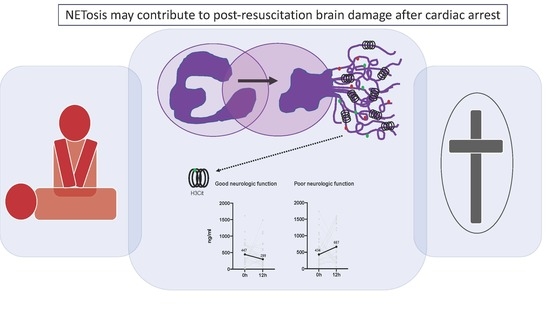
Increased Citrullinated Histone H3 Levels in the Early Post-Resuscitative Period Are Associated with Poor Neurologic Function in Cardiac Arrest Survivors—A Prospective Observational Study
Abstract
:1. Introduction
2. Methods
2.1. Blood Sampling
2.2. Laboratory Analysis of NET Related Biomarker
2.3. Statistical Methods
3. Results
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lilja, G.; Nielsen, N.; Friberg, H.; Horn, J.; Kjaergaard, J.; Nilsson, F.; Pellis, T.; Wetterslev, J.; Wise, M.P.; Bosch, F.; et al. Cognitive function in survivors of out-of-hospital cardiac arrest after target temperature management at 33 degrees C versus 36 degrees C. Circulation 2015, 131, 1340–1349. [Google Scholar] [CrossRef] [PubMed]
- Sugita, A.; Kinoshita, K.; Sakurai, A.; Chiba, N.; Yamaguchi, J.; Kuwana, T.; Sawada, N.; Hori, S. Systemic impact on secondary brain aggravation due to ischemia/reperfusion injury in post-cardiac arrest syndrome: A prospective observational study using high-mobility group box 1 protein. Crit. Care 2017, 21, 247. [Google Scholar] [CrossRef] [PubMed]
- Mongardon, N.; Dumas, F.; Ricome, S.; Grimaldi, D.; Hissem, T.; Pene, F.; Cariou, A. Postcardiac arrest syndrome: From immediate resuscitation to long-term outcome. Ann. Intensive Care 2011, 1, 45. [Google Scholar] [CrossRef] [PubMed]
- Wada, T. Coagulofibrinolytic Changes in Patients with Post-cardiac Arrest Syndrome. Front. Med. (Lausanne) 2017, 4, 156. [Google Scholar] [CrossRef]
- Cho, Y.D.; Park, S.J.; Choi, S.H.; Yoon, Y.H.; Kim, J.Y.; Lee, S.W.; Lim, C.S. The inflammatory response of neutrophils in an in vitro model that approximates the postcardiac arrest state. Ann. Surg. Treat. Res. 2017, 93, 217–224. [Google Scholar] [CrossRef]
- Weiser, C.; Schwameis, M.; Sterz, F.; Herkner, H.; Lang, I.M.; Schwarzinger, I.; Spiel, A.O. Mortality in patients resuscitated from out-of-hospital cardiac arrest based on automated blood cell count and neutrophil lymphocyte ratio at admission. Resuscitation 2017, 116, 49–55. [Google Scholar] [CrossRef]
- Patel, V.H.; Vendittelli, P.; Garg, R.; Szpunar, S.; LaLonde, T.; Lee, J.; Rosman, H.; Mehta, R.H.; Othman, H. Neutrophil-lymphocyte ratio: A prognostic tool in patients with in-hospital cardiac arrest. World J. Crit. Care Med. 2019, 8, 9–17. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, K.N.; Kim, S.H.; Lee, B.K.; Oh, S.H.; Moon, H.K.; Jeung, K.W.; Choi, S.P.; Cho, I.S.; Youn, C.S. Association between the neutrophil-to-lymphocyte ratio and neurological outcomes in patients undergoing targeted temperature management after cardiac arrest. J. Crit. Care 2018, 47, 227–231. [Google Scholar] [CrossRef]
- Boeltz, S.; Amini, P.; Anders, H.J.; Andrade, F.; Bilyy, R.; Chatfield, S.; Cichon, I.; Clancy, D.M.; Desai, J.; Dumych, T.; et al. To NET or not to NET:current opinions and state of the science regarding the formation of neutrophil extracellular traps. Cell Death Differ. 2019, 26, 395–408. [Google Scholar] [CrossRef] [Green Version]
- Kimball, A.S.; Obi, A.T.; Diaz, J.A.; Henke, P.K. The Emerging Role of NETs in Venous Thrombosis and Immunothrombosis. Front. Immunol. 2016, 7, 236. [Google Scholar] [CrossRef] [Green Version]
- Mangold, A.; Alias, S.; Scherz, T.; Hofbauer, T.; Jakowitsch, J.; Panzenbock, A.; Simon, D.; Laimer, D.; Bangert, C.; Kammerlander, A.; et al. Coronary neutrophil extracellular trap burden and deoxyribonuclease activity in ST-elevation acute coronary syndrome are predictors of ST-segment resolution and infarct size. Circ. Res. 2015, 116, 1182–1192. [Google Scholar] [CrossRef] [PubMed]
- Mauracher, L.M.; Posch, F.; Martinod, K.; Grilz, E.; Daullary, T.; Hell, L.; Brostjan, C.; Zielinski, C.; Ay, C.; Wagner, D.D.; et al. Citrullinated histone H3, a biomarker of neutrophil extracellular trap formation, predicts the risk of venous thromboembolism in cancer patients. J. Thromb. Haemost. 2018, 16, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018, 18, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Pertiwi, K.R.; van der Wal, A.C.; Pabittei, D.R.; Mackaaij, C.; van Leeuwen, M.B.; Li, X.; de Boer, O.J. Neutrophil Extracellular Traps Participate in All Different Types of Thrombotic and Haemorrhagic Complications of Coronary Atherosclerosis. Thromb. Haemost. 2018, 118, 1078–1087. [Google Scholar] [CrossRef]
- Yipp, B.G.; Petri, B.; Salina, D.; Jenne, C.N.; Scott, B.N.; Zbytnuik, L.D.; Pittman, K.; Asaduzzaman, M.; Wu, K.; Meijndert, H.C.; et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat. Med. 2012, 18, 1386–1393. [Google Scholar] [CrossRef] [Green Version]
- Fuchs, T.A.; Abed, U.; Goosmann, C.; Hurwitz, R.; Schulze, I.; Wahn, V.; Weinrauch, Y.; Brinkmann, V.; Zychlinsky, A. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007, 176, 231–241. [Google Scholar] [CrossRef]
- Ge, L.; Zhou, X.; Ji, W.J.; Lu, R.Y.; Zhang, Y.; Zhang, Y.D.; Ma, Y.Q.; Zhao, J.H.; Li, Y.M. Neutrophil extracellular traps in ischemia-reperfusion injury-induced myocardial no-reflow: Therapeutic potential of DNase-based reperfusion strategy. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H500–H509. [Google Scholar] [CrossRef]
- Gould, T.J.; Lysov, Z.; Liaw, P.C. Extracellular DNA and histones: Double-edged swords in immunothrombosis. J. Thromb. Haemost. 2015, 13, S82–S91. [Google Scholar] [CrossRef]
- Noubouossie, D.F.; Whelihan, M.F.; Yu, Y.B.; Sparkenbaugh, E.; Pawlinski, R.; Monroe, D.M.; Key, N.S. In vitro activation of coagulation by human neutrophil DNA and histone proteins but not neutrophil extracellular traps. Blood 2017, 129, 1021–1029. [Google Scholar] [CrossRef]
- Silk, E.; Zhao, H.; Weng, H.; Ma, D. The role of extracellular histone in organ injury. Cell Death Dis. 2017, 8, e2812. [Google Scholar] [CrossRef]
- Jorch, S.K.; Kubes, P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat. Med. 2017, 23, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, M.; Lindberg, M.R.; Kennett, M.J.; Xiong, N.; Wang, Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J. Exp. Med. 2010, 207, 1853–1862. [Google Scholar] [CrossRef]
- Nolan, J.P.; Hazinski, M.F.; Aickin, R.; Bhanji, F.; Billi, J.E.; Callaway, C.W.; Castren, M.; de Caen, A.R.; Ferrer, J.M.; Finn, J.C.; et al. Part 1: Executive summary: 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Resuscitation 2015, 95, e1–e31. [Google Scholar] [CrossRef]
- Edgren, E.; Hedstrand, U.; Kelsey, S.; Sutton-Tyrrell, K.; Safar, P. Assessment of neurological prognosis in comatose survivors of cardiac arrest. BRCT I Study Group. Lancet 1994, 343, 1055–1059. [Google Scholar] [CrossRef]
- Cuzick, J. A Wilcoxon-type test for trend. Stat. Med. 1985, 4, 87–90. [Google Scholar] [CrossRef]
- Peberdy, M.A.; Andersen, L.W.; Abbate, A.; Thacker, L.R.; Gaieski, D.; Abella, B.S.; Grossestreuer, A.V.; Rittenberger, J.C.; Clore, J.; Ornato, J.; et al. Inflammatory markers following resuscitation from out-of-hospital cardiac arrest-A prospective multicenter observational study. Resuscitation 2016, 103, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Bro-Jeppesen, J.; Kjaergaard, J.; Wanscher, M.; Nielsen, N.; Friberg, H.; Bjerre, M.; Hassager, C. The inflammatory response after out-of-hospital cardiac arrest is not modified by targeted temperature management at 33 degrees C or 36 degrees C. Resuscitation 2014, 85, 1480–1487. [Google Scholar] [CrossRef] [PubMed]
- Bro-Jeppesen, J.; Kjaergaard, J.; Stammet, P.; Wise, M.P.; Hovdenes, J.; Aneman, A.; Horn, J.; Devaux, Y.; Erlinge, D.; Gasche, Y.; et al. Predictive value of interleukin-6 in post-cardiac arrest patients treated with targeted temperature management at 33 degrees C or 36 degrees C. Resuscitation 2016, 98, 1–8. [Google Scholar] [CrossRef]
- Arnalich, F.; Menendez, M.; Lagos, V.; Ciria, E.; Quesada, A.; Codoceo, R.; Vazquez, J.J.; Lopez-Collazo, E.; Montiel, C. Prognostic value of cell-free plasma DNA in patients with cardiac arrest outside the hospital: An observational cohort study. Crit. Care 2010, 14, R47. [Google Scholar] [CrossRef]
- Grilz, E.; Mauracher, L.M.; Posch, F.; Konigsbrugge, O.; Zochbauer-Muller, S.; Marosi, C.; Lang, I.; Pabinger, I.; Ay, C. Citrullinated histone H3, a biomarker for neutrophil extracellular trap formation, predicts the risk of mortality in patients with cancer. Br. J. Haematol. 2019, 186, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Sulzgruber, P.; Sterz, F.; Schober, A.; Uray, T.; Van Tulder, R.; Hubner, P.; Wallmuller, C.; El-Tattan, D.; Graf, N.; Ruzicka, G.; et al. Editor’s Choice-Progress in the chain of survival and its impact on outcomes of patients admitted to a specialized high-volume cardiac arrest center during the past two decades. Eur. Heart J. Acute Cardiovasc. Care 2016, 5, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Arrich, J.; Holzer, M.; Havel, C.; Mullner, M.; Herkner, H. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database Syst. Rev. 2016, 2, CD004128. [Google Scholar] [CrossRef] [PubMed]
- Adrie, C.; Monchi, M.; Laurent, I.; Um, S.; Yan, S.B.; Thuong, M.; Cariou, A.; Charpentier, J.; Dhainaut, J.F. Coagulopathy after successful cardiopulmonary resuscitation following cardiac arrest: Implication of the protein C anticoagulant pathway. J. Am. Coll. Cardiol. 2005, 46, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Buchtele, N.; Schober, A.; Schoergenhofer, C.; Spiel, A.O.; Mauracher, L.; Weiser, C.; Sterz, F.; Jilma, B.; Schwameis, M. Added value of the DIC score and of D-dimer to predict outcome after successfully resuscitated out-of-hospital cardiac arrest. Eur. J. Intern. Med. 2018, 57, 44–48. [Google Scholar] [CrossRef] [Green Version]
- Tadie, J.M.; Bae, H.B.; Jiang, S.; Park, D.W.; Bell, C.P.; Yang, H.; Pittet, J.F.; Tracey, K.; Thannickal, V.J.; Abraham, E.; et al. HMGB1 promotes neutrophil extracellular trap formation through interactions with Toll-like receptor 4. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 304, L342–L349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, I.; Fukazawa, J.; Yoshida, M. Post-translational methylation of high mobility group box 1 (HMGB1) causes its cytoplasmic localization in neutrophils. J. Biol. Chem. 2007, 282, 16336–16344. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Li, M.; Huang, K.; Zhou, S.; Hu, Y.; Pan, S.; Gu, Y. HMGB1 binding heptamer peptide improves survival and ameliorates brain injury in rats after cardiac arrest and cardiopulmonary resuscitation. Neuroscience 2017, 360, 128–138. [Google Scholar] [CrossRef]
- Lewis, H.D.; Liddle, J.; Coote, J.E.; Atkinson, S.J.; Barker, M.D.; Bax, B.D.; Bicker, K.L.; Bingham, R.P.; Campbell, M.; Chen, Y.H.; et al. Inhibition of PAD4 activity is sufficient to disrupt mouse and human NET formation. Nat. Chem. Biol. 2015, 11, 189–191. [Google Scholar] [CrossRef]
- Martinod, K.; Demers, M.; Fuchs, T.A.; Wong, S.L.; Brill, A.; Gallant, M.; Hu, J.; Wang, Y.; Wagner, D.D. Neutrophil histone modification by peptidylarginine deiminase 4 is critical for deep vein thrombosis in mice. Proc. Natl. Acad. Sci. USA 2013, 110, 8674–8679. [Google Scholar] [CrossRef] [Green Version]
- Martinod, K.; Wagner, D.D. Thrombosis: Tangled up in NETs. Blood 2014, 123, 2768–2776. [Google Scholar] [CrossRef]


| Variable | Total (n = 62) | Good Function CPC 1–2 (n = 30) | Poor Function CPC 3–5 (n = 32) | p-Value |
|---|---|---|---|---|
| Male sex | 49 (79) | 25 (83) | 24 (75) | 0.421 |
| Age, years | 57 (46–67) | 52 (44–61) | 61 (53–70) | 0.030 * |
| Cause of cardiac arrest | 0.338 | |||
| Acute coronary syndrome | 44 (71) | 23 (77) | 21 (66) | |
| Primary arrhythmia | 18 (29) | 7 (23) | 11 (34) | |
| PCI with stenting | 42 (68) | 22 (73) | 20 (63) | 0.362 |
| Resuscitation characteristics | ||||
| CPC prior to cardiac arrest | 1.0 | |||
| CPC 1 | 61 (98) | 30 (100) | 31 (95) | |
| CPC 2 | 1 (2) | 0 | 1 (5) | |
| Location of cardiac arrest | 0.290 | |||
| Place of residence | 35 (57) | 19 (63) | 16 (50) | |
| Public place | 27 (43) | 11 (37) | 16 (50) | |
| Witnessed | 54 (87) | 28 (93) | 26 (81) | 0.258 |
| Basic life support | 44 (71) | 23 (77) | 21 (66) | 0.338 |
| Shockable rhythm | 46 (77) | 27 (93) | 19 (61) | 0.004 * |
| Administration of heparin by EMS | 28 (45.2) | 14 (47) | 14 (44) | 0.795 |
| Epinephrine, mg | 3 (1–4) | 1 (0–4) | 3 (2–5) | 0.004 * |
| Down time, min | 29 (19–47) | 23 (11–36) | 38 (24–50) | 0.011 * |
| Temp at admission, °C | 35.3 (34.8–35.7) | 35.4 (35.0–35.6) | 35.1 (34.7–36) | 0.676 |
| Time from collapse to blood sampling, min | 60 (49–72) | 65 (52–87) | 56 (43–65) | 0.033 * |
| Laboratory values | ||||
| Lactate, mmol/L 0 h | 7 (5–10) | 6 (3–7) | 10 (6–12) | 0.001 * |
| D-dimer, µg/mL 0 h | 8 (3–17) | 4 (2–8) | 14 (8–21) | 0.003 * |
| D-dimer, µg/mL 12 h | 4 (2–6) | 2 (1–4) | 6 (3–8) | 0.009 * |
| Aptt, s 0 h | 47 (36–121) | 48 (33–129) | 46 (37–119) | 0.444 |
| Aptt, s 12 h | 37 (34–42) | 37 (34–42) | 38 (34–45) | 0.487 |
| Prothrombin time, % 0 h | 79 (67–61) | 79 (64–88) | 78 (71–91) | 0.418 |
| Prothrombin time, % 12 h | 77 (66–87) | 80 (68–88) | 76 (63–86) | 0.549 |
| Fibrinogen, mg/dL 0 h | 290 (242–322) | 297 (246–317) | 283 (240–343) | 0.983 |
| Fibrinogen, mg/dL 12 h | 297 (258–350) | 295 (260–346) | 308 (241–359) | 0.502 |
| Platelet count, G/L 0 h | 204 (163–245) | 204 (163–235) | 204 (164–251) | 0.972 |
| Platelet count, G/L 12h | 193 (141–240) | 193 (141–239) | 193 (150–252) | 0.490 |
| CRP, mg/dL 0 h | 0.2 (0.1–0.6) | 0.2 (0.1–0.4) | 0.3 (0.1–0.7) | 0.410 |
| CRP, mg/dL 12 h | 1.6 (0.7–3.1) | 1.0 (0.3–2.8) | 1.7 (1.0–3.3) | 0.057 |
| Neutrophils 0 h, G/L | 8.5 (6.2–12.8) | 7.8 (6.0–12.8) | 9.6 (6.4–13.2) | 0.443 |
| Neutrophils 12 h, G/L | 10.9 (8.5–14.6) | 9.9 (7.7–12.2) | 11.8 (8.8–16) | 0.051 |
| NLR 0 h | 2.5 (1.4–4.4) | 2.6 (1.4–4.7) | 2.4 (1.3–3.8) | 0.375 |
| NLR 12 h | 10.3 (6.1–14.8) | 6.8 (5.7–11.5) | 11.6 (7–18) | 0.046 * |
| cfDNA 0h, ng/mL | 1481 (948–2176) | 1197 (835–1544) | 1898 (1148–2377) | 0.007 * |
| cfDNA 12 h, ng/mL | 555 (436–721) | 489 (404–634) | 593 (516–807) | 0.016 * |
| Nucleosomes 0 h, MoM | 4.4 (2.4–7.1) | 3.8 (1.6–4.9) | 5.6 (2.8–9.7) | 0.032 * |
| Nucleosomes 12 h, MoM | 0.7 (0.3–1.8) | 0.4 (0.2–1.1) | 1.1 (0.5–2.4) | 0.036 * |
| H3Cit 0 h, ng/mL | 447 (228–772) | 447 (229–744) | 434 (205–899) | 0.755 |
| H3Cit 12 h, ng/mL | 386 (207–968) | 299 (146–789) | 667 (300–1201) | 0.047 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mauracher, L.-M.; Buchtele, N.; Schörgenhofer, C.; Weiser, C.; Herkner, H.; Merrelaar, A.; Spiel, A.O.; Hell, L.; Ay, C.; Pabinger, I.; et al. Increased Citrullinated Histone H3 Levels in the Early Post-Resuscitative Period Are Associated with Poor Neurologic Function in Cardiac Arrest Survivors—A Prospective Observational Study. J. Clin. Med. 2019, 8, 1568. https://doi.org/10.3390/jcm8101568
Mauracher L-M, Buchtele N, Schörgenhofer C, Weiser C, Herkner H, Merrelaar A, Spiel AO, Hell L, Ay C, Pabinger I, et al. Increased Citrullinated Histone H3 Levels in the Early Post-Resuscitative Period Are Associated with Poor Neurologic Function in Cardiac Arrest Survivors—A Prospective Observational Study. Journal of Clinical Medicine. 2019; 8(10):1568. https://doi.org/10.3390/jcm8101568
Chicago/Turabian StyleMauracher, Lisa-Marie, Nina Buchtele, Christian Schörgenhofer, Christoph Weiser, Harald Herkner, Anne Merrelaar, Alexander O. Spiel, Lena Hell, Cihan Ay, Ingrid Pabinger, and et al. 2019. "Increased Citrullinated Histone H3 Levels in the Early Post-Resuscitative Period Are Associated with Poor Neurologic Function in Cardiac Arrest Survivors—A Prospective Observational Study" Journal of Clinical Medicine 8, no. 10: 1568. https://doi.org/10.3390/jcm8101568
APA StyleMauracher, L.-M., Buchtele, N., Schörgenhofer, C., Weiser, C., Herkner, H., Merrelaar, A., Spiel, A. O., Hell, L., Ay, C., Pabinger, I., Jilma, B., & Schwameis, M. (2019). Increased Citrullinated Histone H3 Levels in the Early Post-Resuscitative Period Are Associated with Poor Neurologic Function in Cardiac Arrest Survivors—A Prospective Observational Study. Journal of Clinical Medicine, 8(10), 1568. https://doi.org/10.3390/jcm8101568