Effectiveness of Hypertonic Saline Nasal Irrigation for Alleviating Allergic Rhinitis in Children: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Experimental Section
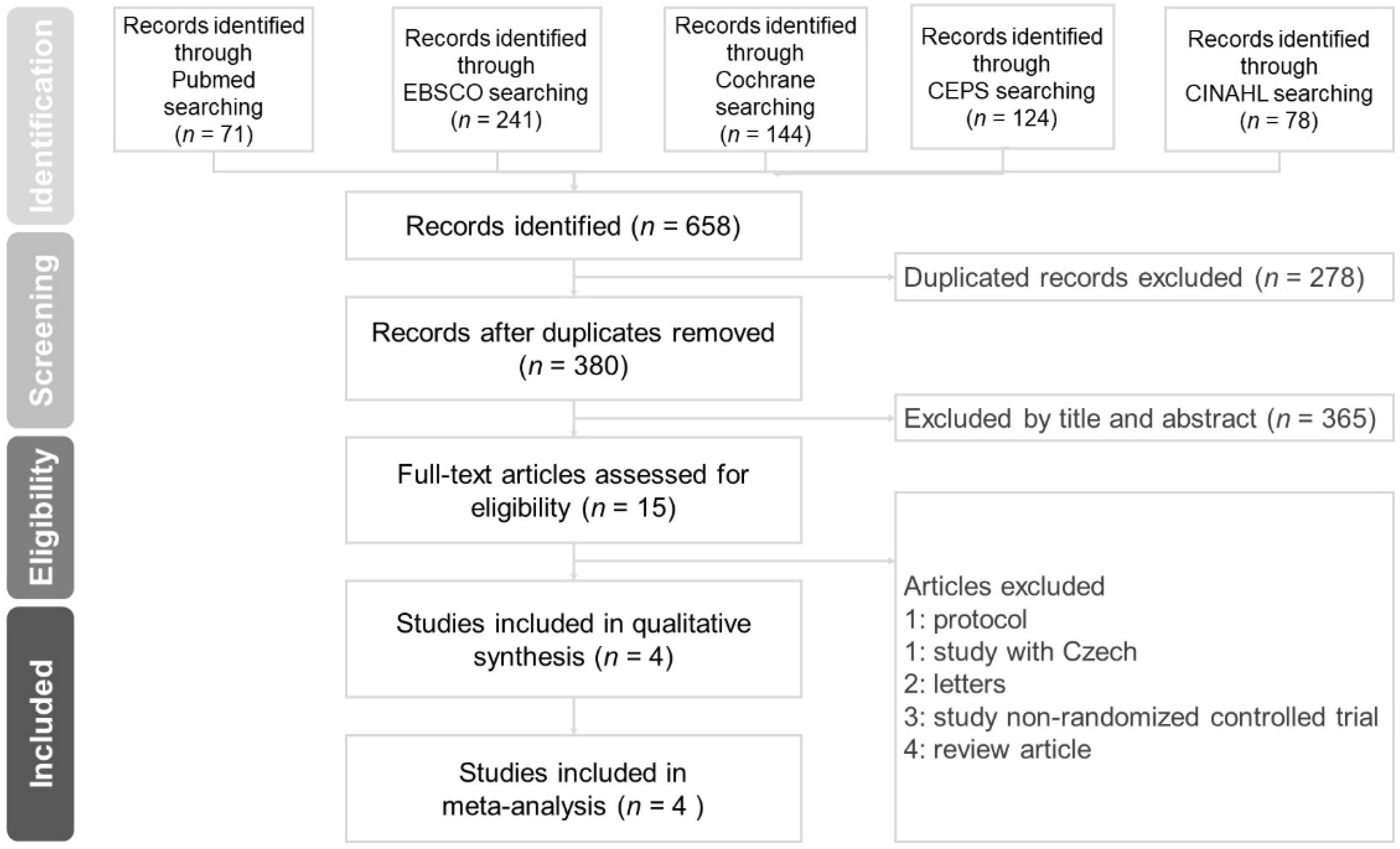
2.1. Research Method, Literature Search Strategy, and Results
2.2. Data Extraction and Validity Assessment
2.3. Data Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pawankar, R. Allergic diseases and asthma: A global public health concern and a call to action. World Allergy Organ. J. 2014, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Bauchau, V.; Durham, S.R. Prevalence and rate of diagnosis of allergic rhinitis in europe. Eur. Respir. J. 2004, 24, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Nathan, R.A.; Meltzer, E.O.; Seiner, J.C.; Storms, W. Prevalence of allergic rhinitis in the united states. J. Allergy Clin. Immunol. 1997, 99, 808–814. [Google Scholar] [CrossRef]
- Bousquet, J.; Khaltaev, N.; Cruz, A.A.; Denburg, J.; Fokkens, W.J.; Togias, A.; Zuberbier, T.; Baena-Cagnani, C.E.; Canonica, G.W.; van Weel, C.; et al. Allergic rhinitis and its impact on asthma (ARIA) 2008 update (in collaboration with the world health organization, ga(2)len and allergen). Allergy 2008, 63 (Suppl. 86), 8–160. [Google Scholar] [CrossRef]
- Small, P.; Keith, P.K.; Kim, H. Allergic rhinitis. Allergy Asthma Clin. Immunol. 2018, 14 (Suppl. 2), 51. [Google Scholar] [CrossRef]
- Wheatley, L.M.; Togias, A. Clinical practice. Allergic rhinitis. N. Engl. J. Med. 2015, 372, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.Y.; Chen, Y.J.; Lin, M.W.; Chen, T.J.; Chu, S.Y.; Chen, C.C.; Lee, D.D.; Chang, Y.T.; Wang, W.J.; Liu, H.N. Prevalence of atopic dermatitis, allergic rhinitis and asthma in taiwan: A national study 2000 to 2007. Acta Dermato-Venereol. 2010, 90, 589–594. [Google Scholar]
- Settipane, R.A. Complications of allergic rhinitis. Allergy Asthma Proc. 1999, 20, 209–213. [Google Scholar] [CrossRef]
- Dykewicz, M.S.; Wallace, D.V.; Baroody, F.; Bernstein, J.; Craig, T.; Finegold, I.; Huang, F.; Larenas-Linnemann, D.; Meltzer, E.; Steven, G.; et al. Treatment of seasonal allergic rhinitis: An evidence-based focused 2017 guideline update. Ann. Allergy Asthma Immunol. 2017, 119, 489–511. [Google Scholar] [CrossRef]
- Sur, D.K.; Plesa, M.L. Treatment of allergic rhinitis. Am. Fam. Phys. 2015, 92, 985–992. [Google Scholar]
- Bousquet, J. Available online: http://120.52.51.13/www.eaaci.org/attachments/768_Aria%20Pocket%20Guide.pdf (accessed on 4 January 2019).
- Ciprandi, G.; Fenoglio, D.; Cirillo, I.; Tosca, M.A.; La Rosa, M.; Licari, A.; Marseglia, A.; Barberi, S.; Marseglia, G.L. Sublingual immunotherapy: An update on immunologic and functional effects. Allergy Asthma Proc. 2007, 28, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Chong, L.Y.; Head, K.; Hopkins, C.; Philpott, C.; Glew, S.; Scadding, G.; Burton, M.J.; Schilder, A.G. Saline irrigation for chronic rhinosinusitis. Cochrane Database Syst. Rev. 2016, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Head, K.; Snidvongs, K.; Glew, S.; Scadding, G.; Schilder, A.G.; Philpott, C.; Hopkins, C. Saline irrigation for allergic rhinitis. Cochrane Database Syst. Rev. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- King, D.; Mitchell, B.; Williams, C.P.; Spurling, G.K. Saline nasal irrigation for acute upper respiratory tract infections. Cochrane Database Syst. Rev. 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Talbot, A.R.; Herr, T.M.; Parsons, D.S. Mucociliary clearance and buffered hypertonic saline solution. Laryngoscope 1997, 107, 500–503. [Google Scholar] [CrossRef] [PubMed]
- Garavello, W.; Romagnoli, M.; Sordo, L.; Gaini, R.M.; Di Berardino, C.; Angrisano, A. Hypersaline nasal irrigation in children with symptomatic seasonal allergic rhinitis: A randomized study. Pediatr. Allergy Immunol. 2003, 14, 140–143. [Google Scholar] [CrossRef]
- Malizia, V.; Fasola, S.; Ferrante, G.; Cilluffo, G.; Montalbano, L.; Landi, M.; Marchese, D.; Passalacqua, G.; La Grutta, S. Efficacy of buffered hypertonic saline nasal irrigation for nasal symptoms in children with seasonal allergic rhinitis: A randomized controlled trial. Int. Arch. Allergy Immunol. 2017, 174, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Marchisio, P.; Varricchio, A.; Baggi, E.; Bianchini, S.; Capasso, M.E.; Torretta, S.; Capaccio, P.; Gasparini, C.; Patria, F.; Esposito, S.; et al. Hypertonic saline is more effective than normal saline in seasonal allergic rhinitis in children. Int. J. Immunopathol. Pharmacol. 2012, 25, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Satdhabudha, A.; Poachanukoon, O. Efficacy of buffered hypertonic saline nasal irrigation in children with symptomatic allergic rhinitis: A randomized double-blind study. Int. J. Pediatr. Otorhinolaryngol. 2012, 76, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Bachert, C.; Hauser, U.; Prem, B.; Rudack, C.; Ganzer, U. Proinflammatory cytokines in allergic rhinitis. Eur. Arch. Oto-Rhino-Laryngol. 1995, 252 (Suppl. 1), 44–49. [Google Scholar] [CrossRef]
- Pawankar, R.; Hayashi, M.; Yamanishi, S.; Igarashi, T. The paradigm of cytokine networks in allergic airway inflammation. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Scadding, G. Cytokine profiles in allergic rhinitis. Curr. Allergy Asthma Rep. 2014, 14, 435. [Google Scholar] [CrossRef] [PubMed]
- König, K.; Klemens, C.; Eder, K.; San Nicoló, M.; Becker, S.; Kramer, M.F.; Gröger, M. Cytokine profiles in nasal fluid of patients with seasonal or persistent allergic rhinitis. Allergy Asthma Clin. Immunol. 2015, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Barham, H.P.; Harvey, R.J. Nasal saline irrigation: Therapeutic or homeopathic. Braz. J. Otorhinolaryngol. 2015, 81, 457–458. [Google Scholar] [CrossRef] [PubMed]
- Bonnomet, A.; Luczka, E.; Coraux, C.; de Gabory, L. Non-diluted seawater enhances nasal ciliary beat frequency and wound repair speed compared to diluted seawater and normal saline. Int. Forum Allergy Rhinol. 2016, 6, 1062–1068. [Google Scholar] [CrossRef] [Green Version]
- Georgitis, J.W. Nasal hyperthermia and simple irrigation for perennial rhinitis. Changes in inflammatory mediators. Chest 1994, 106, 1487–1492. [Google Scholar] [CrossRef]
- Ciprandi, G.; Cirillo, I.; Vizzaccaro, A.; Milanese, M.; Tosca, M.A. Correlation of nasal inflammation and nasal airflow with forced expiratory volume in 1 second in patients with perennial allergic rhinitis and asthma. Ann. Allergy Asthma Immunol. 2004, 93, 575–580. [Google Scholar] [CrossRef]
- Chen, J.-R.; Jin, L.; Li, X.-Y. The effectiveness of nasal saline irrigation (seawater) in treatment of allergic rhinitis in children. Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 1115–1118. [Google Scholar] [CrossRef]
- Gallant, J.N.; Basem, J.I.; Turner, J.H.; Shannon, C.N.; Virgin, F.W. Nasal saline irrigation in pediatric rhinosinusitis: A systematic review. Int. J. Pediatr. Otorhinolaryngol. 2018, 108, 155–162. [Google Scholar] [CrossRef]
- Hermelingmeier, K.E.; Weber, R.K.; Hellmich, M.; Heubach, C.P.; Mösges, R. Nasal irrigation as an adjunctive treatment in allergic rhinitis: A systematic review and meta-analysis. Am. J. Rhinol. Allergy 2012, 26, 119–125. [Google Scholar] [CrossRef]
- Rabago, D.; Zgierska, A. Saline nasal irrigation for upper respiratory conditions. Am. Fam. Phys. 2009, 80, 1117–1119. [Google Scholar]
- Ural, A.; Oktemer, T.K.; Kizil, Y.; Ileri, F.; Uslu, S. Impact of isotonic and hypertonic saline solutions on mucociliary activity in various nasal pathologies: Clinical study. J. Laryngol. Otol. 2009, 123, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Kanjanawasee, D.; Seresirikachorn, K.; Chitsuthipakorn, W.; Snidvongs, K. Hypertonic saline versus isotonic saline nasal irrigation: Systematic review and meta-analysis. Am. J. Rhinol. Allergy 2018, 32, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Shoseyov, D.; Bibi, H.; Shai, P.; Shoseyov, N.; Shazberg, G.; Hurvitz, H. Treatment with hypertonic saline versus normal saline nasal wash of pediatric chronic sinusitis. J. Allergy Clin. Immunol. 1998, 101, 602–605. [Google Scholar] [CrossRef]
- Silver, A.H.; Esteban-Cruciani, N.; Azzarone, G.; Douglas, L.C.; Lee, D.S.; Liewehr, S.; Nazif, J.M.; Agalliu, I.; Villegas, S.; Rhim, H.J.; et al. 3% hypertonic saline versus normal saline in inpatient bronchiolitis: A randomized controlled trial. Pediatrics 2015, 136, 1036–1043. [Google Scholar] [CrossRef]
- Berjis, N.; Sonbolastan, S.M.; Okhovat, S.H.; Narimani, A.A.; Razmjui, J.R. Normal saline versus hypertonic 3% saline: It’s efficacy in non-acute rhinosinusitis. Iran. J. Otorhinolaryngol. 2011, 23, 23–28. [Google Scholar]
- Elkins, M.R.; Bye, P.T. Mechanisms and applications of hypertonic saline. J. R. Soc. Med. 2011, 104 (Suppl. 1), 2–5. [Google Scholar] [CrossRef] [Green Version]
- Rabago, D.; Pasic, T.; Zgierska, A.; Mundt, M.; Barrett, B.; Maberry, R. The efficacy of hypertonic saline nasal irrigation for chronic sinonasal symptoms. Otolaryngol. Head Neck Surg. 2005, 133, 3–8. [Google Scholar] [CrossRef]
- Hauk, L. Treatment of seasonal allergic rhinitis: A guideline from the aaaai/acaai joint task force on practice parameters. Am. Fam. Phys. 2018, 97, 756–757. [Google Scholar]
- Seidman, M.D.; Gurgel, R.K.; Lin, S.Y.; Schwartz, S.R.; Baroody, F.M.; Bonner, J.R.; Dawson, D.E.; Dykewicz, M.S.; Hackell, J.M.; Han, J.K.; et al. Clinical practice guideline: Allergic rhinitis. Otolaryngol. Head Neck Surg. 2015, 152, 1–43. [Google Scholar] [CrossRef]



| Author, Year (Ref.) | Country | Participants (M:F) | Age (y/o) | Intervention Design | Concentration | Volume | Daily Frequency | Duration | Outcome | Results | Adverse Effects |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Garavello, 2003 [17] | Italy | 20 (8:12) | 6–12 | HSNI:no saline irrigation (1:1) | 3% | 2.5 mL each nostril | 3 times per day | 6 weeks |
|
| None reported |
| Marchisio, 2012 [19] | Italy | 220 (137:83) | 5–9 | HSNI:ISNI:no saline irrigation (80:80:60) | 2.7% | 20 mL | Twice daily | 4 weeks |
|
| Two children (one in each treatment group) |
| Satdhabudha, 2012 [20] | Thailand | 81 (49:32) | 6–15 | HSNI:ISNI (40:41) | 1.25% | 240 mL/time | Twice daily | 4 weeks |
|
| 12% (HSNI) and 5% (ISNI) in 3rd visit |
| Malizia, 2017 [18] | Italy | 30 (25:11) | 6–13 | HSNI:ISNI (1:1) | 3% | 5 mL | Twice daily | 3 weeks |
|
| 1 child in ISNI (6%) versus 2 children in HSNI (12%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.-L.; Lin, H.-C.; Lin, C.-Y.; Hsu, T.-F. Effectiveness of Hypertonic Saline Nasal Irrigation for Alleviating Allergic Rhinitis in Children: A Systematic Review and Meta-Analysis. J. Clin. Med. 2019, 8, 64. https://doi.org/10.3390/jcm8010064
Li C-L, Lin H-C, Lin C-Y, Hsu T-F. Effectiveness of Hypertonic Saline Nasal Irrigation for Alleviating Allergic Rhinitis in Children: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2019; 8(1):64. https://doi.org/10.3390/jcm8010064
Chicago/Turabian StyleLi, Chia-Ling, Hsiao-Chuan Lin, Chien-Yu Lin, and Teh-Fu Hsu. 2019. "Effectiveness of Hypertonic Saline Nasal Irrigation for Alleviating Allergic Rhinitis in Children: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 8, no. 1: 64. https://doi.org/10.3390/jcm8010064
APA StyleLi, C.-L., Lin, H.-C., Lin, C.-Y., & Hsu, T.-F. (2019). Effectiveness of Hypertonic Saline Nasal Irrigation for Alleviating Allergic Rhinitis in Children: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 8(1), 64. https://doi.org/10.3390/jcm8010064






