MiR-146a-5p Expression in Peripheral CD14+ Monocytes from Patients with Psoriatic Arthritis Induces Osteoclast Activation, Bone Resorption, and Correlates with Clinical Response
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Isolation and Culture of Peripheral Monocytes
2.3. Osteoclast Formation
2.4. Bone Resorption Assay
2.5. Transient Transfection of miR-146a-5p Inhibitors
2.6. Quantitative Real-Time PCR Analysis for miRNAs
2.7. Statistical Analysis
3. Results
3.1. Subject Demographics
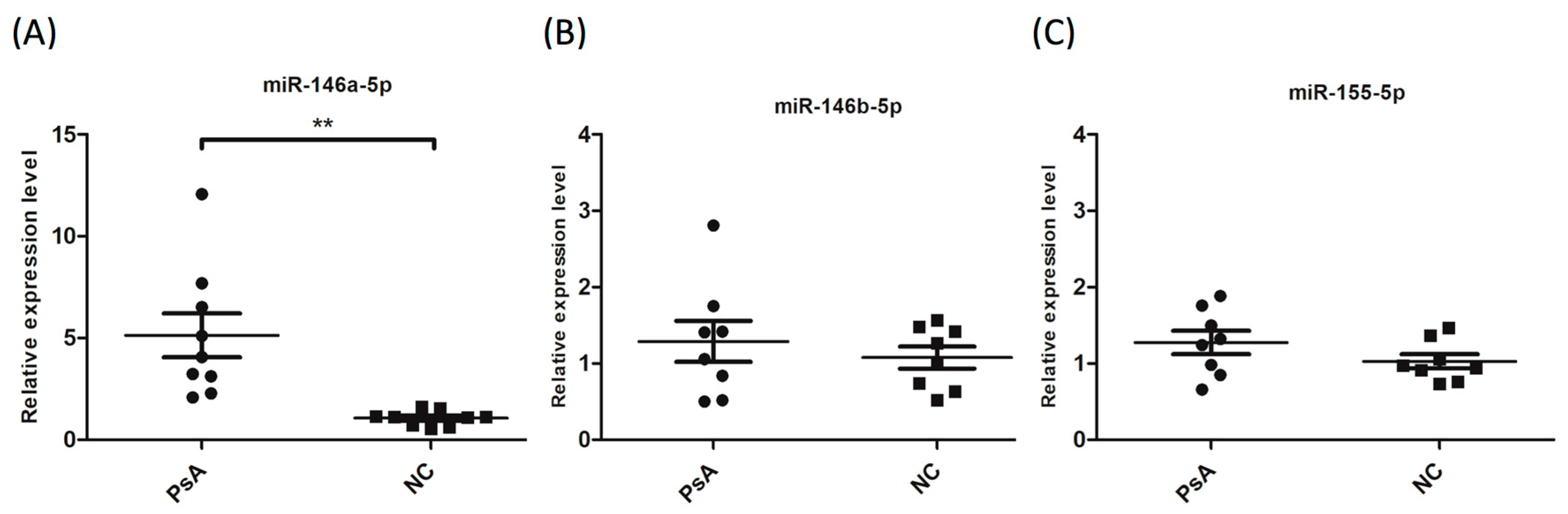
3.2. Upregulation of miR-146a-5p in CD14+ Monocytes from PsA Patients
3.3. The Expression of miR-146a-5p was Significantly Increased in CD14+ Monocytes from PsA Patients Compared to PsO Patients and NCs
3.4. The Increased Osteoclast Differentiation and Enhanced Bone Resorption Activity in PsA Patients are Abrogated by RNA Interference against miR-146a-5p
3.5. miR-146a-5p Expression was Reduced in CD14+ Cells of PsA Patients in Clinical Remission
3.6. The Expression of miR-146a-5p in CD14+ Monocytes from Patients with PsA correlates with Blood CRP Level, but not the PASI Score nor the Presence of Enthesitis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gladman, D.D.; Antoni, C.; Mease, P.; Clegg, D.O.; Nash, P. Psoriatic arthritis: Epidemiology, clinical features, course, and outcome. Ann. Rheum. Dis. 2005, 64, ii14–ii17. [Google Scholar] [CrossRef] [PubMed]
- Ritchlin, C.T.; Colbert, R.A.; Gladman, D.D. Psoriatic Arthritis. New Engl. J. Med. 2017, 376, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Ogdie, A.; Schwartzman, S.; Husni, M.E. Recognizing and managing comorbidities in psoriatic arthritis. Curr. Opin. Rheumatol. 2015, 27, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Kane, D.; Stafford, L.; Bresnihan, B.; FitzGerald, O. A prospective, clinical and radiological study of early psoriatic arthritis: An early synovitis clinic experience. Rheumatology 2003, 42, 1460–1468. [Google Scholar] [CrossRef] [PubMed]
- Haroon, M.; Gallagher, P.; FitzGerald, O. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann. Rheum. Dis. 2015, 74, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Van den Bosch, F.; Coates, L. Clinical management of psoriatic arthritis. Lancet 2018, 391, 2285–2294. [Google Scholar] [CrossRef]
- Taylor, W.; Gladman, D.; Helliwell, P.; Marchesoni, A.; Mease, P.; Mielants, H.; Group, C.S. Classification criteria for psoriatic arthritis: Development of new criteria from a large international study. Arthritis Rheum. 2006, 54, 2665–2673. [Google Scholar] [CrossRef]
- Haroon, M.; Kirby, B.; FitzGerald, O. High prevalence of psoriatic arthritis in patients with severe psoriasis with suboptimal performance of screening questionnaires. Ann. Rheum. Dis. 2013, 72, 736–740. [Google Scholar] [CrossRef]
- Teitelbaum, S.L. Bone resorption by osteoclasts. Science 2000, 289, 1504–1508. [Google Scholar] [CrossRef]
- Massey, H.M.; Flanagan, A.M. Human osteoclasts derive from CD14-positive monocytes. Br. J. Haematol. 1999, 106, 167–170. [Google Scholar] [CrossRef]
- Hemingway, F.; Cheng, X.; Knowles, H.J.; Estrada, F.M.; Gordon, S.; Athanasou, N.A. In vitro generation of mature human osteoclasts. Calcif. Tissue Int. 2011, 89, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Deng, H.W.; Shen, H. Circulating monocytes: An appropriate model for bone-related study. Osteoporos. Int. 2015, 26, 2561–2572. [Google Scholar] [CrossRef] [PubMed]
- Ritchlin, C.T.; Haas-Smith, S.A.; Li, P.; Hicks, D.G.; Schwarz, E.M. Mechanisms of TNF-alpha- and RANKL-mediated osteoclastogenesis and bone resorption in psoriatic arthritis. J. Clin. Invest. 2003, 111, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, A.; Lembo, S.; Di Caprio, R.; Donnarumma, G.; Monfrecola, G.; Balato, N.; Ayala, F.; Balato, A. Psoriatic cutaneous inflammation promotes human monocyte differentiation into active osteoclasts, facilitating bone damage. Eur. J. Immunol. 2017, 47, 1062–1074. [Google Scholar] [CrossRef] [PubMed]
- Rahman, P.; Elder, J.T. Genetic epidemiology of psoriasis and psoriatic arthritis. Ann. Rheum. Dis. 2005, 64, ii37–ii39. [Google Scholar] [CrossRef] [Green Version]
- FitzGerald, O.; Haroon, M.; Giles, J.T.; Winchester, R. Concepts of pathogenesis in psoriatic arthritis: Genotype determines clinical phenotype. Arthritis Res. Ther. 2015, 17, 115. [Google Scholar] [CrossRef]
- McGonagle, D. Imaging the joint and enthesis: Insights into pathogenesis of psoriatic arthritis. Ann. Rheum. Dis. 2005, 64, ii58–ii60. [Google Scholar] [CrossRef]
- Qu, Z.; Li, W.; Fu, B. MicroRNAs in autoimmune diseases. BioMed Res. Int. 2014, 2014, 527895. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, A.; Lunardi, C.; Fiore, P.F.; Tinazzi, E.; Patuzzo, G.; Argentino, G.; Moretta, F.; Puccetti, A.; Dolcino, M. MicroRNA Expression Profiling in Psoriatic Arthritis. BioMed Res. Int. 2018, 2018, 7305380. [Google Scholar] [CrossRef]
- Ciancio, G.; Ferracin, M.; Saccenti, E.; Bagnari, V.; Farina, I.; Furini, F.; Galuppi, E.; Zagatti, B.; Trotta, F.; Negrini, M.; et al. Characterisation of peripheral blood mononuclear cell microRNA in early onset psoriatic arthritis. Clin. Exp. Rheumatol. 2017, 35, 113–121. [Google Scholar] [PubMed]
- Forrest, A.R.; Kanamori-Katayama, M.; Tomaru, Y.; Lassmann, T.; Ninomiya, N.; Takahashi, Y.; de Hoon, M.J.; Kubosaki, A.; Kaiho, A.; Suzuki, M.; et al. Induction of microRNAs, mir-155, mir-222, mir-424 and mir-503, promotes monocytic differentiation through combinatorial regulation. Leukemia 2010, 24, 460–466. [Google Scholar] [CrossRef]
- Wang, J.; Xiang, G.; Mitchelson, K.; Zhou, Y. Microarray profiling of monocytic differentiation reveals miRNA-mRNA intrinsic correlation. J. Cell. Biochem. 2011, 112, 2443–2453. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Xiong, Q.; Ge, W.; Zhang, L. The role of microRNAs in osteoclasts and osteoporosis. RNA Biol. 2014, 11, 1355–1363. [Google Scholar] [CrossRef]
- Cheng, V.K.-F.; Au, P.C.-M.; Tan, K.C.B.; Cheung, C.-L. MicroRNA and Human Bone Health. JBMR Plus 2018, 1–12. [Google Scholar] [CrossRef]
- Zhang, B.; Yi, J.; Zhang, C.L.; Zhang, Q.H.; Xu, J.F.; Shen, H.Q.; Ge, D.W. MiR-146a inhibits proliferation and induces apoptosis in murine osteoblastic MC3T3-E1 by regulating Bcl2. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 3754–3762. [Google Scholar] [PubMed]
- Curtis, A.M.; Fagundes, C.T.; Yang, G.; Palsson-McDermott, E.M.; Wochal, P.; McGettrick, A.F.; Foley, N.H.; Early, J.O.; Chen, L.; Zhang, H.; et al. Circadian control of innate immunity in macrophages by miR-155 targeting Bmal1. Proc. Natl. Acad. Sci. USA 2015, 112, 7231–7236. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.H.; Chuang, H.Y.; Ho, J.C.; Lee, C.H.; Hsiao, C.C. Treatment with TNF-alpha inhibitor rectifies M1 macrophage polarization from blood CD14+ monocytes in patients with psoriasis independent of STAT1 and IRF-1 activation. J. Dermatol. Sci. 2018, 91, 276–284. [Google Scholar] [CrossRef]
- Kurihara, N.; Suda, T.; Miura, Y.; Nakauchi, H.; Kodama, H.; Hiura, K.; Hakeda, Y.; Kumegawa, M. Generation of osteoclasts from isolated hematopoietic progenitor cells. Blood 1989, 74, 1295–1302. [Google Scholar]
- Kvon, E.Z.; Kazmar, T.; Stampfel, G.; Yanez-Cuna, J.O.; Pagani, M.; Schernhuber, K.; Dickson, B.J.; Stark, A. Genome-scale functional characterization of Drosophila developmental enhancers in vivo. Nature 2014, 512, 91–95. [Google Scholar] [CrossRef]
- Kuo, H.C.; Hsieh, K.S.; Ming-Huey Guo, M.; Weng, K.P.; Ger, L.P.; Chan, W.C.; Li, S.C. Next-generation sequencing identifies micro-RNA-based biomarker panel for Kawasaki disease. J. Allergy Clin. Immunol. 2016, 138, 1227–1230. [Google Scholar] [CrossRef] [PubMed]
- Kagiya, T.; Nakamura, S. Expression profiling of microRNAs in RAW264.7 cells treated with a combination of tumor necrosis factor alpha and RANKL during osteoclast differentiation. J. Periodontal Res. 2013, 48, 373–385. [Google Scholar] [CrossRef]
- Nakasa, T.; Shibuya, H.; Nagata, Y.; Niimoto, T.; Ochi, M. The inhibitory effect of microRNA-146a expression on bone destruction in collagen-induced arthritis. Arthritis Rheum. 2011, 63, 1582–1590. [Google Scholar] [CrossRef]
- Li, D.; Duan, M.; Feng, Y.; Geng, L.; Li, X.; Zhang, W. MiR-146a modulates macrophage polarization in systemic juvenile idiopathic arthritis by targeting INHBA. Mol. Immunol. 2016, 77, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Mun, S.H.; Ko, N.Y.; Kim, H.S.; Kim, J.W.; Kim, D.K.; Kim, A.R.; Lee, S.H.; Kim, Y.G.; Lee, C.K.; Lee, S.H.; et al. Interleukin-33 stimulates formation of functional osteoclasts from human CD14(+) monocytes. Cell. Mol. Life Sci. 2010, 67, 3883–3892. [Google Scholar] [CrossRef]
- Lacey, D.L.; Timms, E.; Tan, H.L.; Kelley, M.J.; Dunstan, C.R.; Burgess, T.; Elliott, R.; Colombero, A.; Elliott, G.; Scully, S.; et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998, 93, 165–176. [Google Scholar] [CrossRef]
- Yago, T.; Nanke, Y.; Ichikawa, N.; Kobashigawa, T.; Mogi, M.; Kamatani, N.; Kotake, S. IL-17 induces osteoclastogenesis from human monocytes alone in the absence of osteoblasts, which is potently inhibited by anti-TNF-alpha antibody: A novel mechanism of osteoclastogenesis by IL-17. J. Cell. Biochem. 2009, 108, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.; Takeshita, S.; Barker, J.E.; Kanagawa, O.; Ross, F.P.; Teitelbaum, S.L. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J. Clin. Invest. 2000, 106, 1481–1488. [Google Scholar] [CrossRef]
- Pivarcsi, A.; Meisgen, F.; Xu, N.; Stahle, M.; Sonkoly, E. Changes in the level of serum microRNAs in patients with psoriasis after antitumour necrosis factor-alpha therapy. Br. J. Dermatol. 2013, 169, 563–570. [Google Scholar] [CrossRef]
- Punzi, L.; Podswiadek, M.; Oliviero, F.; Lonigro, A.; Modesti, V.; Ramonda, R.; Todesco, S. Laboratory findings in psoriatic arthritis. Reumatismo 2007, 59, 52–55. [Google Scholar] [CrossRef]




| Patients with PsA (N = 34) | Psoriatic Patients without Arthritis (N = 17) | Normal Control (N = 34) | |
|---|---|---|---|
| Age (years) | 47.8 ± 11.2 | 43.2 ± 11.8 | 44.3 ± 12.1 |
| Female—no. (%) | 9 (26.5) | 5 (29.4) | 15 (44.0) |
| Weight—kg | 73.4 ± 15.1 | 70.1 ± 11.5 | 66.0 ± 11.2 |
| Psoriasis (years) | 15.0 ± 8.3 | 15.1 ± 6.0 | |
| Psoriatic arthritis (years) | 8.1 ± 6.5 | ||
| No. of previous anti-TNFα drugs—no. (%) | 8 (23.5) | 8 (23.5) | |
| Use of methotrexate—no. (%) | 28 (82.4) | 13 (76.5) | |
| Use of leflunomide | 10 (29.4) | ||
| Use of NSAID | 30 (88.2) | ||
| Patients with specific disease characteristics—no. (%) CRP (mg/L) | 11.9 ± 13.2 | ||
| PASI | 14.8 ± 11.3 | ||
| Peripheral arthritis | 21 (61.8) | ||
| Peripheral and axil arthritis | 13 (38.2) | ||
| Dactylitis | 8 (25.8) | ||
| Enthesitis | 12 (41.9) | ||
| Tender-joint count (of 68 joints) | 9.1 ± 9.3 | ||
| Swollen-joint count (of 66 joints) | 3.5 ± 6.8 | ||
| Uveitis | 2 (5.9) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.-H.; Ho, J.-C.; Li, S.-C.; Chen, J.-F.; Hsiao, C.-C.; Lee, C.-H. MiR-146a-5p Expression in Peripheral CD14+ Monocytes from Patients with Psoriatic Arthritis Induces Osteoclast Activation, Bone Resorption, and Correlates with Clinical Response. J. Clin. Med. 2019, 8, 110. https://doi.org/10.3390/jcm8010110
Lin S-H, Ho J-C, Li S-C, Chen J-F, Hsiao C-C, Lee C-H. MiR-146a-5p Expression in Peripheral CD14+ Monocytes from Patients with Psoriatic Arthritis Induces Osteoclast Activation, Bone Resorption, and Correlates with Clinical Response. Journal of Clinical Medicine. 2019; 8(1):110. https://doi.org/10.3390/jcm8010110
Chicago/Turabian StyleLin, Shang-Hung, Ji-Chen Ho, Sung-Chou Li, Jia-Feng Chen, Chang-Chun Hsiao, and Chih-Hung Lee. 2019. "MiR-146a-5p Expression in Peripheral CD14+ Monocytes from Patients with Psoriatic Arthritis Induces Osteoclast Activation, Bone Resorption, and Correlates with Clinical Response" Journal of Clinical Medicine 8, no. 1: 110. https://doi.org/10.3390/jcm8010110
APA StyleLin, S.-H., Ho, J.-C., Li, S.-C., Chen, J.-F., Hsiao, C.-C., & Lee, C.-H. (2019). MiR-146a-5p Expression in Peripheral CD14+ Monocytes from Patients with Psoriatic Arthritis Induces Osteoclast Activation, Bone Resorption, and Correlates with Clinical Response. Journal of Clinical Medicine, 8(1), 110. https://doi.org/10.3390/jcm8010110





