Effects of Hip Structure Analysis Variables on Hip Fracture: A Propensity Score Matching Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Participants
2.3. Biochemical Analyses
2.4. Measurements of the Appendicular Skeletal Muscle Mass and Bone Mineral Density (BMD)
2.5. Definition of Osteoporosis
2.6. Hip Structure Analysis (HSA)
2.7. Statistical Analyses
3. Results
3.1. Demographic Characteristics by Presence of Hip Fracture after Propensity Score Matching
3.2. Hip Structural Analysis (HSA) by the Presence of Hip Fracture
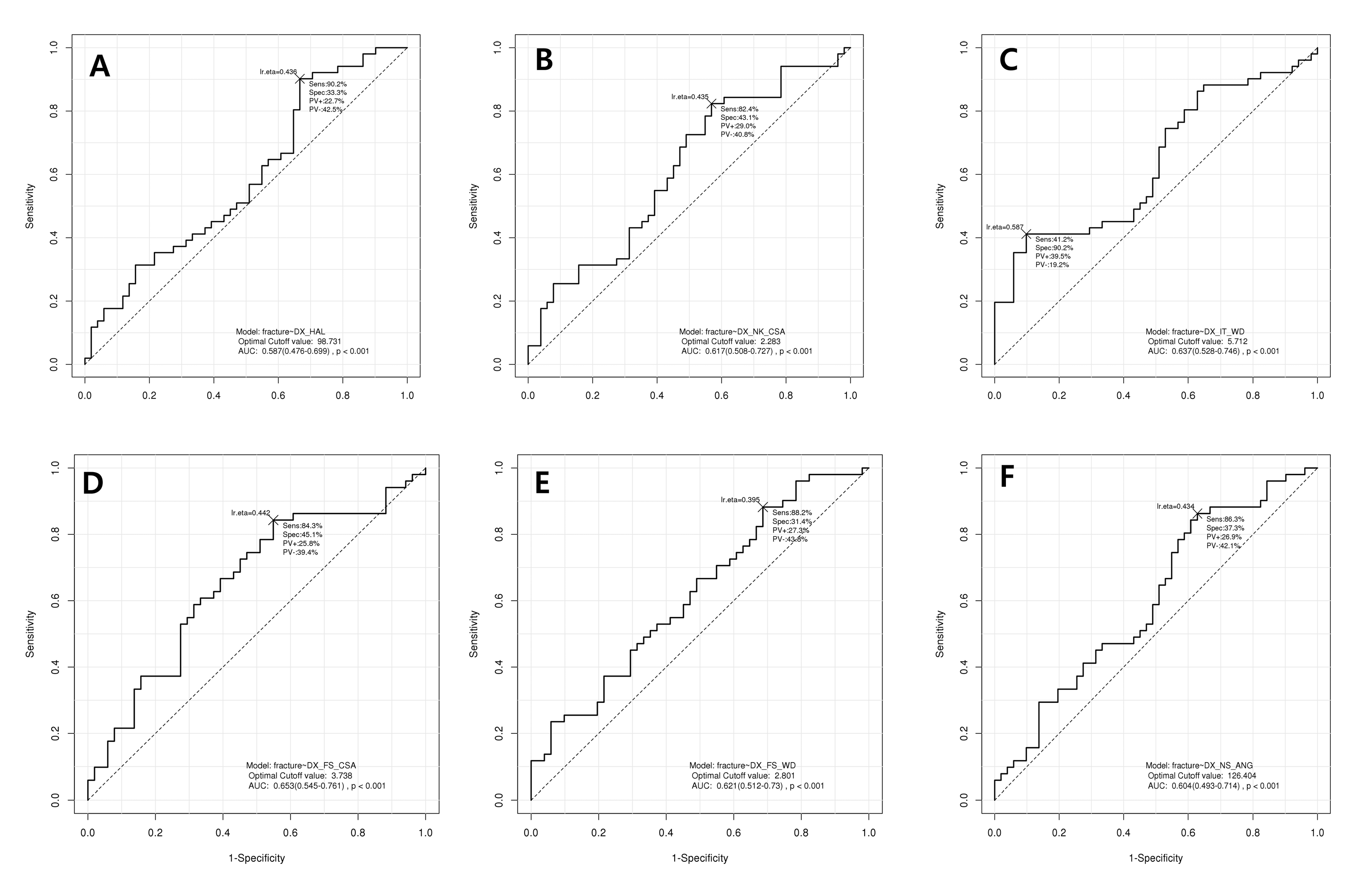
3.3. Receiver Operator Curve (ROC) Analysis for Diagnosis of Hip Fracture Using Hip Structural Analysis
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Christensen, K.; Doblhammer, G.; Rau, R.; Vaupel, J.W. Ageing populations: The challenges ahead. Lancet 2009, 374, 1196–1208. [Google Scholar] [CrossRef]
- Kanis, J.A.; Burlet, N.; Cooper, C.; Delmas, P.D.; Reginster, J.Y.; Borgstrom, F.; Rizzoli, R. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos. Int. 2008, 19, 399–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazrun, A.S.; Tzar, M.N.; Mokhtar, S.A.; Mohamed, I.N. A systematic review of the outcomes of osteoporotic fracture patients after hospital discharge: Morbidity, subsequent fractures, and mortality. Ther. Clin. Risk Manag. 2014, 10, 937–948. [Google Scholar] [PubMed]
- Kwon, H.Y.; Ha, Y.C.; Yoo, J.I. Health-Related Quality of Life in Accordance with Fracture History and Comorbidities in Korean Patients with Osteoporosis. J. Bone Metab. 2016, 23, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Tanawat, A.; Lerkong, N. One-year mortality rate after osteoporotic hip fractures and associated risk factors in Police General Hospital. Osteoporos. Sarcopenia 2015, 1, 75–79. [Google Scholar] [Green Version]
- Curtis, E.M.; Moon, R.J.; Harvey, N.C.; Cooper, C. The impact of fragility fracture and approaches to osteoporosis risk assessment worldwide. Bone 2017, 104, 29–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanis, J.A.; Harvey, N.C.; Johansson, H.; Odén, A.; McCloskey, E.V.; Leslie, W.D. Overview of Fracture Prediction Tools. J. Clin. Densitom. 2017, 20, 444–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques, A.; Lucas, R.; Simões, E.; Verstappen, S.M.M.; Jacobs, J.W.G.; da Silva, J.A.P. Do we need bone mineral density to estimate osteoporotic fracture risk? A 10-year prospective multicentre validation study. RMD Open 2017, 3, e000509. [Google Scholar] [CrossRef] [PubMed]
- Nakata, Y.; Tanaka, K.; Mizuki, T.; Yoshida, T. Body composition measurements by dual-energy X-ray absorptiometry differ between two analysis modes. J. Clin. Densitom. 2004, 7, 443–447. [Google Scholar] [CrossRef]
- Cawthon, P.M. Assessment of Lean Mass and Physical Performance in Sarcopenia. J. Clin. Densitom. 2015, 18, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Midorikawa, T.; Ohta, M.; Torii, S.; Sakamoto, S. Lean Soft Tissue Mass Measured Using Dual-Energy X-ray Absorptiometry Is an Effective Index for Assessing Change in Leg Skeletal Muscle Mass Following Exercise Training. J. Clin. Densitom. 2018, 21, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Yoo, J.-I. Sarcopenia and Hip-Structure Analysis Variables in Korean Elderly Population. J. Clin. Densitom. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Shevroja, E.; Lamy, O.; Kohlmeier, L.; Koromani, F.; Rivadeneira, F.; Hans, D. Use of Trabecular Bone Score (TBS) as a Complementary Approach to Dual-energy X-ray Absorptiometry (DXA) for Fracture Risk Assessment in Clinical Practice. J. Clin. Densitom. 2017, 20, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Schneider, D.L.; Bettencourt, R.; Barrett-Connor, E. The Clinical Utility of Spine Bone Density in Elderly Women. J. Clin. Densitom. 2006, 9, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Ichchou, L.; Allali, F.; Rostom, S.; Bennani, L.; Hmamouchi, I.; Abourazzak, F.Z.; Khazzani, H.; El Mansouri, L.; Abouqal, R.; Hajjaj-Hassouni, N. Relationship between spine osteoarthritis, bone mineral density and bone turn over markers in post menopausal women. BMC Women’s Health 2010, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, M.C.; Lethbridge-Cejku, M.; Tobin, J.D. Bone mineral density and osteoarthritis: Data from the Baltimore Longitudinal Study of Aging. 11 Supported in part by grants from the Arthritis Foundation, Maryland Chapter and the National Institute of Arthritis, Musculoskeletal and Skin Diseases. Osteoarthr. Cartil. 2004, 12, 45–48. [Google Scholar] [CrossRef]
- Cranney, A.; Jamal, S.A.; Tsang, J.F.; Josse, R.G.; Leslie, W.D. Low bone mineral density and fracture burden in postmenopausal women. Cmaj 2007, 177, 575–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broy, S.B.; Cauley, J.A.; Lewiecki, M.E.; Schousboe, J.T.; Shepherd, J.A.; Leslie, W.D. Fracture Risk Prediction by Non-BMD DXA Measures: The 2015 ISCD Official Positions Part 1: Hip Geometry. J. Clin. Densitom. 2015, 18, 287–308. [Google Scholar] [CrossRef]
- Kweon, S.; Kim, Y.; Jang, M.J.; Kim, Y.; Kim, K.; Choi, S.; Chun, C.; Khang, Y.H.; Oh, K. Data resource profile: The Korea National Health and Nutrition Examination Survey (KNHANES). Int. J. Epidemiol. 2014, 43, 69–77. [Google Scholar] [CrossRef]
- Sözen, T.; Özışık, L.; Başaran, N.Ç. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef]
- Beck, T.J.; Looker, A.C.; Ruff, C.B.; Sievanen, H.; Wahner, H.W. Structural trends in the aging femoral neck and proximal shaft: Analysis of the Third National Health and Nutrition Examination Survey dual-energy X-ray absorptiometry data. J. Bone Miner. Res. 2000, 15, 2297–2304. [Google Scholar] [CrossRef] [PubMed]
- Uusi-Rasi, K.; Semanick, L.M.; Zanchetta, J.R.; Bogado, C.E.; Eriksen, E.F.; Sato, M.; Beck, T.J. Effects of teriparatide [rhPTH (1–34)] treatment on structural geometry of the proximal femur in elderly osteoporotic women. Bone 2005, 36, 948–958. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Y.; Kim, D.; Kim, K.M.; Kim, K.J.; Choi, H.S.; Rhee, Y.; Lim, S.K. Age-Related Bone Mineral Density Patterns in Koreans (KNHANES IV). J. Clin. Endocrinol. Metab. 2012, 97, 3310–3318. [Google Scholar] [CrossRef] [Green Version]
- Austin, P.C. A critical appraisal of propensity-score matching in the medical literature between 1996 and 2003. Stat. Med. 2008, 27, 2037–2049. [Google Scholar] [CrossRef] [PubMed]
- Kaptoge, S.; Beck, T.J.; Reeve, J.; Stone, K.L.; Hillier, T.A.; Cauley, J.A.; Cummings, S.R. Prediction of Incident Hip Fracture Risk by Femur Geometry Variables Measured by Hip Structural Analysis in the Study of Osteoporotic Fractures. J. Bone Miner. Res. 2008, 23, 1892–1904. [Google Scholar] [CrossRef] [Green Version]
- Khoo, B.C.C.; Lewis, J.R.; Brown, K.; Prince, R.L. Evaluation of a simplified hip structure analysis method for the prediction of incident hip fracture events. Osteoporos. Int. 2016, 27, 241–248. [Google Scholar] [CrossRef] [PubMed]
- For the Manitoba Bone Density Program; Leslie, W.D.; Pahlavan, P.S.; Tsang, J.F.; Lix, L.M. Prediction of hip and other osteoporotic fractures from hip geometry in a large clinical cohort. Osteoporos. Int. 2009, 20, 1767–1774. [Google Scholar] [CrossRef]
- LaCroix, A.Z.; Beck, T.J.; Cauley, J.A.; Lewis, C.E.; Bassford, T.; Jackson, R.; Wu, G.; Chen, Z. Hip structural geometry and incidence of hip fracture in postmenopausal women: What does it add to conventional bone mineral density? Osteoporos. Int. 2010, 21, 919–929. [Google Scholar] [CrossRef]
- Leslie, W.D.; Luo, Y.; Yang, S.; Goertzen, A.L.; Ahmed, S.; Delubac, I.; Lix, L.M. Fracture Risk Indices from DXA-Based Finite Element Analysis Predict Incident Fractures Independently from FRAX: The Manitoba BMD Registry. J. Clin. Densitom. 2019, 22, 338–345. [Google Scholar] [CrossRef]
- Aldieri, A.; Terzini, M.; Osella, G.; Priola, A.M.; Angeli, A.; Veltri, A.; Audenino, A.L.; Bignardi, C. Osteoporotic Hip Fracture Prediction: Is T-Score-Based Criterion Enough? A Hip Structural Analysis-Based Model. J. Biomech. Eng. 2018, 140, 111004. [Google Scholar] [CrossRef]
- Borggrefe, J.; de Buhr, T.; Shrestha, S.; Marshall, L.M.; Orwoll, E.; Peters, K.; Black, D.M.; Glüer, C.C.; Osteoporotic Fractures in Men (MrOS) Study Research Group. Association of 3D Geometric Measures Derived from Quantitative Computed Tomography with Hip Fracture Risk in Older Men: QCT-DERIVED 3D Geometric Measures Associated with Hip Fracture. J. Bone Miner. Res. 2016, 31, 1550–1558. [Google Scholar] [CrossRef] [PubMed]
- Fujii, M.; Aoki, T.; Okada, Y.; Mori, H.; Kinoshita, S.; Hayashida, Y.; Hajime, M.; Tanaka, K.; Tanaka, Y.; Korogi, Y. Prediction of Femoral Neck Strength in Patients with Diabetes Mellitus with Trabecular Bone Analysis and Tomosynthesis Images. Radiology 2016, 281, 933–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolta, S.; Etcheto, A.; Fechtenbaum, J.; Feydy, A.; Roux, C.; Briot, K. Measurement of Trabecular Bone Score of the Spine by Low-Dose Imaging System (EOS®): A Feasibility Study. J. Clin. Densitom. 2019, 22, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Leslie, W.D.; Luo, Y.; Goertzen, A.L.; Ahmed, S.; Ward, L.M.; Delubac, I.; Lix, L.M. Automated DXA-based finite element analysis for hip fracture risk stratification: A cross-sectional study. Osteoporos. Int. 2018, 29, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, K.G.; McClung, M.; Cummings, S.R. Automated evaluation of hip axis length for predicting hip fracture. J. Bone Miner. Res. 1994, 9, 1065–1070. [Google Scholar] [CrossRef] [PubMed]

| Non-HF (N = 51) | HF (N = 51) | p-Value | |
|---|---|---|---|
| Age (years) | 78.2 ± 6.4 | 77.9 ± 7.3 | 0.807 |
| Sex | 1.000 | ||
| Male | 14 (27.5%) | 15 (29.4%) | |
| Female | 37 (72.5%) | 36 (70.6%) | |
| BMI (kg/m2) | 22.0 ± 3.0 | 21.6 ± 3.4 | 0.500 |
| Osteoporosis | 0.890 | ||
| Normal | 3 (5.9%) | 2 (3.9%) | |
| Osteopenia | 15 (29.4%) | 16 (31.4%) | |
| Osteoporosis | 33 (64.7%) | 33 (64.7%) | |
| SMI (kg/m2) | 6.0 ± 0.8 | 5.8 ± 1.0 | 0.429 |
| VitD (ng/mL) | 17.5 ± 9.0 | 5.8 ± 1.0 | 0.934 |
| Non-HF (N = 51) | HF (N = 51) | p-Value | ||
|---|---|---|---|---|
| Hip axis length (mm) | 102.07 ± 14.15 | 107.31 ± 9.55 | 0.031 | |
| Femur neck | CSA | 2.12 ± 0.46 | 1.93 ± 0.44 | 0.030 |
| WD | 3.37 ± 0.33 | 3.41 ± 0.34 | 0.606 | |
| CT | 0.13 ± 0.03 | 0.11 ± 0.02 | 0.004 | |
| Intertrochanteric area | CSA | 3.28 ± 0.88 | 3.18 ± 0.88 | 0.535 |
| WD | 5.29 ± 0.38 | 5.57± 0.58 | 0.005 | |
| CT | 0.26 ± 0.07 | 0.24 ± 0.06 | 0.076 | |
| Femur shaft | CSA | 3.57 ± 0.78 | 3.18 ± 0.83 | 0.016 |
| WD | 2.92 ± 0.23 | 3.05 ± 0.23 | 0.010 | |
| CT | 0.47 ± 0.11 | 0.38 ± 0.09 | <0.001 | |
| NSA (°) | 128.85 ± 5.81 | 131.11 ± 5.29 | 0.043 | |
| HSA | Cut-Off Point | Sensitivity | Specificity | AUC | p-Value |
|---|---|---|---|---|---|
| HAL | 98.73 | 90.2% | 33.3% | 0.587 | <0.001 |
| NK_CSA | 2.283 | 82.4% | 43.1% | 0.617 | <0.001 |
| IT_WD | 5.712 | 41.2% | 90.2% | 0.637 | <0.001 |
| FS_CSA | 3.738 | 84.3% | 45.1% | 0.653 | <0.001 |
| FS_WD | 2.801 | 88.2% | 31.4% | 0.621 | <0.001 |
| NSA | 126.40 | 86.3% | 37.3% | 0.604 | <0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ha, Y.-C.; Yoo, J.-I.; Yoo, J.; Park, K.S. Effects of Hip Structure Analysis Variables on Hip Fracture: A Propensity Score Matching Study. J. Clin. Med. 2019, 8, 1507. https://doi.org/10.3390/jcm8101507
Ha Y-C, Yoo J-I, Yoo J, Park KS. Effects of Hip Structure Analysis Variables on Hip Fracture: A Propensity Score Matching Study. Journal of Clinical Medicine. 2019; 8(10):1507. https://doi.org/10.3390/jcm8101507
Chicago/Turabian StyleHa, Yong-Chan, Jun-Il Yoo, Jeongkyun Yoo, and Ki Soo Park. 2019. "Effects of Hip Structure Analysis Variables on Hip Fracture: A Propensity Score Matching Study" Journal of Clinical Medicine 8, no. 10: 1507. https://doi.org/10.3390/jcm8101507
APA StyleHa, Y.-C., Yoo, J.-I., Yoo, J., & Park, K. S. (2019). Effects of Hip Structure Analysis Variables on Hip Fracture: A Propensity Score Matching Study. Journal of Clinical Medicine, 8(10), 1507. https://doi.org/10.3390/jcm8101507






