Association Between HDL Cholesterol and QTc Interval: A Population-Based Epidemiological Study
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Measurements
2.2.1. Clinical Evaluation
2.2.2. Laboratory Measurements
2.2.3. Electrocardiogram Analysis
2.3. Statistical Analysis
3. Results
4. Discussion
5. Study Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Imaizumi, S.; Miura, S.; Nakamura, K.; Kiya, Y.; Uehara, Y.; Zhang, B.; Matsuo, Y.; Urata, H.; Ideishi, M.; Rye, K.A.; et al. Antiarrhythmogenic effect of reconstituted high-density lipoprotein against ischemia/reperfusion in rats. J. Am. Coll. Cardiol. 2008, 51, 1604–1612. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, S.; Okumura, M.; Tanaka, F.; Sato, T.; Kagami, A.; Tada, N.; Nagano, M. Ischemia-reperfusion arrhythmias and lipids: Effect of human high- and low-density lipoproteins on reperfusion arrhythmias. Cardiovasc. Drugs Ther. 1991, 5, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Kunutsor, S.K.; Zaccardi, F.; Karppi, J.; Kurl, S.; Laukkanen, J.A. Is High Serum LDL/HDL Cholesterol Ratio an Emerging Risk Factor for Sudden Cardiac Death? Findings from the KIHD Study. J. Atheroscler. Thromb. 2017, 24, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Wannamethee, G.; Shaper, A.G.; Macfarlane, P.W.; Walker, M. Risk factors for sudden cardiac death in middle-aged British men. Circulation 1995, 91, 1749–1756. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Fabritz, L. High-density lipoprotein shortens the ventricular action potential. A novel explanation for how statins prevent sudden arrhythmic death? J. Am. Coll. Cardiol. 2011, 58, 45–47. [Google Scholar] [CrossRef]
- Liu, Y.B.; Wu, C.C.; Lee, C.M.; Chen, W.J.; Wang, T.D.; Chen, P.S.; Lee, Y.T. Dyslipidemia is associated with ventricular tachyarrhythmia in patients with acute ST-segment elevation myocardial infarction. J. Formos. Med. Assoc. 2006, 105, 17–24. [Google Scholar] [CrossRef]
- Boudi, F.B.; Kalayeh, N.; Movahed, M.R. High-Density Lipoprotein Cholesterol (HDL-C) Levels Independently Correlates with Cardiac Arrhythmias and Atrial Fibrillation. J. Intensive Care Med. 2018. [Google Scholar] [CrossRef]
- Li, Z.Z.; Du, X.; Guo, X.Y.; Tang, R.B.; Jiang, C.; Liu, N.; Chang, S.S.; Yu, R.H.; Long, D.Y.; Bai, R.; et al. Association Between Blood Lipid Profiles and Atrial Fibrillation: A Case-Control Study. Med. Sci. Monit. 2018, 24, 3903–3908. [Google Scholar] [CrossRef]
- Yao, H.; Jiang, L.; Lin, X.; Liang, Z.G. Fluvastatin combined with benazepril may contribute to the favorable prognosis of patients with atrial fibrillation. Biomed. Pharmacother. 2016, 83, 687–692. [Google Scholar] [CrossRef]
- Annoura, M.; Ogawa, M.; Kumagai, K.; Zhang, B.; Saku, K.; Arakawa, K. Cholesterol paradox in patients with paroxysmal atrial fibrillation. Cardiology 1999, 92, 21–27. [Google Scholar] [CrossRef]
- Hayashi, M.; Shimizu, W.; Albert, C.M. The spectrum of epidemiology underlying sudden cardiac death. Circ. Res. 2015, 116, 1887–1906. [Google Scholar] [CrossRef] [PubMed]
- Straus, S.M.; Kors, J.A.; De Bruin, M.L.; van der Hooft, C.S.; Hofman, A.; Heeringa, J.; Deckers, J.W.; Kingma, J.H.; Sturkenboom, M.C.; Stricker, B.H.; et al. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J. Am. Coll. Cardiol. 2006, 47, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Roden, D.M. Keep the QT interval: It is a reliable predictor of ventricular arrhythmias. Heart Rhythm 2008, 5, 1213–1215. [Google Scholar] [CrossRef] [PubMed]
- Den Ruijter, H.M.; Franssen, R.; Verkerk, A.O.; van Wijk, D.F.; Vaessen, S.F.; Holleboom, A.G.; Levels, J.H.; Opthof, T.; Sungnoon, R.; Stroes, E.S.; et al. Reconstituted high-density lipoprotein shortens cardiac repolarization. J. Am. Coll. Cardiol. 2011, 58, 40–44. [Google Scholar] [CrossRef] [PubMed]
- O’Neal, W.T.; Singleton, M.J.; Roberts, J.D.; Tereshchenko, L.G.; Sotoodehnia, N.; Chen, L.Y.; Marcus, G.M.; Soliman, E.Z. Association Between QT-Interval Components and Sudden Cardiac Death: The ARIC Study (Atherosclerosis Risk in Communities). Circ. Arrhythm. Electrophysiol. 2017, 10, e005485. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.S.; Thomas, K.L.; Broderick, S.; Shaw, L.K.; Velazquez, E.J.; Al-Khatib, S.M.; Daubert, J.P. Race and gender variation in the QT interval and its association with mortality in patients with coronary artery disease: Results from the Duke Databank for Cardiovascular Disease (DDCD). Am. Heart J. 2012, 164, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Beinart, R.; Zhang, Y.; Lima, J.A.; Bluemke, D.A.; Soliman, E.Z.; Heckbert, S.R.; Post, W.S.; Guallar, E.; Nazarian, S. The QT interval is associated with incident cardiovascular events: The MESA study. J. Am. Coll. Cardiol. 2014, 64, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Korantzopoulos, P.; Liberopoulos, E.; Barkas, F.; Kei, A.; Goudevenos, J.A.; Elisaf, M. No association between high-density lipoprotein levels and ventricular repolarization indexes in subjects with primary hypercholesterolemia. Scand. J. Clin. Lab. Investig. 2014, 74, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. PLoS Med. 2007, 4, e297. [Google Scholar] [CrossRef] [PubMed]
- TRUE Consortium. Recommended Standards for Assessing Blood Pressure in Human Research where Blood Pressure or Hypertension Is a Major Focus. Kidney Int. Rep. 2017, 16, 733–738. [Google Scholar]
- Salvadé, I.; Schätti-Stählin, S.; Violetti, E.; Schönholzer, C.; Cereghetti, C.; Zwahlen, H.; Berwert, L.; Burnier, M.; Gabutti, L. A prospective observational study comparing a non-operator dependent automatic PWV analyser to pulse pressure, in assessing arterial stiffness in hemodialysis. BMC Nephrol. 2015, 16, 62. [Google Scholar] [CrossRef] [PubMed]
- Milan, A.; Zocaro, G.; Leone, D.; Tosello, F.; Buraioli, I.; Schiavone, D.; Veglio, F. Current assessment of pulse wave velocity: Comprehensive review of validation studies. J. Hypertens. 2019, 37, 1547–1557. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Rautaharju, P.M.; Surawicz, B.; Gettes, L.S. AHA/ACCF/HRS Recommendations for the Standardization and Interpretation of the Electrocardiogram Part IV: The ST Segment, T and U Waves, and the QT Interval A Scientific Statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society Endorsed by the International Society for Computerized Electrocardiology. J. Am. Coll. Cardiol. 2009, 53, 982–999. [Google Scholar] [PubMed]
- Dettori, J.R.; Norvell, D.C.; Chapman, J.R. The Sin of Missing Data: Is All Forgiven by Way of Imputation? Glob. Spine J. 2018, 8, 892–894. [Google Scholar] [CrossRef]
- Maguy, A.; Hebert, T.E.; Nattel, S. Involvement of lipid rafts and caveolae in cardiac ion channel function. Cardiovasc. Res. 2006, 69, 798–807. [Google Scholar] [CrossRef]
- Abi-Char, J.; Maguy, A.; Coulombe, A.; Balse, E.; Ratajczak, P.; Samuel, J.L.; Nattel, S.; Hatem, S.N. Membrane cholesterol modulates Kv1.5 potassium channel distribution and function in rat cardiomyocytes. J. Physiol. 2007, 582, 1205–1217. [Google Scholar] [CrossRef]
- Levitan, I.; Singh, D.K.; Rosenhouse-Dantsker, A. Cholesterol binding to ion channels. Front. Physiol. 2014, 5, 65. [Google Scholar] [CrossRef]
- Balijepalli, R.C.; Kamp, T.J. Caveolae, ion channels and cardiac arrhythmias. Prog. Biophys. Mol. Biol. 2008, 98, 149. [Google Scholar] [CrossRef]
- Baartscheer, A.; Schumacher, C.A.; Wekker, V.; Verkerk, A.O.; Veldkamp, M.W.; van Oort, R.J.; Elzenaar, I.; Ottenhoff, R.; van Roomen, C.; Aerts, H.; et al. Dyscholesterolemia Protects Against Ischemia-Induced Ventricular Arrhythmias. Circ. Arrhythm. Electrophysiol. 2015, 8, 1481–1490. [Google Scholar] [CrossRef]
- Levitan, I.; Fang, Y.; Rosenhouse-Dantsker, A.; Romanenko, V. Cholesterol and ion channels. Subcell. Biochem. 2010, 51, 509–549. [Google Scholar]
- Trépanier-Boulay, V.; St-Michel, C.; Tremblay, A.; Fiset, C. Gender-based differences in cardiac repolarization in mouse ventricle. Circ. Res. 2001, 89, 437–444. [Google Scholar] [CrossRef]
- Yarnoz, M.J.; Curtis, A.B. More reasons why men and women are not the same (gender differences in electrophysiology and arrhythmias). Am. J. Cardiol. 2008, 101, 1291–1296. [Google Scholar] [CrossRef]
- Sims, C.; Reisenweber, S.; Viswanathan, P.C.; Choi, B.R.; Walker, W.H.; Salama, G. Sex, age, and regional differences in L type calcium current are important determinants of arrhythmia phenotype in rabbit hearts with drug-induced long QT type 2. Circ. Res. 2008, 102, e86–e100. [Google Scholar] [CrossRef]
- Chu, S.H.; Sutherland, K.; Beck, J.; Kowalski, J.; Goldspink, P.; Schwertz, D. Sex differences in expression of calcium-handling proteins and beta-adrenergic receptors in rat heart ventricle. Life Sci. 2005, 76, 2735–2749. [Google Scholar] [CrossRef]
- Furukawa, T.; Kurokawa, J. Regulation of cardiac ion channels via non-genomic action of sex steroid hormones: Implication for the gender difference in cardiac arrhythmias. Pharmacol. Ther. 2007, 115, 106–115. [Google Scholar] [CrossRef]
- Legato, M.J. Dyslipidaemia, gender, and the role of high density lipoprotein cholesterol: Implications for therapy. Am. J. Cardiol. 2000, 86, 15L–18L. [Google Scholar] [CrossRef]
- Gordon, D.J.; Probstfield, J.L.; Garrison, R.J.; Neaton, J.D.; Castelli, W.P.; Knoke, J.D.; Jacobs, D.R., Jr.; Bangdiwala, S.; Tyroler, H.A. High-density lipoprotein cholesterol and cardiovascular disease: Four prospective American studies. Circulation 1989, 79, 8–15. [Google Scholar] [CrossRef]
- Watanabe, H.; Tanabe, N.; Yagihara, N.; Watanabe, T.; Aizawa, Y.; Kodama, M. Association between lipid profile and risk of atrial fibrillation. Circ. J. 2011, 75, 2767–2774. [Google Scholar] [CrossRef]
- Nakagawa, M.; Ooie, T.; Ou, B.; Ichinose, M.; Takahashi, N.; Hara, M.; Yonemochi, H.; Saikawa, T. Gender differences in autonomic modulation of ventricular repolarization in humans. J. Cardiovasc. Electrophysiol. 2005, 16, 278–284. [Google Scholar] [CrossRef]
- Zhang, Y.; Ouyang, P.; Post, W.S.; Dalal, D.; Vaidya, D.; Blasco-Colmenares, E.; Soliman, E.Z.; Tomaselli, G.F.; Guallar, E. Sex-steroid hormones and electrocardiographic QT-interval duration: Findings from the Third National Health and Nutrition Examination Survey and the Multi-Ethnic Study of Atherosclerosis. Am. J. Epidemiol. 2011, 174, 403–411. [Google Scholar] [CrossRef]
- Wilkins, J.T.; Ning, H.; Stone, N.J.; Criqui, M.H.; Zhao, L.; Greenland, P.; Lloyd-Jones, D.M. Coronary heart disease risks associated with high levels of HDL cholesterol. J. Am. Heart Assoc. 2014, 3, e000519. [Google Scholar] [CrossRef]
- Van der Steeg, W.A.; Holme, I.; Boekholdt, S.M.; Larsen, M.L.; Lindahl, C.; Stroes, E.S.G.; Tikkanen, M.J.; Wareham, N.J.; Faergeman, O.; Olsson, A.G.; et al. High-density lipoprotein cholesterol, high-density lipoprotein particle size, and apolipoprotein A-I: Significance for cardiovascular risk: The IDEAL and EPIC-Norfolk studies. J. Am. Coll. Cardiol. 2008, 51, 634–642. [Google Scholar] [CrossRef]
- Madsen, C.M.; Varbo, A.; Nordestgaard, B.G. Extreme high high-density lipoprotein cholesterol is paradoxically associated with high mortality in men and women: Two prospective cohort studies. Eur. Heart J. 2017, 38, 2478–2486. [Google Scholar] [CrossRef]
- Voight, B.F.; Peloso, G.M.; Orho-Melander, M.; Frikke-Schmidt, R.; Barbalic, M.; Jensen, M.K. Plasma HDL cholesterol and risk of myocardial infarction: A mendelian randomisation study. Lancet 2012, 380, 572–580. [Google Scholar] [CrossRef]
- Zanoni, P.; Khetarpal, S.A.; Larach, D.B.; Hancock-Cerutti, W.F.; Millar, J.S.; Cuchel, M.; DerOhannessian, S.; Kontush, A.; Surendran, P.; Saleheen, D.; et al. Rare variant in scavenger receptor BI raises HDL cholesterol and increases risk of coronary heart disease. Science 2016, 351, 1166–1171. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Brewer, H.B., Jr.; Ansell, B.J.; Barter, P.; Chapman, M.J.; Heinecke, J.W.; Kontush, A.; Tall, A.R.; Webb, N.R. Dysfunctional HDL and atherosclerotic cardiovascular disease. Nat. Rev. Cardiol. 2016, 13, 48–60. [Google Scholar] [CrossRef]
- Arsenault, B.J.; Despre’s, J.P. HDL cholesterol is not HDL—Don’t judge the book by its cover. Nat. Rev. Cardiol. 2012, 9, 557–558. [Google Scholar] [CrossRef]
- Adams, V.; Besler, C.; Fischer, T.; Riwanto, M.; Noack, F.; Höllriegel, R.; Oberbach, A.; Jehmlich, N.; Völker, U.; Winzer, E.B.; et al. Exercise training in patients with chronic heart failure promotes restoration of high-density lipoprotein functional properties. Circ. Res. 2013, 113, 1345–1355. [Google Scholar] [CrossRef]
- Wu, L.; Parhofer, K.G. Diabetic dyslipidemia. Metabolism 2014, 63, 1469–1479. [Google Scholar] [CrossRef]
- Arslan, E.; Yiğiner, O.; Yavaşoğlu Ozçelik, F.; Kardeşoğlu, E.; Nalbant, S. Effect of uncomplicated obesity on QT interval in young men. Pol. Arch. Med. Wewn. 2010, 120, 209–213. [Google Scholar]
- Reiner, Z.; Catapano, A.L.; De Backer, G.; Graham, I.; Taskinen, M.-R.; Wiklund, O.; Agewall, S.; Alegria, E.; Chapman, M.J.; Durrington, P.; et al. ESC/EAS Guidelines for the management of dyslipidaemias: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur. Heart J. 2011, 32, 1769–1818. [Google Scholar]
- Hamer, M.; O’Donovan, G.; Stamatakis, E. High-Density Lipoprotein Cholesterol and Mortality: Too Much of a Good Thing? Arterioscler. Thromb. Vasc. Biol. 2018, 38, 669–672. [Google Scholar] [CrossRef]
- Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [CrossRef]
- Farrer, S. Beyond Statins: Emerging Evidence for HDL-Increasing Therapies and Diet in Treating Cardiovascular Disease. Adv. Prev. Med. 2018, 2018, 6024747. [Google Scholar] [CrossRef]

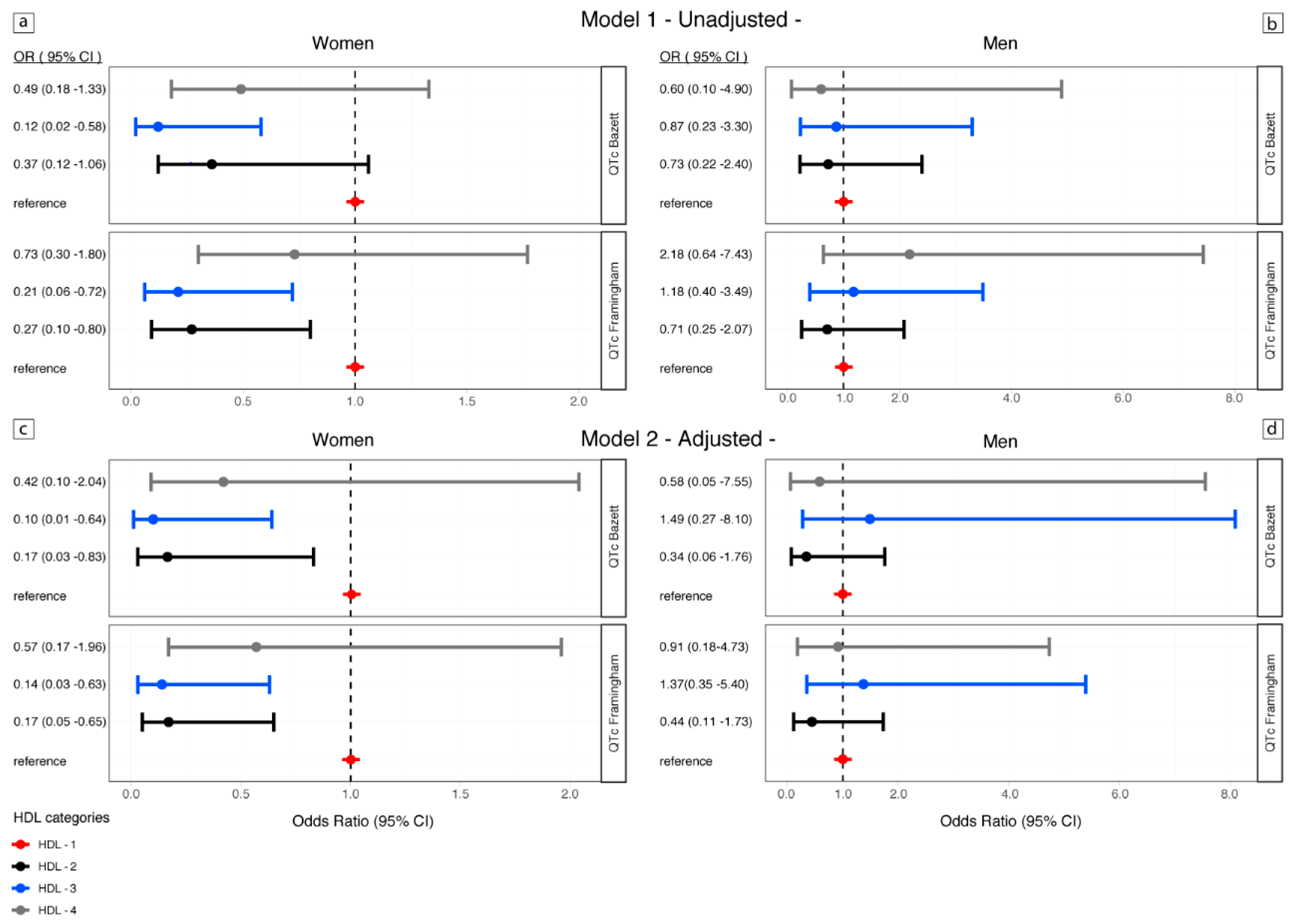
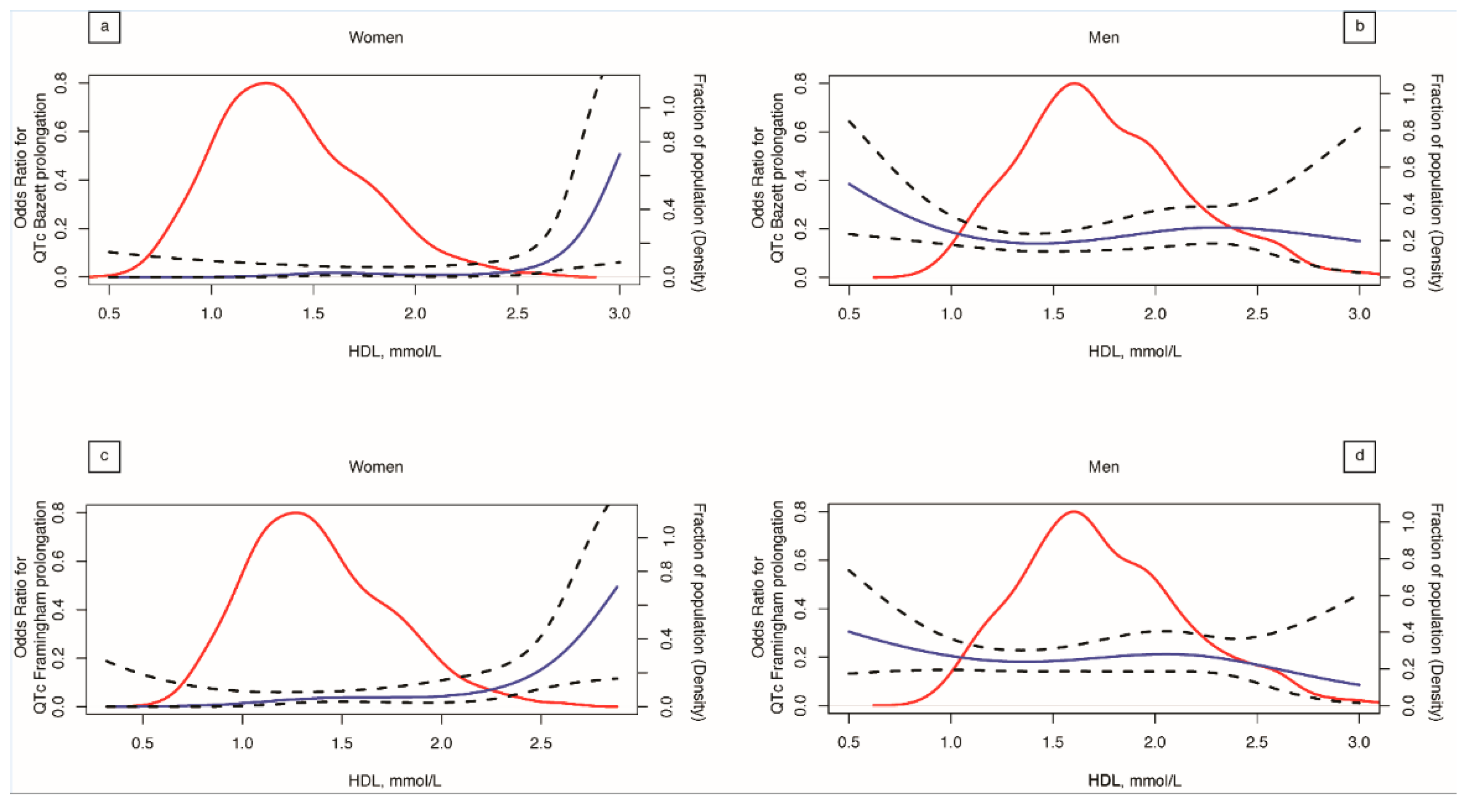
| Clinical Characteristics | Number or Median | (25th–75th Percentile) or % |
|---|---|---|
| Age, years | 51 | 42–59 |
| Gender, Females | 619 | 57.1 (%) |
| Weight, Kg | 70 | 59–81 |
| Height, cm | 168 | 162–176 |
| BMI, Kg/m2 | 24 | 22–27 |
| Waist/Hip, cm | 0.91 | 0.86–0.95 |
| Systolic Blood Pressure in-office, mmHg | 129 | 119–140 |
| Diastolic Blood Pressure in-office, mmHg | 80 | 74–88 |
| Medical History | ||
| Smoking | 217 | 20 (%) |
| Family history of CVD | 265 | 24 (%) |
| Hypercholesterolemia | 128 | 12 (%) |
| Hypertension | 144 | 13 (%) |
| Diabetes mellitus | 18 | 2 (%) |
| Statin therapy | 130 | 13 (%) |
| Laboratory Characteristics | ||
| Total Cholesterol, mmol/L | 5.3 | 4.6–6.0 |
| LDL, mmol/L | 3.5 | 2.9–4.2 |
| HDL, mmol/L | 1.6 | 1.3–1.9 |
| HDL, <25th percentile (<1.3 mmol/L) | 339 | 31 (%) |
| HDL, 25th to 50th percentile (1.4–1.6 mmol/L) | 304 | 28 (%) |
| HDL, 50th to 75th percentile (1.7–1.9 mmol/L) | 231 | 21 (%) |
| HDL, >75th percentile (>2.0 mmol/L) | 209 | 19 (%) |
| Triglycerides, mmol/L | 0.90 | 0.7–1.3 |
| Magnesium, mmol/L | 0.83 | 0.83–0.87 |
| Calcium, mmol/L | 1.21 | 1.18–1.22 |
| Potassium, mmol/24 h | 35 | 26–49 |
| Sodium, mmol/L/24 h | 165 | 115–233 |
| Albumin, mg/24 h | 25.2 | 21.1-28.9 |
| Creatinin, μmol/L | 16.5 | 11.2–23.5 |
| Glomerular filtration rate, mL/min/1.73 m² (CKD-EPI creatinine equation) | 96.1 | 85.7–105.8 |
| Blood Urea Nitrogen, mmol/L | 354 | 278–445 |
| Cystatin, mg/L | 0.80 | 0.73–0.89 |
| Hemoglobin A1c, (%) | 5.3 | 5.1–5.5 |
| Glycemia, mmol/L | 5.8 | 5.5–6.2 |
| Ambulatory Blood Pressure Monitoring | ||
| Systolic Blood Pressure, mmHg/24 h | 117 | 111–126 |
| Diastolic Blood Pressure, mmHg/24 h | 73 | 68–80 |
| Heart Rate, beats/min/24 h | 67 | 65–75 |
| Pulse Wave Velocity, m/sec/24/h | 6.9 | 5.93–8.0 |
| ECG Variables | ||
| Heart Rate, beats/min | 66 | 59–73 |
| PR Interval, ms | 152 | 140–168 |
| QRS Interval, ms | 86 | 80–94 |
| QT, ms | 406 | 390–426 |
| QTcFram, ms | 420 | 408–432 |
| QTcBazz, ms | 427 | 413–441 |
| Women | Men | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 Unadjusted | Model 2 Adjusted | Model 1 Unadjusted | Model 2 Adjusted | |||||||||
| QTcBazz, ms | β-coef | SE | p-value | β-coef | SE | p-value | β-coef | SE | p-value | β-coef | SE | p-value |
| HDL, <25th percentile (≤1.3 mmol/L) | Reference | Reference | ||||||||||
| HDL, 25th to 50th percentile (1.4–1.6 mmol/L) | –11.306 | 4.625 | 0.016 | –11.697 | 4.333 | <0.001 | –2.047 | 4.297 | 0.635 | 1.197 | 4.025 | 0.625 |
| HDL, 50th to 75th percentile (1.7–1.9 mmol/L) | –12.347 | 4.875 | 0.012 | –11.786 | 4.719 | 0.014 | –8.450 | 5.098 | 0.100 | –1.133 | 5.039 | 0.792 |
| HDL, >75th percentile (≥2.0 mmol/L) | –5.047 | 4.658 | 0.280 | –4.937 | 4.626 | 0.288 | 3.162 | 7.343 | 0.667 | 10.447 | 6.805 | 0.128 |
| QTcFram, ms | ||||||||||||
| HDL, <25th percentile (≤1.3 mmol/L) | Reference | Reference | ||||||||||
| HDL, 25th to 50th percentile (1.4–1.6 mmol/L) | –10.186 | 4.016 | 0.012 | –10.908 | 4.151 | 0.010 | –0.440 | 3.759 | 0.907 | 2.007 | 3.967 | 0.614 |
| HDL, 50th to 75th percentile (1.7–1.9 mmol/L) | –12.048 | 4.233 | <0.001 | –11.002 | 4.521 | 0.016 | –3.423 | 4.459 | 0.444 | –1.298 | 4.966 | 0.794 |
| HDL, > 75 th percentile (≥2.0 mmol/L) | –5.985 | 4.045 | 0.141 | –4.368 | 4.432 | 0.326 | 14.072 | 6.423 | 0.030 | 10.644 | 6.076 | 0.116 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Giorno, R.; Gabutti, S.; Troiani, C.; Stefanelli, K.; Falciano, R.; Graziano, E.; Rochat Negro, T.; Gabutti, L. Association Between HDL Cholesterol and QTc Interval: A Population-Based Epidemiological Study. J. Clin. Med. 2019, 8, 1527. https://doi.org/10.3390/jcm8101527
Del Giorno R, Gabutti S, Troiani C, Stefanelli K, Falciano R, Graziano E, Rochat Negro T, Gabutti L. Association Between HDL Cholesterol and QTc Interval: A Population-Based Epidemiological Study. Journal of Clinical Medicine. 2019; 8(10):1527. https://doi.org/10.3390/jcm8101527
Chicago/Turabian StyleDel Giorno, Rosaria, Sofia Gabutti, Chiara Troiani, Kevyn Stefanelli, Raffaele Falciano, Elisa Graziano, Tommaso Rochat Negro, and Luca Gabutti. 2019. "Association Between HDL Cholesterol and QTc Interval: A Population-Based Epidemiological Study" Journal of Clinical Medicine 8, no. 10: 1527. https://doi.org/10.3390/jcm8101527
APA StyleDel Giorno, R., Gabutti, S., Troiani, C., Stefanelli, K., Falciano, R., Graziano, E., Rochat Negro, T., & Gabutti, L. (2019). Association Between HDL Cholesterol and QTc Interval: A Population-Based Epidemiological Study. Journal of Clinical Medicine, 8(10), 1527. https://doi.org/10.3390/jcm8101527





