Tissue-Specific miRNAs Regulate the Development of Thoracic Aortic Aneurysm: The Emerging Role of KLF4 Network
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Samples
2.2. Study Design
3. Results
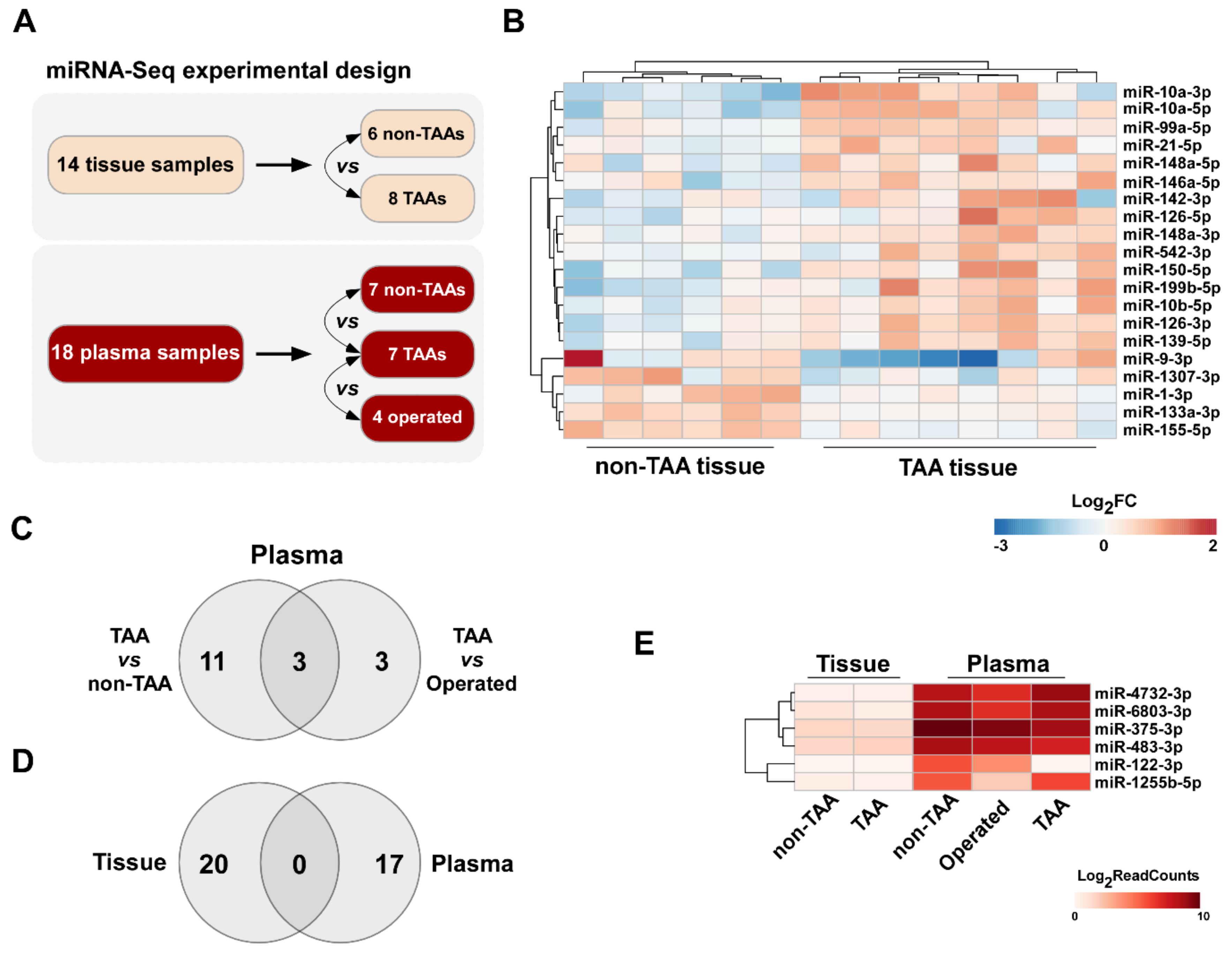
3.1. Differential miRNA Expression Analysis in TAA Tissue and Blood Plasma Samples
3.2. Validation of Selected miRNAs in TAA Tissue and Plasma Samples by qRT-PCR
3.3. Functional Analysis of miRNA Target Genes Involved in TAA Development
3.4. Number of VSMCs Expressing KLF4 Dramatically Increases during TAA Development
4. Discussion
4.1. miRNA Expression Patterns in Tissues May Be Influenced by Aneurysmal Location and Sex
4.2. Circulating miRNA Profile Does Not Match to Aneurysmal Signature of TAA Tissues
4.3. miRNA Target Analysis Reveals KLF4 As a Key Factor for the TAA Development in vivo
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kuzmik, G.A.; Sang, A.X.; Elefteriades, J.A. Natural history of thoracic aortic aneurysms. J. Vasc. Surg. 2012, 56, 565–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elefteriades, J.A.; Farkas, E.A. Thoracic Aortic Aneurysm Clinically Pertinent Controversies and Uncertainties. J. Am. Coll. Cardiol. 2010, 55, 841–857. [Google Scholar] [CrossRef] [PubMed]
- Quintana, R.A.; Taylor, W.R. Cellular Mechanisms of Aortic Aneurysm Formation. Circ. Res. 2019, 124, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Olsson, C.; Thelin, S.; Ståhle, E.; Ekbom, A.; Granath, F. Thoracic Aortic Aneurysm and Dissection. Circulation 2006, 114, 2611–2618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Allmen, R.S.; Anjum, A.; Powell, J.T. Incidence of Descending Aortic Pathology and Evaluation of the Impact of Thoracic Endovascular Aortic Repair: A Population-based Study in England and Wales from 1999 to 2010. Eur. J. Vasc. Endovasc. Surg. 2013, 45, 154–159. [Google Scholar] [CrossRef] [Green Version]
- Cheung, K.; Boodhwani, M.; Chan, K.L.; Beauchesne, L.; Dick, A.; Coutinho, T. Thoracic Aortic Aneurysm Growth: Role of Sex and Aneurysm Etiology. J. Am. Heart Assoc. 2017, 6, e003792. [Google Scholar] [CrossRef]
- Nicolini, F.; Vezzani, A.; Corradi, F.; Gherli, R.; Benassi, F.; Manca, T.; Gherli, T. Gender differences in outcomes after aortic aneurysm surgery should foster further research to improve screening and prevention programmes. Eur. J. Prev. Cardiol. 2018, 25, 32–41. [Google Scholar] [CrossRef]
- Owens, G.K. Regulation of Differentiation of Vascular Smooth-Muscle Cells. Physiol. Rev. 1995, 75, 487–517. [Google Scholar] [CrossRef]
- Owens, G.K.; Kumar, M.S.; Wamhoff, B.R. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol. Rev. 2004, 84, 767–801. [Google Scholar] [CrossRef]
- Wang, Z.G.; Wang, D.Z.; Pipes, G.C.T.; Olson, E.N. Myocardin is a master regulator of smooth muscle gene expression. Proc. Natl. Acad. Sci. USA 2003, 100, 7129–7134. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Sinha, S.; McDonald, O.G.; Shang, Y.T.; Hoofnagle, M.H.; Owens, G.K. Kruppel-like factor 4 abrogates myocardin-induced activation of smooth muscle gene expression. J. Biol. Chem. 2005, 280, 9719–9727. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.F.; Yang, X.H.; Friesel, R.E.; Vary, C.P.H.; Liaw, L. Mechanisms of TGF-beta-Induced Differentiation in Human Vascular Smooth Muscle Cells. J. Vasc. Res. 2011, 48, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Chuang, J.C.; Jones, P.A. Epigenetics and microRNAs. Pediatr. Res. 2007, 61, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Small, E.M.; Frost, R.J.A.; Olson, E.N. MicroRNAs Add a New Dimension to Cardiovascular Disease. Circulation 2010, 121, 1022–1032. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.S.; Jin, J.P.; Wang, J.Q.; Zhang, Z.G.; Freedman, J.H.; Zheng, Y.; Cai, L. miRNAS in cardiovascular diseases: Potential biomarkers, therapeutic targets and challenges. Acta Pharmacol. Sin. 2018, 39, 1073–1084. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.F.; Li, G.H. Role of Specific MicroRNAs in Regulation of Vascular Smooth Muscle Cell Differentiation and the Response to Injury. J. Cardiovasc. Transl. Res. 2010, 3, 246–250. [Google Scholar] [CrossRef] [Green Version]
- Merlet, E.; Atassi, F.; Motiani, R.K.; Mougenot, N.; Jacquet, A.; Nadaud, S.; Capiod, T.; Trebak, M.; Lompre, A.M.; Marchand, A. miR-424/322 regulates vascular smooth muscle cell phenotype and neointimal formation in the rat. Cardiovasc. Res. 2013, 98, 458–468. [Google Scholar] [CrossRef] [Green Version]
- Dong, N.N.; Wang, W.; Tian, J.W.; Xie, Z.L.; Lv, B.; Dai, J.N.; Jiang, R.; Huang, D.; Fang, S.H.; Tian, J.T.; et al. MicroRNA-182 prevents vascular smooth muscle cell dedifferentiation via FGF9/PDGFR signaling. Int. J. Mol. Med. 2017, 39, 791–798. [Google Scholar] [CrossRef]
- Jones, J.A.; Stroud, R.E.; O’Quinn, E.C.; Black, L.E.; Barth, J.L.; Elefteriades, J.A.; Bavaria, J.E.; Gorman, J.H.; Gorman, R.C.; Spinale, F.G.; et al. Selective MicroRNA Suppression in Human Thoracic Aneurysms Relationship of miR-29a to Aortic Size and Proteolytic Induction. Circ-Cardiovasc. Genet. 2011, 4, 605–613. [Google Scholar] [CrossRef]
- Licholai, S.; Blaz, M.; Kapelak, B.; Sanak, M. Unbiased Profile of MicroRNA Expression in Ascending Aortic Aneurysm Tissue Appoints Molecular Pathways Contributing to the Pathology. Ann. Thorac. Surg. 2016, 102, 1245–1252. [Google Scholar] [CrossRef] [Green Version]
- Boileau, A.; Lindsay, M.E.; Michel, J.B.; Devaux, Y. Epigenetics in Ascending Thoracic Aortic Aneurysm and Dissection. Aorta 2018, 6, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moushi, A.; Michailidou, K.; Soteriou, M.; Cariolou, M.; Bashiardes, E. MicroRNAs as possible biomarkers for screening of aortic aneurysms: A systematic review and validation study. Biomarkers 2018, 23, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Raffort, J.; Lareyre, F.; Clement, M.; Mallat, Z. Micro-RNAs in abdominal aortic aneurysms: Insights from animal models and relevance to human disease. Cardiovasc. Res. 2016, 110, 165–177. [Google Scholar] [CrossRef]
- Butkytė, S.; Čiupas, L.; Jakubauskienė, E.; Vilys, L.; Mocevicius, P.; Kanopka, A.; Vilkaitis, G. Splicing-dependent expression of microRNAs of mirtron origin in human digestive and excretory system cancer cells. Clin. Epigenet. 2016, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Torella, D.; Iaconetti, C.; Catalucci, D.; Ellison Georgina, M.; Leone, A.; Waring Cheryl, D.; Bochicchio, A.; Vicinanza, C.; Aquila, I.; Curcio, A.; et al. MicroRNA-133 Controls Vascular Smooth Muscle Cell Phenotypic Switch In Vitro and Vascular Remodeling In Vivo. Circ. Res. 2011, 109, 880–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonas, S.; Izaurralde, E. Towards a molecular understanding of microRNA-mediated gene silencing. Nat. Rev. Genet. 2015, 16, 421. [Google Scholar] [CrossRef] [PubMed]
- Huusko, T.; Salonurmi, T.; Taskinen, P.; Liinamaa, J.; Juvonen, T.; Paakko, P.; Savolainen, M.; Kakko, S. Elevated messenger RNA expression and plasma protein levels of osteopontin and matrix metalloproteinase types 2 and 9 in patients with ascending aortic aneurysms. J. Thorac. Cardiovasc. Surg. 2013, 145, 1117–1123. [Google Scholar] [CrossRef] [Green Version]
- Lesauskaite, V.; Tanganelli, P.; Sassi, C.; Neri, E.; Diciolla, F.; Ivanoviene, L.; Epistolato, M.C.; Lalinga, A.V.; Alessandrini, C.; Spina, D. Smooth muscle cells of the media in the dilatative pathology of ascending thoracic aorta: Morphology, immunoreactivity for osteopontin, matrix metalloproteinases, and their inhibitors. Hum. Pathol. 2001, 32, 1003–1011. [Google Scholar] [CrossRef]
- Spin, J.M.; Li, D.Y.; Maegdefessel, L.; Tsao, P.S. Non-coding RNAs in aneurysmal aortopathy. Vascul. Pharmacol. 2018, 114, 110–121. [Google Scholar] [CrossRef]
- Li, Y.H.; Maegdefessel, L. Non-coding RNA Contribution to Thoracic and Abdominal Aortic Aneurysm Disease Development and Progression. Front. Physiol. 2017, 8, 429. [Google Scholar] [CrossRef]
- Ruddy, J.M.; Jones, J.A.; Spinale, F.G.; Ikonomidis, J.S. Regional heterogeneity within the aorta: Relevance to aneurysm disease. J. Thorac. Cardiovasc. Surg. 2008, 136, 1123–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkatesh, P.; Phillippi, J.; Chukkapalli, S.; Rivera-Kweh, M.; Velsko, I.; Gleason, T.; VanRyzin, P.; Aalaei-Andabili, S.H.; Ghanta, R.K.; Beaver, T.; et al. Aneurysm-Specific miR-221 and miR-146a Participates in Human Thoracic and Abdominal Aortic Aneurysms. Int. J. Mol. Sci. 2017, 18, 875. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liang, K.; Zou, G.; Chen, X.; Shi, S.; Wang, G.; Zhang, K.; Li, K.; Zhai, S. Inhibition of miR-155 attenuates abdominal aortic aneurysm in mice by regulating macrophage-mediated inflammation. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef] [PubMed]
- Welten, S.M.J.; Goossens, E.A.C.; Quax, P.H.A.; Nossent, A.Y. The multifactorial nature of microRNAs in vascular remodelling. Cardiovasc. Res. 2016, 110, 6–22. [Google Scholar] [CrossRef] [PubMed]
- Deaton, R.A.; Gan, Q.; Owens, G.K. Sp1-dependent activation of KLF4 is required for PDGF-BB-induced phenotypic modulation of smooth muscle. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H1027–H1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.R.; Xie, C.Q.; Sun, X.; Ritchie, R.P.; Zhang, J.F.; Chen, Y.E. miR-10a Contributes to Retinoid Acid-induced Smooth Muscle Cell Differentiation. J. Biol. Chem. 2010, 285, 9383–9389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sokolis, D.P.; Iliopoulos, D.C. Impaired mechanics and matrix metalloproteinases/inhibitors expression in female ascending thoracic aortic aneurysms. J. Mech. Behav. Biomed. 2014, 34, 154–164. [Google Scholar] [CrossRef]
- Guay, C.; Regazzi, R. Exosomes as new players in metabolic organ cross-talk. Diabetes Obes. Metab. 2017, 19, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Xu, Y.; Hao, J.; Wang, S.; Li, C.; Meng, S. MiR-122 in hepatic function and liver diseases. Protein Cell 2012, 3, 364–371. [Google Scholar] [CrossRef] [Green Version]
- Bandiera, S.; Pfeffer, S.; Baumert, T.F.; Zeisel, M.B. miR-122–A key factor and therapeutic target in liver disease. J. Hepatol. 2015, 62, 448–457. [Google Scholar] [CrossRef]
- Olijhoek, J.K.; van der Graaf, Y.; Banga, J.-D.; Algra, A.; Rabelink, T.J.; Visseren, F.L. The Metabolic Syndrome is associated with advanced vascular damage in patients with coronary heart disease, stroke, peripheral arterial disease or abdominal aortic aneurysm. Eur. Heart J. 2004, 25, 342–348. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandra, Y.; Devanna, P.; Limana, F.; Straino, S.; Di Carlo, A.; Brambilla, P.G.; Rubino, M.; Carena, M.C.; Spazzafumo, L.; De Simone, M.; et al. Circulating microRNAs are new and sensitive biomarkers of myocardial infarction. Eur. Heart J. 2010, 31, 2765–2773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devaux, Y.; Salgado-Somoza, A.; Dankiewicz, J.; Boileau, A.; Stammet, P.; Schritz, A.; Zhang, L.; Vausort, M.; Gilje, P.; Erlinge, D.; et al. Incremental Value of Circulating MiR-122-5p to Predict Outcome after Out of Hospital Cardiac Arrest. Theranostics 2017, 7, 2555–2564. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Micaelo, N.; Beltrán-Debón, R.; Baiges, I.; Faiges, M.; Alegret, J.M. Specific circulating microRNA signature of bicuspid aortic valve disease. J. Transl. Med. 2017, 15, 76. [Google Scholar] [CrossRef] [PubMed]
- Kraenkel, N.; Kuschnerus, K.; Briand, S.; Luescher, T.F.; Landmesser, U. miR-483 impairs endothelial homeostasis and response to vascular injury: Upregulation by high-glucose and in patients with type-2 diabetes. Eur. Heart J. 2013, 34, 762. [Google Scholar] [CrossRef]
- Takeda, N.; Hara, H.; Fujiwara, T.; Kanaya, T.; Maemura, S.; Komuro, I. TGF-β Signaling-Related Genes and Thoracic Aortic Aneurysms and Dissections. Int. J. Mol. Sci. 2018, 19, 2125. [Google Scholar] [CrossRef]
- Zhang, P.; Hou, S.Y.; Chen, J.C.; Zhang, J.S.; Lin, F.Y.; Ju, R.J.; Cheng, X.; Ma, X.W.; Song, Y.; Zhang, Y.Y.; et al. Smad4 Deficiency in Smooth Muscle Cells Initiates the Formation of Aortic Aneurysm. Circ. Res. 2016, 118, 388–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Li, Q.L.; Jiao, Y.; Qin, L.F.; Ali, R.; Zhou, J.; Ferruzzi, J.; Kim, R.W.; Geirsson, A.; Dietz, H.C.; et al. Tgfbr2 disruption in postnatal smooth muscle impairs aortic wall homeostasis. J. Clin. Investig. 2014, 124, 755–767. [Google Scholar] [CrossRef]
- Gomez, D.; Coyet, A.; Ollivier, V.; Jeunemaitre, X.; Jondeau, G.; Michel, J.B.; Vranckx, R. Epigenetic control of vascular smooth muscle cells in Marfan and non-Marfan thoracic aortic aneurysms. Cardiovasc. Res. 2011, 89, 446–456. [Google Scholar] [CrossRef]
- Jones, J.A.; Barbour, J.R.; Stroud, R.E.; Bouges, S.; Stephens, S.L.; Spinale, F.G.; Ikonomidis, J.S. Altered Transforming Growth Factor-Beta Signaling in a Murine Model of Thoracic Aortic Aneurysm. J. Vasc. Res. 2008, 45, 457–468. [Google Scholar] [CrossRef] [Green Version]
- Leeper, N.J.; Raiesdana, A.; Kojima, Y.; Chun, H.J.; Azuma, J.; Maegdefessel, L.; Kundu, R.K.; Quertermous, T.; Tsao, P.S.; Spin, J.M. MicroRNA-26a is a novel regulator of vascular smooth muscle cell function. J. Cell. Physiol. 2011, 226, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Frismantiene, A.; Philippova, M.; Erne, P.; Resink, T.J. Smooth muscle cell-driven vascular diseases and molecular mechanisms of VSMC plasticity. Cell. Signal. 2018, 52, 48–64. [Google Scholar] [CrossRef] [PubMed]
- Salmon, M.; Johnston, W.F.; Woo, A.; Pope, N.H.; Su, G.; Upchurch, G.R.; Owens, G.K.; Ailawadi, G. KLF4 Regulates Abdominal Aortic Aneurysm Morphology and Deletion Attenuates Aneurysm Formation. Circulation 2013, 128, S163–S174. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Tissue | Plasma | ||||
|---|---|---|---|---|---|
| Variables | non-TAA (n = 6) | TAA (n = 8) | non-TAA (n = 7) | TAA (n = 7) | Operated (n = 4) |
| Age, years ± SD | 47 ± 5 | 62 ± 10 | 54 ± 12 | 63 ± 11 | 64 ± 12 |
| Sex, male (%) | 4 (67 %) | 6 (75 %) | 4 (57 %) | 5 (71 %) | 3 (75 %) |
| Ascending aortic diameter, mm | 36 ± 0.7 * | 50 ± 3 | 35 ± 3 | 53 ± 5 | 52 ± 4 |
| Aortic valve stenosis (%) | 0 (0 %) | 3 (38 %) | 1 (14 %) | 2 (29 %) | 1 (25 %) |
| Bicuspid aortic valve (%) | 0 (0 %) | 5 (63 %) | 0 (0 %) | 4 (57 %) | 2 (50 %) |
| Aortic valve insufficiency (%) | 0 (0 %) | 5 (63 %) | 1 (14 %) | 3 (43 %) | 1 (25 %) |
| Hypertension (%) | 2 (100 %) * | 7 (88 %) | 4 (57 %) | 6 (86 %) | 4 (100 %) |
| Smokers (%) | 2 (100 %) * | 1 (13 %) | 1 (14 %) | 1 (14 %) | 0 (0 %) |
| Diabetes (%) | 0 (0 %) | 1 (13 %) | 0 (0%) | 3 (43 %) | 1 (25 %) |
| Groups | Number of miRNAs | Upregulated | Downregulated |
|---|---|---|---|
| Tissue | |||
| TAA vs. non-TAA | 20 | 15 | 5 |
| Plasma | |||
| TAA vs. non-TAA | 14 | 3 | 11 |
| TAA v.s Op | 6 | 4 | 2 |
| TAA vs. non-TAA + Op | 10 | 2 | 8 |
| No. | miRNAs | Fold Change | p Value |
|---|---|---|---|
| Upregulated | |||
| 1 | hsa-miR-10a-3p | 2.69 | 2.05E–06 |
| 2 | hsa-miR-10a-5p | 2.45 | 8.63E–07 |
| 3 | hsa-miR-150-5p | 2.21 | 2.05E–05 |
| 4 | hsa-miR-199b-5p | 2.12 | 1.19E–04 |
| 5 | hsa-miR-126-5p | 1.89 | 7.95E–04 |
| 6 | hsa-miR-126-3p | 1,88 | 2.10E–05 |
| 7 | hsa-miR-139-5p | 1.74 | 7.22E–04 |
| 8 | hsa-miR-148a-3p | 1.71 | 3.44E–05 |
| 9 | hsa-miR-10b-5p | 1.70 | 7.78E–04 |
| 10 | hsa-miR-148a-5p | 1.70 | 0.0112 |
| 11 | hsa-miR-99a-5p | 1.68 | 1.76E–05 |
| 12 | hsa-miR-21-5p | 1.67 | 1.10E–03 |
| 13 | hsa-miR-146a-5p | 1.67 | 0.002 |
| 14 | hsa-miR-142-3p | 1.66 | 0.020 |
| 15 | hsa-miR-542-3p | 1.64 | 0.009 |
| Downregulated | |||
| 16 | hsa-miR-1-3p | −1.59 | 0.001 |
| 17 | hsa-miR-133a-3p | −1.64 | 2.96E–07 |
| 18 | hsa-miR-1307-3p | −1.68 | 0.011 |
| 19 | hsa-miR-9-3p | −1.79 | 0.021 |
| 20 | hsa-miR-155-5p | −1.88 | 7.34E−08 |
| Group | No. | miRNA | Regulation | Fold Change | p Value |
|---|---|---|---|---|---|
| TAA vs. non-TAA | 1 | hsa-miR-146b-3p | up | 9.11 | 0.044 |
| 2 | hsa-miR-1255b-5p | up | 8.87 | 0.015 | |
| 3 | hsa-miR-889-3p | up | 7.95 | 0.047 | |
| 4 | hsa-miR-375-3p | down | –2.38 | 0.036 | |
| 5 | hsa–miR-30a-5p | down | –2.54 | 0.033 | |
| 6 | hsa-miR-483-3p | down | –2.68 | 0.015 | |
| 7 | hsa-miR-23b-3p | down | –2.79 | 0.017 | |
| 8 | hsa-miR-140-3p | down | –4.01 | 0.010 | |
| 9 | hsa-miR-100-5p | down | –9.17 | 0.003 | |
| 10 | hsa-miR-145-5p | down | –17.36 | 1.44E–04 | |
| 11 | hsa-miR-143-3p | down | –17.74 | 3.27E–05 | |
| 12 | hsa–miR-23b-5p | down | –24.93 | 0.013 | |
| 13 | hsa-miR-122-3p | down | –69.32 | 3.31E–04 | |
| 14 | hsa-miR-34a-5p | down | –71.95 | 4.01E–05 | |
| TAA vs. Operated | 1 | hsa-miR-1255b-5p | up | 9.7203 | 0.045 |
| 2 | hsa-miR-4732-3p | up | 3.9801 | 0.050 | |
| 3 | hsa-miR-6803-3p | up | 3.4495 | 0.011 | |
| 4 | hsa-miR-22-3p | up | 2.5198 | 0.029 | |
| 5 | hsa-miR-122-3p | down | –18.4085 | 0.024 | |
| 6 | hsa-miR-23b-5p | down | –44.7992 | 0.001 | |
| TAA vs. non-TAA & Operated | 1 | hsa-miR-1255b-5p | up | 11.68 | 0.004 |
| 2 | hsa-miR-22-3p | up | 1.73 | 0.034 | |
| 3 | hsa-miR-375-3p | down | –2.12 | 0.049 | |
| 4 | hsa-miR-483-3p | down | –2.29 | 0.035 | |
| 5 | hsa-miR-23b-3p | down | –2.36 | 0.024 | |
| 6 | hsa-miR-143-3p | down | –3.83 | 0.012 | |
| 7 | hsa-miR-145-5p | down | –4.83 | 0.019 | |
| 8 | hsa-miR-23b-5p | down | –29.67 | 0.003 | |
| 9 | hsa-miR-34a-5p | down | –48.62 | 6.26E–05 | |
| 10 | hsa-miR-122-3p | down | –53.67 | 2.31E–04 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gasiulė, S.; Stankevičius, V.; Patamsytė, V.; Ražanskas, R.; Žukovas, G.; Kapustina, Ž.; Žaliaduonytė, D.; Benetis, R.; Lesauskaitė, V.; Vilkaitis, G. Tissue-Specific miRNAs Regulate the Development of Thoracic Aortic Aneurysm: The Emerging Role of KLF4 Network. J. Clin. Med. 2019, 8, 1609. https://doi.org/10.3390/jcm8101609
Gasiulė S, Stankevičius V, Patamsytė V, Ražanskas R, Žukovas G, Kapustina Ž, Žaliaduonytė D, Benetis R, Lesauskaitė V, Vilkaitis G. Tissue-Specific miRNAs Regulate the Development of Thoracic Aortic Aneurysm: The Emerging Role of KLF4 Network. Journal of Clinical Medicine. 2019; 8(10):1609. https://doi.org/10.3390/jcm8101609
Chicago/Turabian StyleGasiulė, Stasė, Vaidotas Stankevičius, Vaiva Patamsytė, Raimundas Ražanskas, Giedrius Žukovas, Žana Kapustina, Diana Žaliaduonytė, Rimantas Benetis, Vaiva Lesauskaitė, and Giedrius Vilkaitis. 2019. "Tissue-Specific miRNAs Regulate the Development of Thoracic Aortic Aneurysm: The Emerging Role of KLF4 Network" Journal of Clinical Medicine 8, no. 10: 1609. https://doi.org/10.3390/jcm8101609
APA StyleGasiulė, S., Stankevičius, V., Patamsytė, V., Ražanskas, R., Žukovas, G., Kapustina, Ž., Žaliaduonytė, D., Benetis, R., Lesauskaitė, V., & Vilkaitis, G. (2019). Tissue-Specific miRNAs Regulate the Development of Thoracic Aortic Aneurysm: The Emerging Role of KLF4 Network. Journal of Clinical Medicine, 8(10), 1609. https://doi.org/10.3390/jcm8101609





