Bifidobacterium longum BL-88 | 2.6 × 1014 CFU, 2 g/day | PBO | 161/150 | 65.06 ± 11.73 | 78 (52) | CRC |
| 13 | [51] | Liu 2015 (China) | Serum zonulin levels and postoperative infectious complications | DB | 16 | 5 | Colectomy + resection for metastatic tumor/segmental hepatectomy | 6/10 | Lactobacillus plantarum CGMCC No. 1258, Lactobacillus acidophilus LA-11, Bifidobacterium longum BL-88 | 2.6 × 1014 CFU, 2 g/day | PBO | 134/117 | 62.84 ± 17.17 | 70 (59.83) | Colon cancer + Colorectal liver metastases |
| 14 | [52] | Mangell 2012 (Sweden) | Intestinal load of potentially pathogenic bacteria, BT, and cell proliferation | DB | 13 | 4 | Colonic resection | 8/5 | Lactobacillus plantarum 299v | 1011 CFU | PBO | 72/64 | 72 # | 36 (56.25) | Adenocarcinoma |
| 15 | [53] | Mcnaught 2002 (U.K.) | BT, gastric colonization, and septic complications | OL | 9 | 1 | Major abdominal surgery | 7–12/4–9 | Lactobacillus plantarum 299v | 107/mL; preoperatively 4000 mL, postoperatively 800 mL | No intervention | 129/129 | 68.5 # | 75 (58.14) | CRC |
| 16 | [65] | Mizuta 2016 (Japan) | Immune functions, systemic inflammatory responses, postoperative infectious complications | SB | ≤28 | 2 | CRC resection | 7–14/7 | Bifidobacterium Longum BB536 | 5 × 1010 CFU, 2 g | No intervention | 60/60 | 70.01 ± 9.96 | 35 (58.33) | CRC |
| 17 | [54] | Nomura 2007 (Japan) | Surgical outcome | NR | ≥3 | 1 | Pancreaticoduodenectomy, Whipple | 3–15/until discharge | Enterococcus faecealis T-110, Clostridium butyricum TO-A, Bacillus mesentericus TO-A | 6 × 107 CFU | No intervention | 70/64 | 66 # | 39 (60.94) | Pancreatico-billiarty disease |
| 18 | [55] | Okazaki 2013 (Japan) | Gut microbiota, infectious complications | OL | 17 | 1 | Abdominal surgery | 7/10 | Lactobacillus casei Strain Shirota and BBG-01, Bifidobacterium breve Strain Yakult; Prebiotic: GOS | Biolactis powder (1 g/day) and BBG-01 (1 g/day), GOS: 5 g, 3 × day | No intervention | 53/48 | 78.5 # | 26 (54.17) | Upper digestive illness |
| 19 | [63] | Rammohan 2015 (India) | Postoperative infectious complications, clinical outcome | SB (patients) | 15 | 3 | Frey procedure for chronic hepatitis | 5/10 | Streptococcus faecalis T-110, Clostridium butyricum TOA, Bacillus mesentericus TO-A, Lactobacillus sporogenes; Prebiotic: FOS | 60 million, 4 million, 2 million, 100 million, | PBO | 79/75 | 43.29 ± 8.96 | 48 (64) | Chronic hepatitis |
| 20 | [72] | Rayes 2007 (Germany/U.K.) | Postoperative bacterial infection | DB | 9 | 2 | Pylorus-preserving Pancreatoduodenectomy | 1/8 | Pediacoccus pentosaceus 5–33:3; Leuconostoc mesenteroides 77:1; Lactobacillus paracasei subspecies paracasei F19; Lactobacillus plantarum 2362; Prebiotic: bioactive fibers—2.5 g of each betaglucan, inulin, pectin, and resistant starch, | 1010, 10 g | Fiber | 89/80 | 58.5 ± NR | 45 (56.3) | Carcinoma (pancreas) |
| 21 | [71] | Rayes 2005 (Germany/U.K.) | Infectious complications | DB | 14 | 3 | LT | 0/14 | Pediacoccus pentosaceus 5–33:3; Leuconostoc mesenteroides 77:1; Lactobacillus paracasei subspecies paracasei F19; Lactobacillus plantarum 2362; Prebiotic: bioactive fibers—2.5 g of each betaglucan, inulin, pectin, and resistant starch | 1010, 20 g | Fiber | 66/66 | 51.5 ± 2 | 38 (57.6) | Na |
| 22 | [70] | Rayes 2002 a (Multicenter) | Early postoperative infections | OL | 12 | 0 | LT | 0/12 | Lactobacillus plantarum 299v; 2 × day | 1 × 109, oat fibers | PBO + fiber | 105/69 | 48.47 ± 2.49 | 30 (47.6) | Na |
| 23 | [69] | Rayes 2002 (Germany) | Postoperative bacterial infection, clinical outcome | OL | 4 | 0 | Major abdominal surgery | 0/4 | Lactobacillus plantarum 299; Prebiotic: oat fiber | 1 × 109 | PBO + fiber | 90/60 | 60.5 ± 13.59 | 30 (50) | Liver, pancreatic, gastric resection |
| 24 | [73] | Rayes 2012 (Germany) | Liver regeneration after hepatectomy | DB | 11 | 2 | Hepatectomy | 1/10 | Pediacoccus pentosaceus 5–33:3; Leuconostoc mesenteroides 77:1; Lactobacillus paracasei subspecies paracasei F19; Lactobacillus plantarum 2362; Prebiotic: bioactive fibers—2.5 g of each betaglucan, inulin, pectin, and resistant starch | 1010, 20 g | Fiber | 19/19 | 60.05 ± 13.89 | 14 (73.7) | Colorectal metastasis |
| 25 | [62] | Reddy 2007 (Denmark/U.K.) | Prevalence of Enterobacteriaceae, inflammatory response including septic morbidity | OL | 1 | Elective CRC surgery | 1/0 | Lactobacillus acidophilus La5, Lactobacillus bulgaricus, Bifidobacterium lactis, BB-12, Streptococcus thermophilus; Prebiotic: oligorfructose | 4 × 109 CFU, 15 g, 2 × day | Neomycin + MBP | 88/42 | 70.6 # | 22 (52.4) | Anterior resection | |
| 26 | [61] | Sadahiro 2014 (Japan) | Incisional SSI, organ/space SSI, remote infection, leakage, CD toxin | DB | 18 | 6 | Curative resection of CRC | 7/11 | Bifidobacterium bifidum; Prebiotic: multooligossacharide | 1 × 109/day | Antibiotic, mechanical bowel preparation | 294/194 | 66.7 ± 10.72 | 107 (55.2) | CRC |
| 27 | [60] | Sommacal 2015 (Brazil) | Postoperative morbidity and mortality | DB | 14 | 7 | Periampullary cancer: resective and palliative surgery | 4/10 | Lactobacillus acidophilus 10, Lactobacillus rhamnosus HS 111, Lactobacillus casei 10, Bifidobacterium bifidum; Prebiotic: FOS | 1 × 109 CFU, 1 × 109 CFU, 1 × 109 CFU, 1 × 109 CFU, 100 mg | PBO | 48/46 | 59.5 # | NR | Periampullary cancer |
| 28 | [67] | Sugawara 2006 (Japan) | Intestinal barrier function, immune responses, systemic inflammatory responses, microflora, and surgical outcome | OL | 28 | 2 | Liver and extrahepatic bile duct resection with hepaticojejunostomy | 14/14 | Lactobacillus casei strain Shirota, Bifidobacterium breve strain Yakult; Prebiotic: GOS | 80 mL: 4 × 1010; 100 mL: 1 × 1010; 15 g/day | Synbiotic only post-operatively | 101/81 | 63.15 ± 8.84 | 46 (56.79) | Perihilar cholangiocarcinoma |
| 29 | [59] | Tanaka 2012 (Japan) | Postoperative infections | SB | 21 | 3 | Oesophagectomy | 1/21 | Lactobacillus casei strain Shirota, Bifidobacterium breve strain Yakult; Prebiotic: GOS | 1 × 1010/g, 1 × 1010/g; (PRE:3 g/day; POST: 2 g/day) GOS (PRE:15 g, POST:10 g) | Streptococcus faecalis | 64/64 | 62.15 ± 7.74 | 51 (79.7) | Oesophagal cancer |
| 30 | [58] | Usami 2011 (Japan) | Intestinal integrity, systemic inflammatory response, and microflora, surgical outcome | OL | 26 | 4 | Hepatic surgery | 14/12 | Lactobacillus casei strain Shirota, Bifidobacterium breve strain Yakult; Prebiotic: GOS | 1 × 108/g, 1 × 108/g; 10 g | No intervention | 67/61 | 65.42 ± 9.86 | 55 (90.2) | Primary or metastatic liver cancer |
| 31 | [39] | Yang 2016 (China) | Postoperative infections | DB | 12 | 5 | Radical CRC resection | 5/7 | Bifidobacterium longum, Lactobacillus acidophilus Enterococcus faecalis | ≥1.0 × 107 CFU/g, ≥1.0 × 107 CFU/g, ≥1.0 × 107 CFU/g) | PBO | 79/60 | 63.03 ± 11.70 | 27 (45) | CRC |
| 32 | [57] | Yokoyama 2014 (Japan) | Intestinal microenvironment, BT to mlns, postoperative bacteraemia | OL | 21 | 5 | Oesophagectomy | 7/14 | PRE:Lactobacillus casei strain Strain Shirota, Bifidobacterium breve strain Strain Yakult; Prebiotic: 15 g GOS; POST:Lactobacillus casei strain Strain Shirota Bifidobacterium breve strain Strain Yakult; Prebiotic: 15 g GOS | PRE: 4 × 1010, 1 × 1010, 15 g; POST: 1 × 108/g; 1 × 108/g; 15 g | No intervention | 42/42 | 65.5 # | 37 (88.1) | Oesophagal cancer |
| 33 | [66] | Yokoyama 2016 (Japan) | BT to mlns and blood, postoperative infectious complications | OL | 7 | 2 | Pancreatoduodenectomy | 7/0 | Lactobacillus casei Strain Shirota, Bifidobacterium breve strain Strain Yakult; Prebiotic: GOS | 80 mL: 4 × 1010; 100 mL: 1 × 1010; 15 g/day | No intervention | 45/44 | 65 # | 12 (27.27) | Pancreatic cancer |
| 34 | [56] | Zhang 2012 (China) | Postoperative infections and related complications | DB | 3 | 5 | Radical CRC resection with laparotomy | 3/0 | Bifidobacterium longum, Lactobacillus acidophilus, Enterococcus faecalis | 0.21 g (108 CFU/g) | PBO | 60/60 | 64.5 # | 24 (60) | CRC |
| 35 | [68] | Zhang 2013 (Australia) | Assessing the impact on bacterial sepsis and wound complications | OL | ? | 2 | LT | 0/? | Lactobacillus Acidophilus LA-14, Lactobacillus Plantarum 115, Bifidobacterium Lactis BL-04, Lactobacillus Casei LC-11, Lactobacillus Rhamnosus LR-32, Lactobacillus Brevis lbr-35; Prebiotic: fiber | 15.5 × 109; 5.0 × 109; 2.0 × 109; 1.5 × 109; 1.5 × 109; 1.5 × 109 CFU | Fiber | 67/67 | 56.01 ± 10.98 | 36 (53.73) | NR |
| Reference | Country | Gut Microbiota Changes after the Surgery/Intervention |
|---|---|---|
| Aisu 2015 | Japan | Probiotic group: the mean proportion of Bifidobacterium increased between 4.6 ± 1.97 and 9.1 ± 1.89%. No-probiotic group: the mean proportion of Bifidobacterium decreased between 7.06(1.95)% And 5.53(±1.93) |
| Eguchi 2011 | Japan | No significant changes in bacterial species abundance between the groups. In 25% of patients under immunosuppression Enterococcus spp evident in both groups |
| Grąt 2017 | Poland | Probiotic group:Bacteroides spp. count increased in comparison to pre-trial values (p = 0.008). Enterococcus spp. abundance significantly increased (p = 0.04) and a tendency towards increased number of Lactobacillus spp. (p = 0.07) as compared to no-probiotic group |
| Kanazawa 2005 | Japan | Synbiotic group: beneficial bacteria (including Lactobacillus and Bifidobacterium) count increased after surgery, in comparison to controls (p < 0.05). No-synbiotic group: harmful microorganisms (including Enterobacteriaceae, Pseudomonas, and Candida) increased in comparison to synbiotic group (p < 0.05). Enterococci abundance increased after surgery in both groups, with no significant intergroup differences. |
| Komatsu 2016 | Japan | Synbiotic group: Total bacteria, dominant obligate anaerobes (such as Clostridium leptum subgroup or Bifidobacterium), and facultative anaerobes (Lactobacillus species) significantly increased. The abundance of Enterobacteriaceae, Staphylococcus (MSCNS), and Pseudomonas decreased compared to the control group (p < 0.05). Bifidobacterium and L. casei subgroup numbers and C. perfringens, L. gasseri subgroup, L. reuteri subgroup, L. ruminis subgroup, and L. sakei subgroup increased and decreased respectively regarding preoperative concentrations (p < 0.05). No synbiotic group: total bacteria, dominant obligate anaerobes (C. coccoides group, C. leptum subgroup, Bacteroides fragilis group, Bifidobacterium, Prevotella, and Lactobacillus species) counts decreased while the numbers of Enterobacteriaceae, Staphylococcus (MSCNS), Pseudomonas, and C. difficile increased in comparison to the preoperative values (p < 0.05). |
| Liu 2010 | China | Probiotic group:Bifidobacterium count increased in comparison to controls and preoperative values. Enterobacteriaceae, Pseudomonas, and Candida numbers were decreased compared to placebo group (p < 0.05). Probiotic bacterial richness was enhanced when compared to healthy volunteers and the control group (p < 0.05). A higher similarity to the healthy volunteers compared with the control group (p < 0.05). No probiotic group: Enterobacteriaceae, Pseudomonas and Candida numbers increased compared to probiotic group (p < 0.05) Enterococci abundance increased in both groups. |
| Mangell 2012 | Sweden | Probiotic group:Enterobacteriaceae count increased significantly in comparison to placebo (p < 0.001) but not regarding preoperatively values. |
| Mizuta 2016 | Japan | Probiotic group:Firmicutes decreased (62.31% vs. 56.51%) and Actinobacteria increased (0.7% vs. 1.71%) in comparison to control group (p < 0.05). No-probiotic group: Bacteroidetes (24.52% vs. 32.8%) and Proteobacteria (1.74% vs. 3.54%) numbers increased and Firmicutes (66.57% vs. 56.82%) and unclassified bacterial groups (0.5% vs. 0.37%) abundance decreased compared to before the surgery period. |
| Okazaki 2013 | Japan | Synbiotic group: Before surgery Bifidobacteria count and numbers of Enterobacteriaceae and Pseudomonas were significantly increased and decreased, respectively, in comparison to the pre-trial values and the control group (p < 0.05). Bifidobacterium abundance was significantly increased while Enterobacteriaceae and Staphylococcus bacteria counts decreased postoperatively in comparison to controls. No-synbiotic group: Bifidobacterium number gradually decreased |
| Sugawara 2006 | Japan | Pre-and post-operative probiotic group: Bifidobacterium number increased significantly after preoperative treatment (p < 0.05), as well as Lactobacillus but with no statistical difference (p > 0.05). Bifidobacterium abundance 1 day before hepatectomy was higher and lower for Candida in comparison to the only pre-surgery probiotic group. Anaerobic bacteria numbers were unchanged before and after surgery between the two groups, without intergroup differences. |
| Tanaka 2012 | Japan | Synbiotic group: Bifidobacterium and total Lactobacillus numbers were significantly higher (p < 0.01) when compared to controls. Postoperatively (day 7) the abundance of Clostridium coccoides group (p < 0.01); C. leptum subgroup (p < 0.01); Bacteroides fragilis group (p < 0.05); Bifidobacterium (p < 0.01); Atopobium cluster (p < 0.05), Prevotella (p < 0.01), and Lactobacillus (p < 0.01) significantly decreased when compared to the pre-operative time point. Bifidobacterium and Lactobacillus species count were not decreased, but were higher when compared to controls. Enterobacteriaceae, Staphylococcus, and Pseudomonas species numbers were significantly lower in comparison to the second group patients. Collectively (3 weeks post-surgery) Bifidobacterium abundance was significantly higher and Enterobacteriaceae count was lower in the synbiotic group (p < 0.05). |
| Usami 2011 | Japan | Synbiotic group: Fecal anaerobic bacteria, including Bacteroidaceae, as well as Bifidobacterium genus were decreased compared to before the trial (post-operative days 6–8). The numbers of Candida were increased in this time point. In contrast, two weeks after the surgery, these numbers started to resemble values before hepatectomy (Bacteroidaceae: 10.0 ± 0.4 vs. 10.1 ± 0.3, Bifidobacterium: 10.0 ± 0.7 vs. 10.0 ± 0.6, Candida: 3.4 ± 1.4 vs. 3.1 ± 1.0 log10 CFU/g of feces. No-synbiotic group: Two weeks after the surgery, particular bacteria numbers started to resemble values before hepatectomy (Bacteroidaceae: 10.0 ± 0.5 vs. 9.9 ± 0.4, Bifidobacterium: 9.8 ± 0.8 vs. 9.5 ± 0.7, Candida: 4.1 ± 1.6 vs. 4.1 ± 1.9 log10 CFU/g of feces. Subgroup comparison between normal liver and chronic liver damage, including chronic hepatitis, liver fibrosis, and cirrhosis in either group found no significant differences |
| Yokoyama 2014 | Japan | Synbiotic group: A week post-surgery, Bifidobacterium and Lactobacillus counts increased and Enterobacteriaceae and Pseudomonas decreased in comparison to pre-operative values and the control group (p < 0.05). The numbers of Staphylococus, Pseudomonas, and Enterobacteriaceae were significantly decreased 21 days post-surgery when compared to the no-synbiotic group and pre-surgery time (except for Pseudomonas) No-synbiotic group: Pseudomonas, Staphylococcus, and Enterobacteriaceae levels were increased post-operatively in comparison to the intervention group (p < 0.05). |
| Zhang 2012 | China | Probiotic group: During preoperative treatment (3 days before surgery), the reversal of the Bifidobacterium/E. coli ratio inversion in comparison to day–6 (0.26 ± 0.32 and 1.26 ± 0.28 log10/g, respectively, p < 0.001) and controls (1.26 ± 0.28 and 0.27 ± 0.34 log10/g, respectively, p < 0.001). Postoperatively decreased E coli count compared to controls (8.29 ± 0.27 log10/g and 9.67 ± 0.17 log10/g, respectively, p < 0.001), and B. longum increased (8.43 ± 0.17 log10/g and 7.94 ± 0.11 log10/g, respectively; p < 0.001). No-probiotic group: Postoperative Bifidobacterium/E. coli ratio inversion in comparison to 6 days before surgery (0.14 ± 0.20 and 0.26 ± 0.32, respectively, p < 0.001) and probiotic group (0.14 ± 0.20 and 1.73 ± 0.22, p < 0.001). |
| Outcome | SMD (95% CI) | Z-Value | References | Heterogeneity | Tau | Intercept (95% CI) † | Meta-Regression Coefficients |
|---|---|---|---|---|---|---|---|
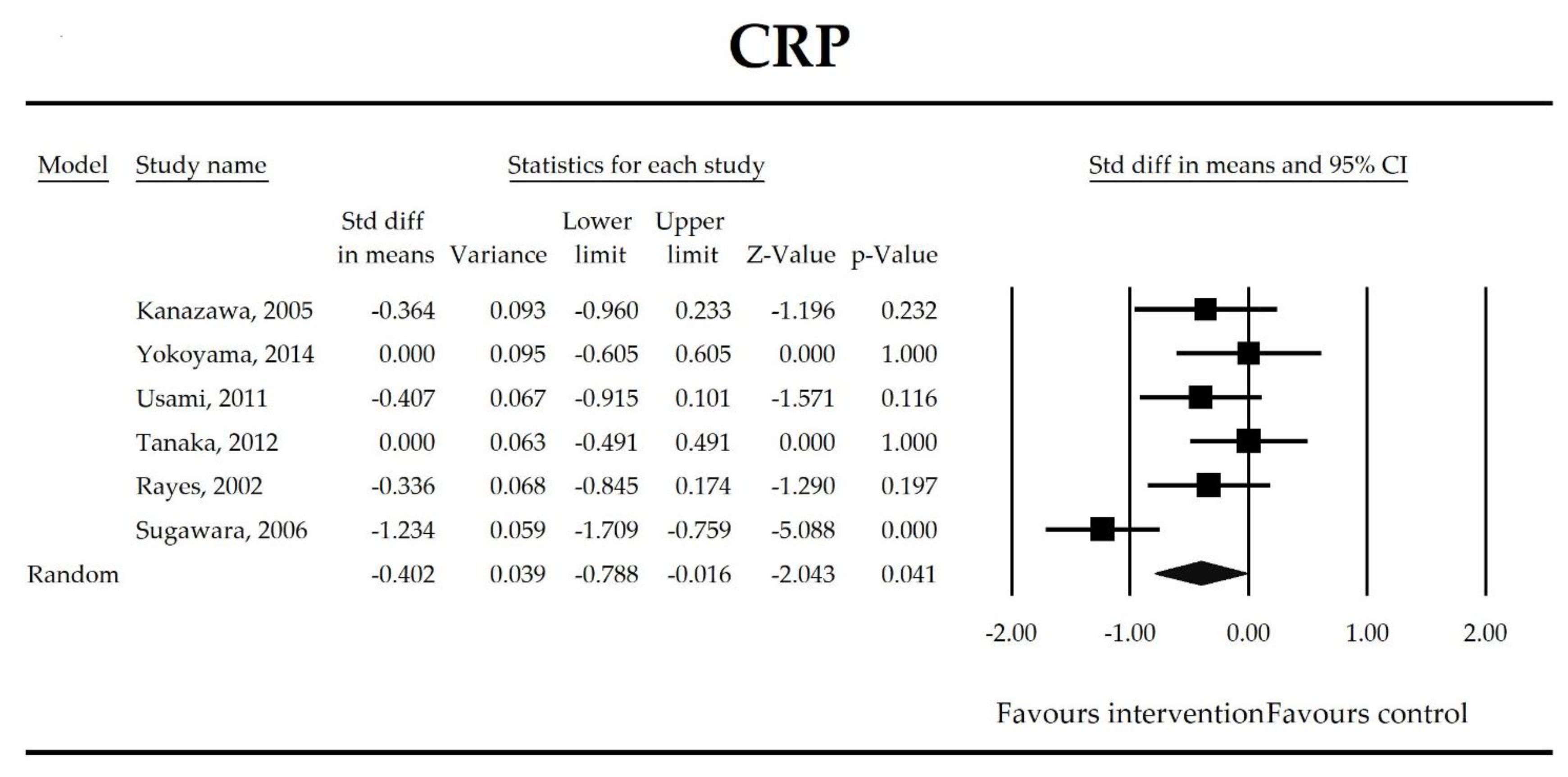
| CRP | −0.40 (−0.79, −0.02) | −2.04 p = 0.041 | Kanazawa, 2005 Yokoyama, 2014 Usami, 2011 Tanaka, 2012 Rayes, 2002 Sugawara, 2006 | Q = 16.1 p = 0.007 (df = 5) I2 = 69 | τ2 = 0.159 τ = 0.399 | 8.59 (−13.42, 30.59) p = 0.339 | Dose: −0.32 (p = 0.158) Intervention: NOT ESTIMABLE Operation (Hepatobiliary vs. Gut): −0.69 (p = 0.075), (Mixed vs. Gut): −0.34, p = 0.515 ROB (Low vs. High): −0.28 (p = 0.539) Duration: −0.02 (p = 0.477) Timing (Post vs. Peri): 0.08 (p = 0.871) |
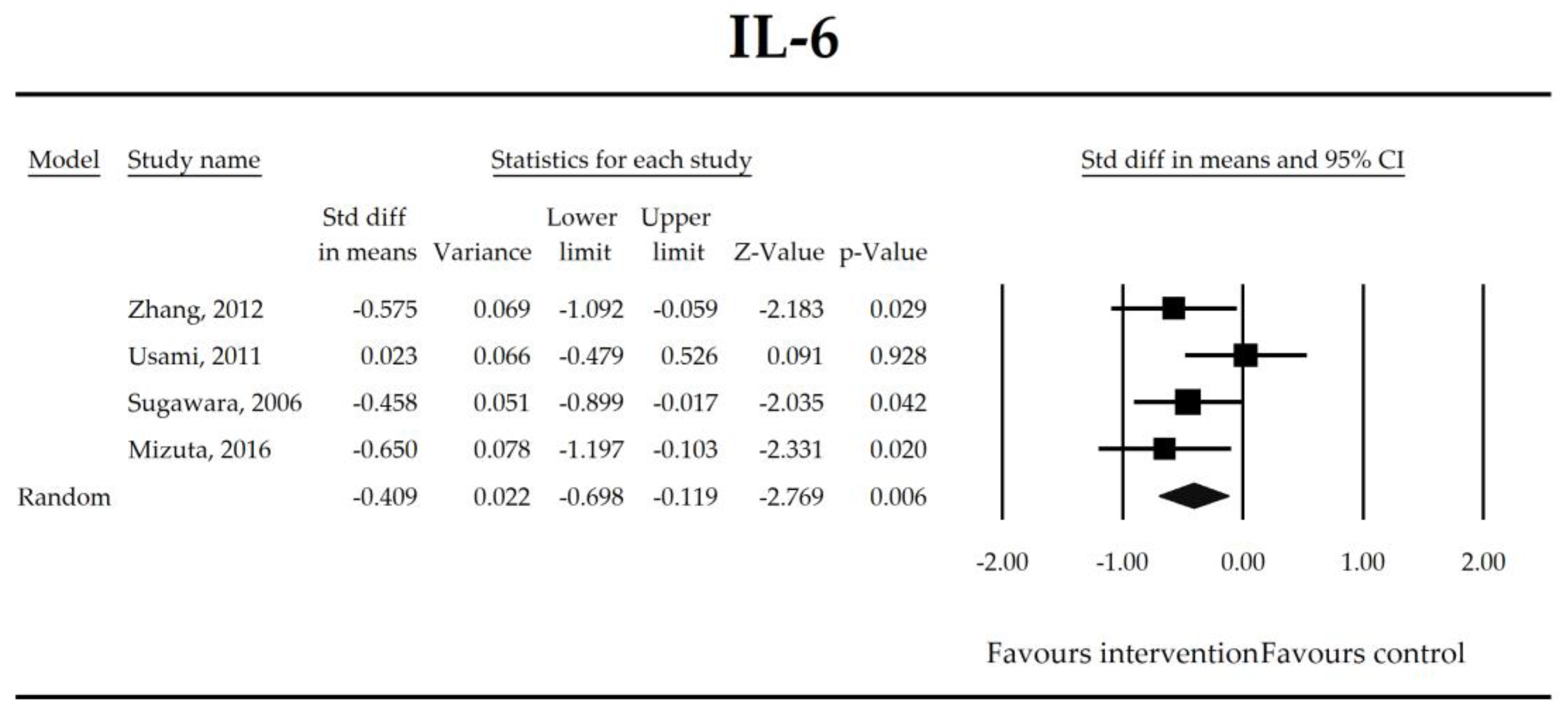
| IL-6 | −0.41 (−0.70, −0.12) | −2.77 p = 0.006 | Zhang, 2012 Usami, 2011 Sugawara, 2006 Mizuta, 2016 | Q = 4.03 p = 0.258 (df = 3) I2 = 25.6 | τ2 = 0.022 τ = 0.150 | −2.18 (−39.73, 35.38) p = 0.826 | Dose: −0.09 (p = 0.538) Intervention (Synbiotic vs. Probiotic): 0.36 (p = 0.159) Operation (Hepatobiliary vs. Gut): 0.36 (p = 0.159) ROB (Low vs. High): −0.27 (p = 0.383) Duration: 0.01 (p = 0.231) Timing (Pre vs. Peri): −0.22 (p = 0.580) |
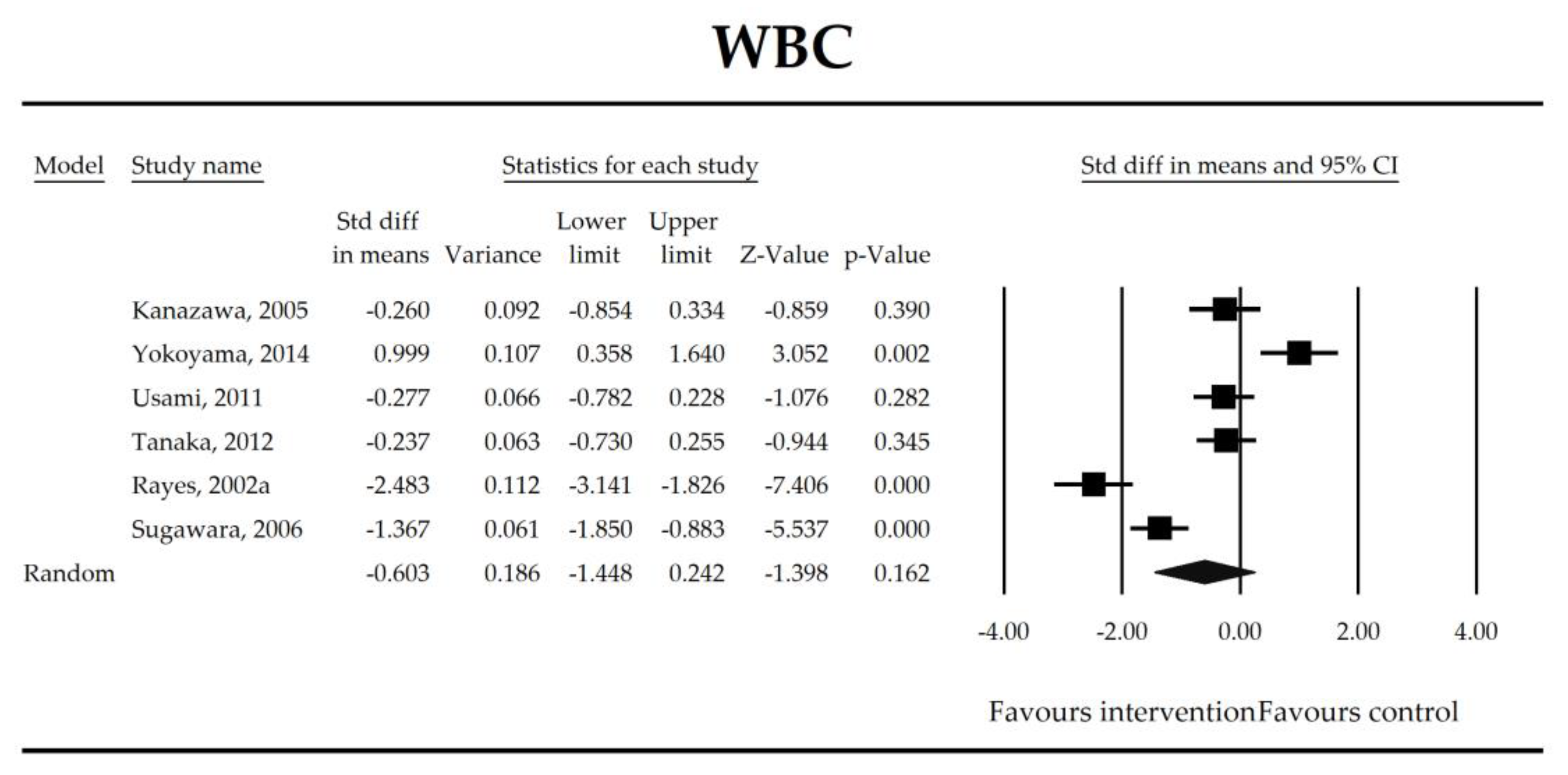
| WBC | −0.60 (−1.45, 0.24) | −1.40 p = 0.162 | Kanazawa, 2005 Yokoyama, 2014 Usami, 2011 Tanaka, 2012 Rayes, 2002a Sugawara, 2006 | Q = 70 p < 0.0001 (df = 5) I2 = 93 | τ2 = 1.033 τ = 1.016 | 0.09 (−38.14, 38.32) p = 0.995 | Dose: −0.03 (p = 0.965) Intervention: NOT ESTIMABLE Operation (Mixed vs. Gut): −1.45 (p = 0.078) ROB (Low vs. High): −1.42 (p = 0.089) Duration: 0.05 (p = 0.515) Timing (Post vs. Peri): −1.13 (p = 0.223) |
| L/M | −0.28 (−0.82, 0.27) | −1.00 p = 0.316 | Kanazawa, 2005 Liu, 2010 Liu, 2013 Sugawara, 2006 | Q = 19.5 p = 0.0002 (df = 3) I2 = 85 | τ2 = 0.257 τ = 0.507 | 8.66 (−14.75, 32.07) p = 0.252 | Dose: −0.28 (p = 0.323) Intervention (Synbiotic vs. Probiotic): 0.46 (p = 0.435) Operation (Mixed vs. Gut): 0.46 (p = 0.435) ROB (Low vs. High): 0.46 (p = 0.435) Duration: −0.002 (p = 0.968) Timing (Post vs. Peri): 0.59 (p = 0.376) |
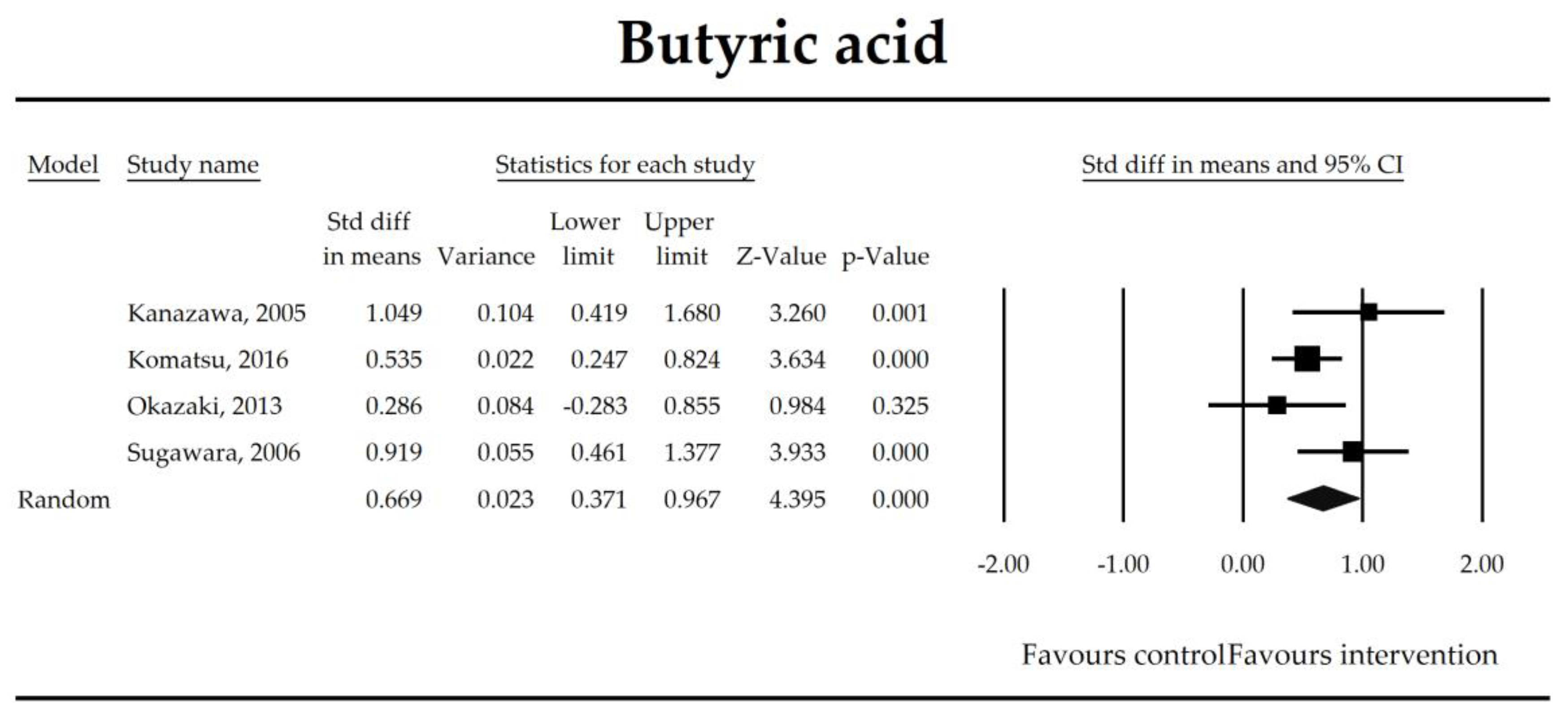
| Butyrate | 0.67 (0.37, 0.97) | 4.40 p = 0.00001 | Kanazawa, 2005 Komatsu, 2016 Okazaki, 2013 Sugawara, 2006 | Q = 5.04 p = 0.169 (df = 3) I2 = 40.4 | τ2 = 0.037 τ = 0.193 | 1.37 (−8.79, 11.53) p = 0.622 | Dose: NOT ESTIMABLE Intervention: NOT ESTIMABLE Operation: NOT ESTIMABLE ROB (Low vs. High): 0.22 (p = 0.572) Duration: 0.02 (p = 0.510) Timing (Post vs. Peri): 0.45 (p = 251) |
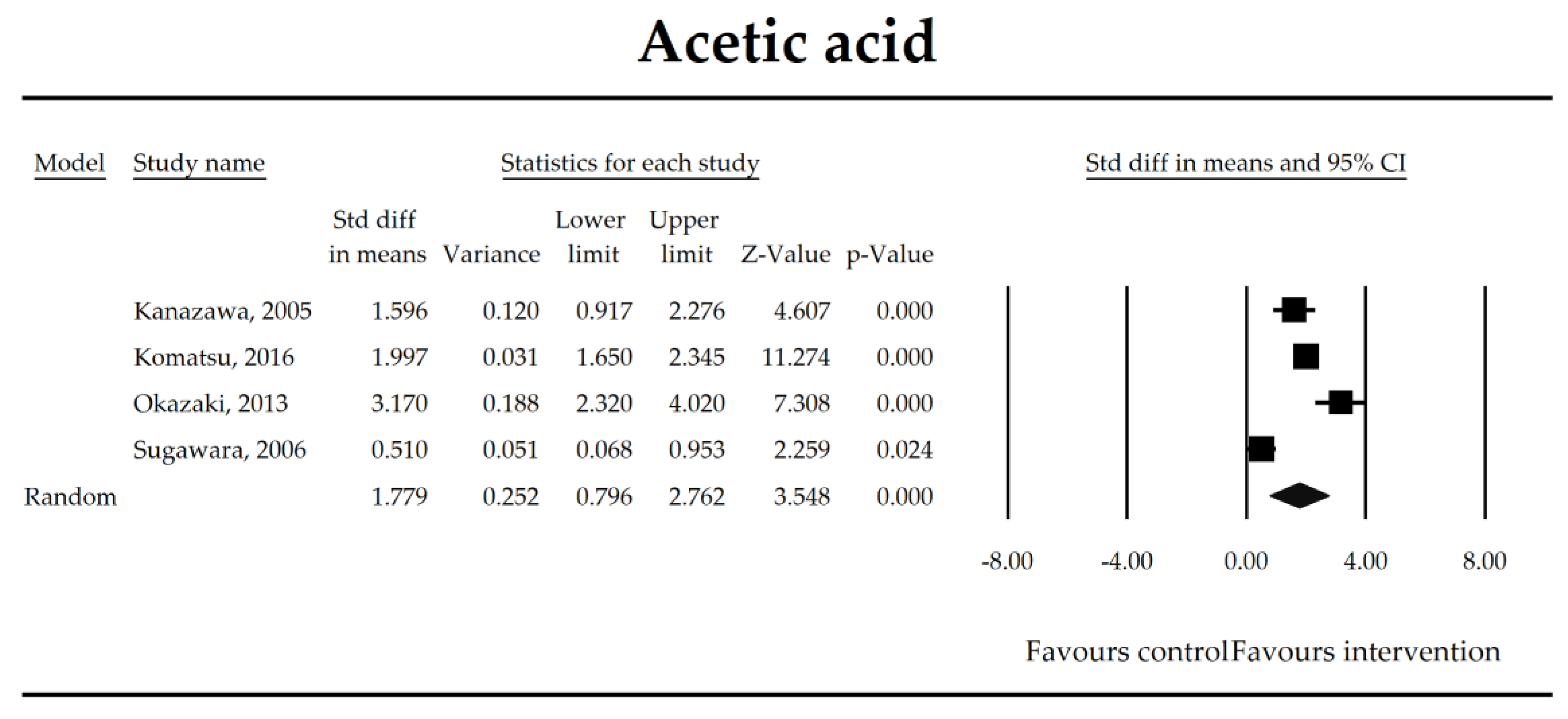
| Acetate | 1.78 (0.80, 2.76) | 3.55 p = 0.0004 | Kanazawa, 2005 Komatsu, 2016 Okazaki, 2013 Sugawara, 2006 | Q = 41.4 p < 0.0001 (df = 3) I2 = 93 | τ2 = 0.912 τ = 0.955 | 2.65 (−26.40, 31.71) p = 0.732 | Dose: NOT ESTIMABLE Intervention: NOT ESTIMABLE Operation: NOT ESTIMABLE ROB (Low vs. High): −0.27 (p = 0.851) Duration: −0.10 (p = 0.118) Timing (Post vs. Peri): −0.25 (p = 0.850) |
| Propionate | 0.46 (0.18, 0.73) | 3.23 p = 0.001 | Kanazawa, 2005 Komatsu, 2016 Okazaki, 2013 Sugawara, 2006 | Q = 4.58 p = 0.206 (df = 3) I2 = 34.4 | τ2 = 0.028 τ = 0.166 | −1.99 (−11.22, 7.24) p = 0.451 | Dose: NOT ESTIMABLE Intervention: NOT ESTIMABLE Operation: NOT ESTIMABLE ROB (Low vs. High): −0.38 (p = 0.074) Duration: −0.04 (p = 0.049) Timing (Post vs. Peri): 0.18 (p = 0.675) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skonieczna-Żydecka, K.; Kaczmarczyk, M.; Łoniewski, I.; Lara, L.F.; Koulaouzidis, A.; Misera, A.; Maciejewska, D.; Marlicz, W. A Systematic Review, Meta-Analysis, and Meta-Regression Evaluating the Efficacy and Mechanisms of Action of Probiotics and Synbiotics in the Prevention of Surgical Site Infections and Surgery-Related Complications. J. Clin. Med. 2018, 7, 556. https://doi.org/10.3390/jcm7120556
Skonieczna-Żydecka K, Kaczmarczyk M, Łoniewski I, Lara LF, Koulaouzidis A, Misera A, Maciejewska D, Marlicz W. A Systematic Review, Meta-Analysis, and Meta-Regression Evaluating the Efficacy and Mechanisms of Action of Probiotics and Synbiotics in the Prevention of Surgical Site Infections and Surgery-Related Complications. Journal of Clinical Medicine. 2018; 7(12):556. https://doi.org/10.3390/jcm7120556
Chicago/Turabian StyleSkonieczna-Żydecka, Karolina, Mariusz Kaczmarczyk, Igor Łoniewski, Luis F. Lara, Anastasios Koulaouzidis, Agata Misera, Dominika Maciejewska, and Wojciech Marlicz. 2018. "A Systematic Review, Meta-Analysis, and Meta-Regression Evaluating the Efficacy and Mechanisms of Action of Probiotics and Synbiotics in the Prevention of Surgical Site Infections and Surgery-Related Complications" Journal of Clinical Medicine 7, no. 12: 556. https://doi.org/10.3390/jcm7120556
APA StyleSkonieczna-Żydecka, K., Kaczmarczyk, M., Łoniewski, I., Lara, L. F., Koulaouzidis, A., Misera, A., Maciejewska, D., & Marlicz, W. (2018). A Systematic Review, Meta-Analysis, and Meta-Regression Evaluating the Efficacy and Mechanisms of Action of Probiotics and Synbiotics in the Prevention of Surgical Site Infections and Surgery-Related Complications. Journal of Clinical Medicine, 7(12), 556. https://doi.org/10.3390/jcm7120556









