Enhanced Efficacy of High Dose Oral Vancomycin Therapy in Clostridium difficile Diarrhea for Hospitalized Adults Not Responsive to Conventional Oral Vancomycin Therapy: Antibiotic Stewardship Implications
Abstract
:1. Introduction
2. Methods
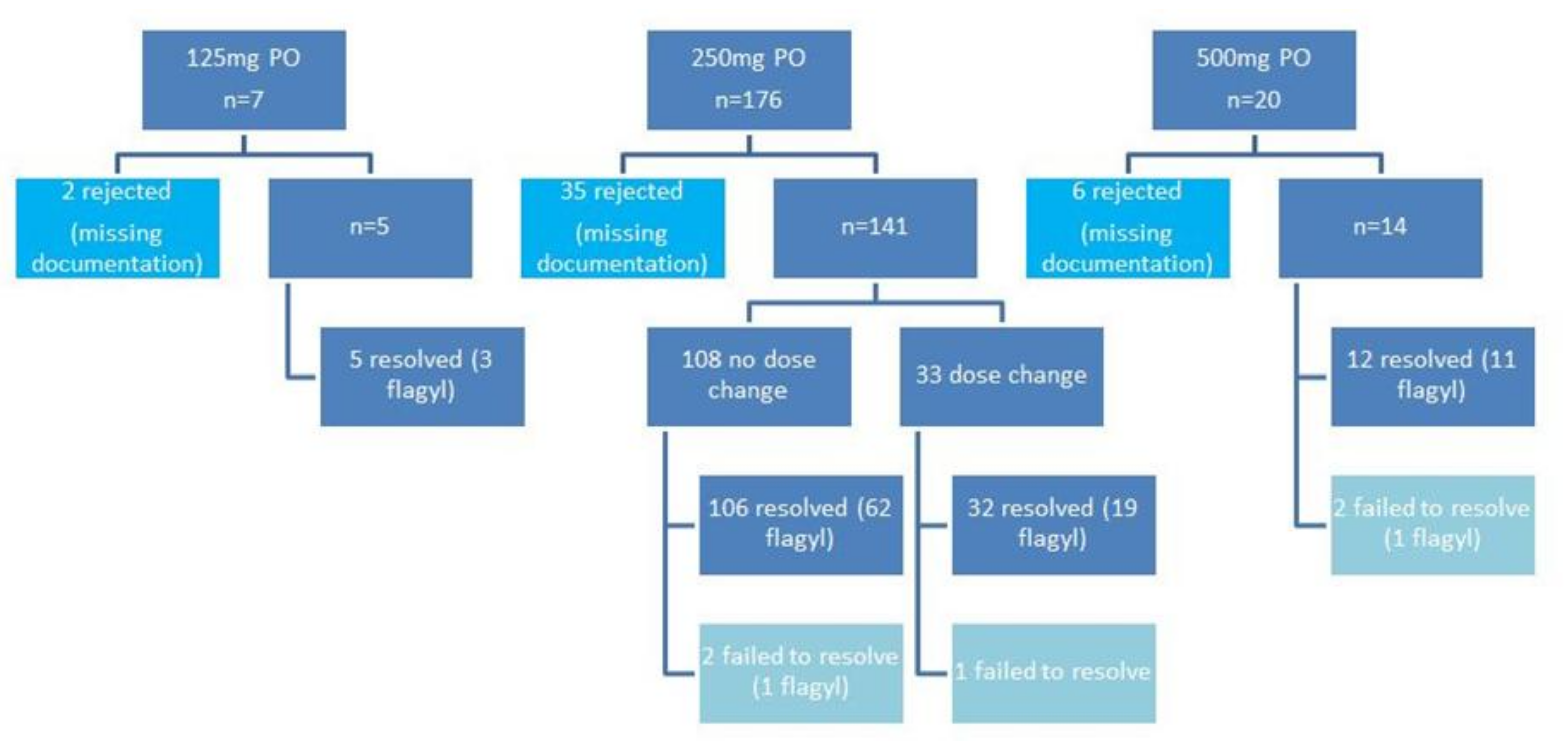
3. Results
3.1. Conventional Dosing Group
3.2. High Dose Escalation Group
3.3. High Dose Group
4. Discussion
Author Contributions
Conflicts of Interest
References
- Nelson, R.L.; Suda, K.J.; Evans, C.T. Antibiotic treatment for Clostridium difficile-associated diarrhea in adults. Cochrane Database Syst. Rev. 2017, 3, 4610. [Google Scholar]
- Al-Jashaami, L.S.; DuPont, H.L. Management of Clostridium difficile Infection. Gastroenterol. Hepat. 2016, 12, 609–616. [Google Scholar]
- Lessa, F.C.; Mu, Y.; Bamberg, W.M.; Beldavs, Z.G.; Dumyati, G.K.; Dunn, J.R.; Farley, M.M.; Holzbauer, S.M.; Meek, J.I.; Phipps, E.C.; et al. Burden of C. difficile infection in the United States. N. Engl. J. Med. 2015, 372, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Depestel, D.D.; Aronoff, D.M. Epidemiology of Clostridium difficile infection. J. Pharm. Pract. 2013, 26, 464–475. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Surveillance for community-associated Clostridium difficile—Connecticut, 2006. MMWR Morb. Mortal. Wkly. Rep. 2008, 57, 340–343. [Google Scholar]
- Wilcox, M.H.; Mooney, L.; Bendall, R.; Settle, C.D.; Fawley, W.N. A case-controlled study of community-associated Clostridium difficile infection. J. Antimicrob. Chemother. 2008, 62, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Lucado, J.; Gould, C.; Elixhauser, A. Clostridium difficile Infections (CDI) in Hospital Stays, 2009: Healthcare Cost and Utilization Project, Statistical Brief #124; Agency for Healthcare Research and Quality: Rockville, MD, USA.
- McDonald, L.C.; Owings, M.; Jernigan, D.B. Clostridium difficile infection in patients discharged from US short-stay hospitals, 1996–2003. Emerg. Infect. Dis. 2006, 12, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.; Bauer, M.P.; Baines, S.D.; Corver, J.; Fawley, W.N.; Goorhuis, B.; Kuijper, E.J.; Wilcox, M.H. The changing epidemiology of Clostridium difficile infections. Clin. Microbiol. Rev. 2010, 23, 529–549. [Google Scholar] [CrossRef] [PubMed]
- Elixhauser, A.; Jhung, M.A. Clostridium difficile-Associated Disease in U.S. Hospitals, 1993–2005: Healthcare Cost and Utilization Project, Statistical Brief #50; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2008.
- Miller, B.A.; Chen, L.F.; Sexton, D.J.; Anderson, D.J. Comparison of the burdens of hospital-onset, healthcare facility-associated Clostridium difficile infection and of healthcare-associated infection due to methicillin-resistant Staphylococcus aureus in community hospitals. Infect. Control Hosp. Epidemiol. 2011, 32, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Hensgens, M.P.; Goorhuis, A.; Dekkers, O.M.; Kuiiper, E.J. Time interval of increased risk for Clostridium difficile infection after exposure to antibiotics. J. Antimicrob. Chemother. 2012, 67, 742–748. [Google Scholar] [CrossRef] [PubMed]
- McDonald, L.C.; Lessa, F.; Sievert, D.; Wise, M.; Herrera, R.; Gould, C.; Malpiedi, P.; Dudeck, M.; Srinivasan, A.; Fridkin, S.; et al. Vital signs: Preventing Clostridium difficile infections. MMWR Morb. Mortal. Wkly. Rep. 2012, 61, 157–162. [Google Scholar]
- Cunningham, R.; Dale, B.; Undy, B.; Gaunt, N. Proton pump inhibitors as a risk factor for Clostridium difficile diarrhoea. J. Hosp. Infect. 2003, 54, 243–245. [Google Scholar] [CrossRef]
- Aseeri, M.; Schroeder, T.; Kramer, J.; Zackula, R. Gastric acid suppression by proton pump inhibitors as a risk factor for Clostridium difficile-associated diarrhea in hospitalized patients. Am. J. Gastroenterol. 2008, 103, 2308–2313. [Google Scholar] [CrossRef] [PubMed]
- Parmar, S.; Bhatt, V.; Yang, J.; Zhang, Q.; Schuster, M. A retrospective review of metronidazole and vancomycin in the management of Clostridium difficile infection in patients with hematologic malignancies. J. Oncol. Pharm. Pract. 2014, 20, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Eddi, R.; Malik, M.N.; Shakrov, R.; Baddoura, W.J.; Chandran, C.; Debari, V.A. Chronic kidney disease as a risk factor for Clostridium difficile infection. Nephrology 2010, 15, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Raines, D.L.; Lopez, F.A. Clostridium difficile infection in non-HIV-immunocompromised patients and in HIV-infected patients. Curr. Gastroenterol. Rep. 2011, 13, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Dubberke, E.R.; Riddle, D.J. Clostridium difficile in solid organ transplant recipients. Am. J. Transplant. 2009, 9, s35–s40. [Google Scholar] [CrossRef] [PubMed]
- Cunha, B.A. Nosocomial diarrhea. Crit. Care Clin. 1998, 14, 329–338. [Google Scholar] [CrossRef]
- Bliss, D.Z.; Johnson, S.; Savik, K.; Clabots, C.R.; Wilard, K.; Gerding, D.N. Acquisition of Clostridium difficile and Clostridium difficile-associated diarrhea in hospitalized patients receiving tube feeding. Ann. Intern. Med. 1998, 129, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Caines, C.; Gill, M.V.; Cunha, B.A. Non-Clostridium difficile nosocomial diarrhea in the intensive care unit. Heart Lung 1997, 26, 83–84. [Google Scholar] [CrossRef]
- Cohen, S.H.; Gerding, D.N.; Johnson, S.; Kelly, C.P.; Loo, V.G.; McDonald, L.; Pepin, J.; Wilcox, M.H. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect. Control Hosp. Epidemiol. 2010, 31, 431–455. [Google Scholar] [CrossRef] [PubMed]
- Rybak, M. The pharmacokinetics and pharmacodynamics properties of vancomycin. Clin. Infect. Dis. 2006, 42, S35–S39. [Google Scholar] [CrossRef] [PubMed]
- Cunha, C.B.; Cunha, B.A. Antibiotic Essentials, 15th ed.; JayPee Medical Publishers: New Delhi, India, 2017; pp. 86–91. [Google Scholar]
- De Lalla, F.; Nicolin, R.; Rinaldi, E.; Scarpellini, P.; Rigoli, R.; Manfrin, V.; Tramarin, A. Prospective study of oral Teicoplanin versus oral vancomycin for therapy of pseudomembranous colitis and Clostridium difficile-associated diarrhea. Antimicrob. Agents Chemother. 1992, 36, 2192–2196. [Google Scholar] [CrossRef] [PubMed]
- Wenisch, J.M.; Schmid, D.; Kuo, H.W.; Allerberger, F.; Michl, V.; Tesik, P.; Tucek, G.; Laferl, H.; Wenisch, C. Prospective observational study comparing three different treatment regimens in patients with Clostridium difficile infection. Antimicrob. Agents Chemother. 2012, 56, 1974–1978. [Google Scholar] [CrossRef] [PubMed]
- Teasley, D.G.; Gerding, D.N.; Olson, M.M.; Peterson, L.R.; Gebhard, R.L.; Schwartz, M.J.; Lee, J.T., Jr. Prospective randomised trial of metronidazole versus vancomycin for Clostridium difficile associated diarrhoea. Lancet 1983, 2, 1043–1046. [Google Scholar] [CrossRef]
- Bagdasarian, N.; Rao, K.; Malani, P.N. Diagnosis and treatment of C. difficile in adults: A systematic review. JAMA 2015, 313, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Al-Nassir, W.N.; Sethi, A.K.; Nerandzic, M.M.; Bobulsky, G.S.; Jump, R.L.; Donskey, C.J. Comparison of clinical and microbiological response to treatment of C. difficile-associated disease with metronidazole and vancomycin. Clin. Infect. Dis. 2008, 47, 56–62. [Google Scholar] [CrossRef] [PubMed]


| Conventional Dosing (n = 113) | High Dose (n = 14) | Dose Escalation (n = 33) | |
|---|---|---|---|
| Age (mean, years) | 69.6 | 64.4 | 68.4 |
| Female (%) | 57 | 50 | 48 |
| Concurrent metronidazole (n, %) | 65, 58 | 11, 79 | 19, 58 |
| Number of stool/day at diagnosis (mean) | 5.60 | 8.80 | 6.45 |
| Number of stool/day at 72 h (mean) | 2.68 | 3.60 | 5.50 |
| Number of stool/day at 72 h following dose escalation (mean) | N/A | N/A | 2.5 |
| Days to clinical resolution (mean) | 5.08 | 5.40 | 10.00 |
| Days to clinical resolution from dose escalation (mean) | N/A | N/A | 4.2 |
| Total vancomycin doses (mean) | 53.4 | 53.0 | 64.4 (250 mg: 21.9; 500 mg: 37.3) |
| Total days of vancomycin therapy (mean) | 14.20 | 13.10 | 17.97 |
| Total days of vancomycin therapy from dose escalation (mean) | N/A | N/A | 11.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cunha, B.A.; Sessa, J.; Blum, S. Enhanced Efficacy of High Dose Oral Vancomycin Therapy in Clostridium difficile Diarrhea for Hospitalized Adults Not Responsive to Conventional Oral Vancomycin Therapy: Antibiotic Stewardship Implications. J. Clin. Med. 2018, 7, 75. https://doi.org/10.3390/jcm7040075
Cunha BA, Sessa J, Blum S. Enhanced Efficacy of High Dose Oral Vancomycin Therapy in Clostridium difficile Diarrhea for Hospitalized Adults Not Responsive to Conventional Oral Vancomycin Therapy: Antibiotic Stewardship Implications. Journal of Clinical Medicine. 2018; 7(4):75. https://doi.org/10.3390/jcm7040075
Chicago/Turabian StyleCunha, Burke A., Julia Sessa, and Sharon Blum. 2018. "Enhanced Efficacy of High Dose Oral Vancomycin Therapy in Clostridium difficile Diarrhea for Hospitalized Adults Not Responsive to Conventional Oral Vancomycin Therapy: Antibiotic Stewardship Implications" Journal of Clinical Medicine 7, no. 4: 75. https://doi.org/10.3390/jcm7040075
APA StyleCunha, B. A., Sessa, J., & Blum, S. (2018). Enhanced Efficacy of High Dose Oral Vancomycin Therapy in Clostridium difficile Diarrhea for Hospitalized Adults Not Responsive to Conventional Oral Vancomycin Therapy: Antibiotic Stewardship Implications. Journal of Clinical Medicine, 7(4), 75. https://doi.org/10.3390/jcm7040075




