Left Ventricular Assist Devices 101: Shared Care for General Cardiologists and Primary Care
Abstract
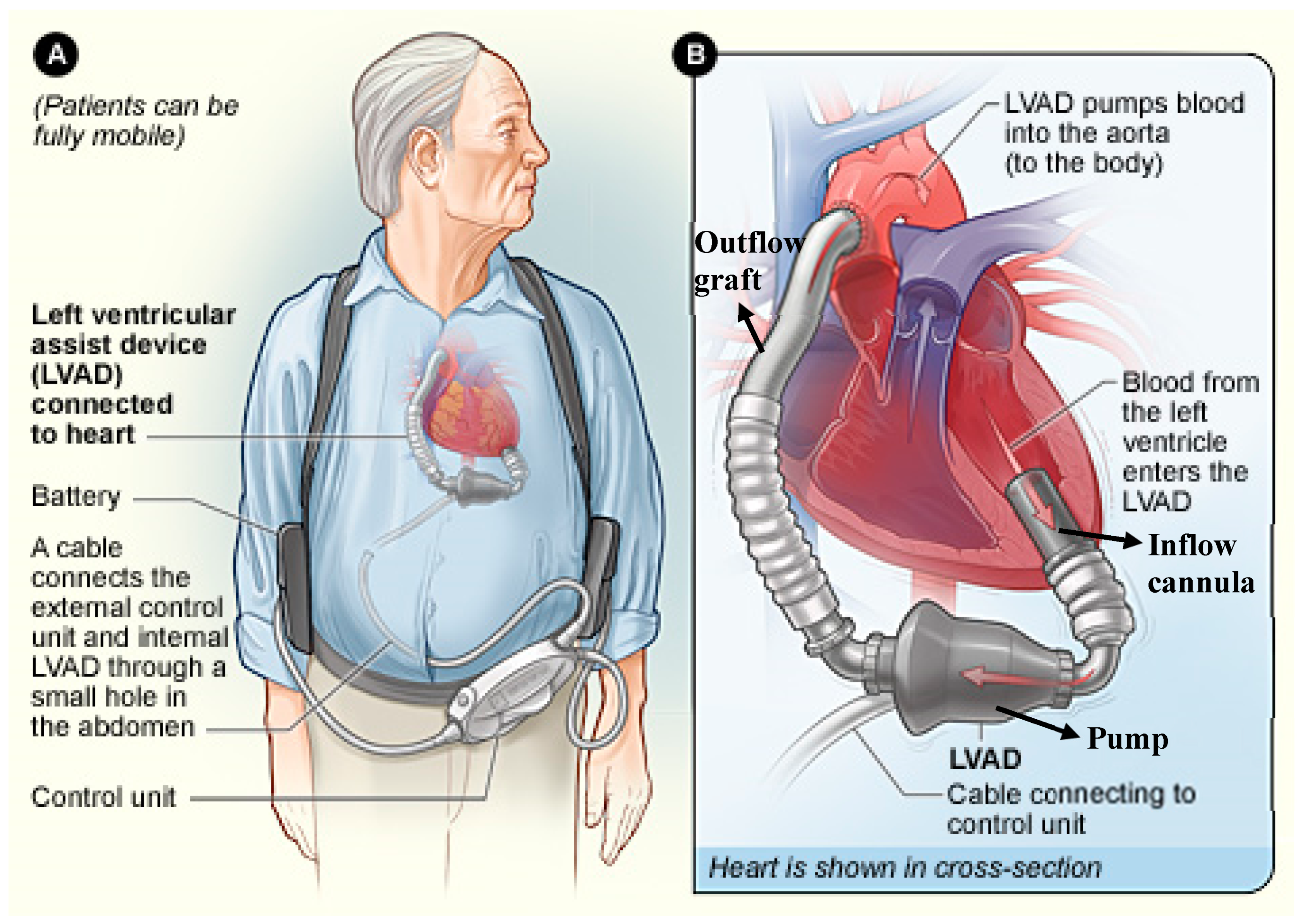
:1. Introduction
Evolution of LVAD Therapy
2. Engineering and Pump Technology
Design and Function
3. Patient Selection and Outcomes
- LVEF < 25%
- NYHA IIIb–IV symptoms for at least 45 of the last 60 days
- Refractory heart failure symptoms despite optimal medical and device therapy
- Peak VO2 < 14 mL/kg/min
- Continued need for IV inotropic therapy due to symptomatic hypotension, worsening end organ function, or persistent pulmonary edema
- IV inotropic medication use for ≥14 days
- Intra-aortic balloon pump support for ≥7 days
5. Long-Term Management
5.1. Patient Assessment
5.1.1. History and Physical Examination
5.1.2. Electrocardiogram
5.2. Device Interrogation
5.2.1. HeartMate II and HeartMate 3
- Hazard alarm: Flashing red heart or battery
- “Low flow”The display will state to call the hospital contact. This warning suggests that the pump flow is estimated to be <2.5 Lpm. Ensure the driveline is intact, connections are secure, the controller is connected to a power source and the batteries have enough charge if the patient is on batteries. Assess the patient for causes of low flow such as hypertension (particularly in the case of HeartMate 3), significant volume loss, right-sided heart failure and pump thrombosis.
- Advisory: Flashing yellow wrench or diamond
- This warning suggests that there is a mechanical, electrical or software issue with the system.
5.2.2. Heart Ware
- High priority: Flashing red triangle
- “VAD stopped”The display will state to connect the driveline or change the controller. Ensure the driveline is intact, connections are secure, the controller is connected to a power source and the batteries have enough charge if the patient is on batteries. Assess for other causes of VAD dysfunction, including pump thrombosis.
- “Critical battery”It indicates less than 5 min of battery remaining. Immediately replace the existing batteries with fully charged ones, or switch to the Power Module.
- Medium priority: Flashing yellow triangle
- “High watt”The display will state to call for medical assistance. This suggests that the pump has exceeded the high power alarm threshold. This could be due to a variety of causes, such as pump thrombosis, or an electrical malfunction and requires evaluation by a VAD specialist.
- Low priority: Solid yellow triangle
5.3. Diagnostic Laboratory and Imaging Tests
5.4. Medical Management
5.4.1. Heart failure therapy
5.4.2. Hypertension
5.5. Device Therapy
5.6. Antithrombotic Therapy
6. Lifestyle Recommendations
7. Procedures for Patients on LVAD Therapy
8. Complications of LVADs
8.1. LVAD Infections
8.2. Gastrointestinal (GI) Bleed
8.3. Ventricular Arrhythmias
8.4. LVAD Malfunction
8.5. Pump Thrombosis
8.6. Neurological Emergencies
8.7. Heart Failure
9. Emergency Care
Unresponsive Patients and Cardiopulmonary Resuscitation
- Assess responsiveness. If there is no response, call for help.
- Check if the patient is breathing and assist ventilation as needed with supplemental oxygen, airway adjuncts and intubation, as deemed necessary. End-tidal CO2 should be monitored.
- Determine if adequate perfusion is being maintained by assessing mental status, skin color, temperature and capillary refill. It is important to remember that LVAD supported patients may not have a palpable pulse or recognizable BP with an automatic machine, even with adequate perfusion.
- Assess if the LVAD is working by auscultating for an LVAD hum over the precordium. If there is a mechanical hum, then the device is likely working effectively. Ensure connections to the controller are secure, and ensure power supply is adequate.
- If the VAD is not functioning, and MAP is ≤ 50 mmHg, then chest compressions and rescue breaths should be initiated per the basic life support protocol. If a connection issue is identified, then the connection should be reestablished, provided the period of LVAD discontinuation is known to be less than 30 minutes. If there has been a prolonged period of LVAD discontinuation, thrombus formation is likely and restarting the device could result in a fatal embolism.
- If the LVAD is not functioning, and no connection problems are identified, a system controller change-out may be required. This should only be done by a trained provider or caregiver. Family members are trained to make this controller change.
- If the LVAD is still not functioning, chest compressions should be continued, defibrillation and medications should be administered per the advanced cardiac life support algorithms. Temporary mechanical support, including extracorporeal membrane oxygenation may be needed.
- If the LVAD is functioning, but the patient is unconscious with MAP ≤ 50 mmHg or PETCO2 < 20 mmHg, external chest compressions are indicated. If the LVAD is functioning, only gentle compressions are required, as only RV compressions are needed (the LVAD will continue to provide systemic flow). With an arterial line, the compressions can be adjusted as needed to maintain a perfusing arterial pressure. Overly vigorous chest compressions should be avoided, particularly in the early postoperative phase, as there is risk of dislodging the device and causing myocardial injury and hemorrhage. Defibrillation and medications should be administered per the advanced cardiac life support algorithms.
- If the LVAD is functioning and both respirations and perfusion appear adequate and the patient is unconscious, assess for causes of unconsciousness like stroke, coma (e.g., hypo- or hyperglycemia), or sedation.
- It is reasonable to provide standard post-cardiac arrest care, including targeted temperature management and early percutaneous coronary intervention, when indicated. Of note, patients with an LVAD need adequate anticoagulation, which may be difficult to monitor during therapeutic hypothermia.
10. Conclusions
Author Contributions
Conflicts of Interest
References
- Ahn, S.A.; Jong, P.; Yusuf, S.; Bangdiwala, S.I.; Pouleur, H.G.; Rousseau, M.F. Early versus delayed enalapril in patients with left ventricular systolic dysfunction: Impact on morbidity and mortality 15 years after the SOLVD trial. J. Am. Coll. Cardiol. 2006, 47, 1904–1905. [Google Scholar] [CrossRef]
- Otterstad, J.E.; Ford, I. The effect of carvedilol in patients with impaired left ventricular systolic function following an acute myocardial infarction. How do the treatment effects on total mortality and recurrent myocardial infarction in CAPRICORN compare with previous beta-blocker trials? Eur. J. Heart Fail. 2002, 4, 501–506. [Google Scholar]
- Breithardt, G. MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy): Cardiac resynchronization therapy towards early management of heart failure. Eur. Heart J. 2009, 30, 2551–2553. [Google Scholar] [CrossRef]
- Bleumink, G.S.; Knetsch, A.M.; Sturkenboom, M.C.; Straus, S.M.; Hofman, A.; Deckers, J.W.; Witteman, J.C.; Stricker, B.H. Quantifying the heart failure epidemic: Prevalence, incidence rate, lifetime risk and prognosis of heart failure The Rotterdam Study. Eur. Heart J. 2004, 25, 1614–1619. [Google Scholar] [CrossRef]
- Levy, W.C.; Mozaffarian, D.; Linker, D.T.; Farrar, D.J.; Miller, L.W.; Investigators, R. Can the Seattle heart failure model be used to risk-stratify heart failure patients for potential left ventricular assist device therapy? J. Heart Lung Transpl. 2009, 28, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Rose, E.A.; Gelijns, A.C.; Moskowitz, A.J.; Heitjan, D.F.; Stevenson, L.W.; Dembitsky, W.; Long, J.W.; Ascheim, D.D.; Tierney, A.R.; Levitan, R.G.; et al. Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure Study, Long-term use of a left ventricular assist device for end-stage heart failure. N. Engl. J. Med. 2001, 345, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Colvin, M.; Smith, J.M.; Skeans, M.A.; Edwards, L.B.; Uccellini, K.; Snyder, J.J.; Israni, A.K.; Kasiske, B.L. OPTN/SRTR 2015 Annual Data Report: Heart. Am. J. Transpl. 2017, 17 (Suppl. 1), 286–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stehlik, J.; Edwards, L.B.; Kucheryavaya, A.Y.; Benden, C.; Christie, J.D.; Dobbels, F.; Kirk, R.; Rahmel, A.O.; Hertz, M.I. The Registry of the International Society for Heart and Lung Transplantation: Twenty-eighth Adult Heart Transplant Report—2011. J. Heart Lung Transpl. 2011, 30, 1078–1094. [Google Scholar] [CrossRef] [PubMed]
- Zimpfer, D.; Zrunek, P.; Sandner, S.; Schima, H.; Grimm, M.; Zuckermann, A.; Wolner, E.; Wieselthaler, G. Post-transplant survival after lowering fixed pulmonary hypertension using left ventricular assist devices. Eur. J. Cardiothorac. Surg. 2007, 31, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Kormos, R.L.; Cowger, J.; Pagani, F.D.; Teuteberg, J.J.; Goldstein, D.J.; Jacobs, J.P.; Higgins, R.S.; Stevenson, L.W.; Stehlik, J.; Atluri, P.; et al. The Society of Thoracic Surgeons Intermacs database annual report: Evolving indications, outcomes, and scientific partnerships. J. Heart Lung Transpl. 2019, 38, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Liotta, D.; Hall, C.W.; Henly, W.S.; Cooley, D.A.; Crawford, E.S.; Debakey, M.E. Prolonged Assisted Circulation during and after Cardiac or Aortic Surgery. Prolonged Partial Left Ventricular Bypass by Means of Intracorporeal Circulation. Am. J. Cardiol. 1963, 12, 399–405. [Google Scholar] [CrossRef]
- Giridharan, G.A.; Lee, T.J.; Ising, M.; Sobieski, M.A.; Koenig, S.C.; Gray, L.A.; Slaughter, M.S. Miniaturization of mechanical circulatory support systems. Artif. Organs 2012, 36, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Fukamachi, K.; Golding, L.; Moazami, N.; Starling, R.C. Left ventricular assist devices: From the bench to the clinic. Cardiology 2013, 125, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Giridharan, G.A.; Koenig, S.C.; Soucy, K.G.; Choi, Y.; Pirbodaghi, T.; Bartoli, C.R.; Monreal, G.; Sobieski, M.A.; Schumer, E.; Cheng, A.; et al. Left ventricular volume unloading with axial and centrifugal rotary blood pumps. ASAIO J. 2015, 61, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Mehra, M.R.; Naka, Y.; Uriel, N.; Goldstein, D.J.; Cleveland, J.C., Jr.; Colombo, P.C.; Walsh, M.N.; Milano, C.A.; Patel, C.B.; Jorde, U.P.; et al. A Fully Magnetically Levitated Circulatory Pump for Advanced Heart Failure. N. Engl. J. Med. 2017, 376, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Saeed, D.; Westenfeld, R.; Maxhera, B.; Keymel, S.; Sherif, A.; Sadat, N.; Petrov, G.; Albert, A.; Lichtenberg, A. Prevalence of De Novo Aortic Valve Insufficiency in Patients After HeartWare VAD Implantation with an Intermittent Low-Speed Algorithm. ASAIO J. 2016, 62, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.A.; Rauf, A.; Stoker, S.; Alharethi, R.; Kfoury, A.G. Marital status and survival in left ventricular assist device patient populations. J. Heart Lung Transpl. 2015, 34, 619–621. [Google Scholar] [CrossRef] [PubMed]
- Kormos, R.L.; Cowger, J.; Pagani, F.D.; Teuteberg, J.J.; Goldstein, D.J.; Jacobs, J.P.; Higgins, R.S.; Stevenson, L.W.; Stehlik, J.; Atluri, P.; et al. The Society of Thoracic Surgeons Intermacs Database Annual Report: Evolving Indications, Outcomes, and Scientific Partnerships. Ann. Thorac. Surg. 2019, 107, 341–353. [Google Scholar] [CrossRef]
- Mehra, M.R.; Uriel, N.; Naka, Y.; Cleveland, J.C., Jr.; Yuzefpolskaya, M.; Salerno, C.T.; Walsh, M.N.; Milano, C.A.; Patel, C.B.; Hutchins, S.W.; et al. A Fully Magnetically Levitated Left Ventricular Assist Device-Final Report. N. Engl. J. Med. 2019, 380, 1618–1627. [Google Scholar] [CrossRef]
- Rogers, J.G.; Butler, J.; Lansman, S.L.; Gass, A.; Portner, P.M.; Pasque, M.K.; Pierson, R.N., 3rd; Investigators, I.N. Chronic mechanical circulatory support for inotrope-dependent heart failure patients who are not transplant candidates: Results of the INTrEPID Trial. J. Am. Coll. Cardiol. 2007, 50, 741–747. [Google Scholar] [CrossRef]
- Miller, L.W.; Pagani, F.D.; Russell, S.D.; John, R.; Boyle, A.J.; Aaronson, K.D.; Conte, J.V.; Naka, Y.; Mancini, D.; Delgado, R.M.; et al. Use of a continuous-flow device in patients awaiting heart transplantation. N. Engl. J. Med. 2007, 357, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, M.S.; Rogers, J.G.; Milano, C.A.; Russell, S.D.; Conte, J.V.; Feldman, D.; Sun, B.; Tatooles, A.J.; Delgado, R.M., 3rd; Long, J.W.; et al. HeartMate, Advanced heart failure treated with continuous-flow left ventricular assist device. N. Engl. J. Med. 2009, 361, 2241–2251. [Google Scholar] [CrossRef] [PubMed]
- Starling, R.C.; Naka, Y.; Boyle, A.J.; Gonzalez-Stawinski, G.; John, R.; Jorde, U.; Russell, S.D.; Conte, J.V.; Aaronson, K.D.; McGee, E.C., Jr.; et al. Results of the post-U.S. Food and Drug Administration-approval study with a continuous flow left ventricular assist device as a bridge to heart transplantation: A prospective study using the INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support). J. Am. Coll. Cardiol. 2011, 57, 1890–1898. [Google Scholar] [PubMed]
- Jorde, U.P.; Kushwaha, S.S.; Tatooles, A.J.; Naka, Y.; Bhat, G.; Long, J.W.; Horstmanshof, D.A.; Kormos, R.L.; Teuteberg, J.J.; Slaughter, M.S.; et al. Results of the destination therapy post-food and drug administration approval study with a continuous flow left ventricular assist device: A prospective study using the INTERMACS registry (Interagency Registry for Mechanically Assisted Circulatory Support). J. Am. Coll. Cardiol. 2014, 63, 1751–1757. [Google Scholar] [CrossRef]
- Aaronson, K.D.; Slaughter, M.S.; Miller, L.W.; McGee, E.C.; Cotts, W.G.; Acker, M.A.; Jessup, M.L.; Gregoric, I.D.; Loyalka, P.; Frazier, O.H.; et al. HeartWare Ventricular Assist Device Bridge to Transplant, Use of an intrapericardial, continuous-flow, centrifugal pump in patients awaiting heart transplantation. Circulation 2012, 125, 3191–3200. [Google Scholar] [CrossRef]
- Rogers, J.G.; Pagani, F.D.; Tatooles, A.J.; Bhat, G.; Slaughter, M.S.; Birks, E.J.; Boyce, S.W.; Najjar, S.S.; Jeevanandam, V.; Anderson, A.S.; et al. Intrapericardial Left Ventricular Assist Device for Advanced Heart Failure. N. Engl. J. Med. 2017, 376, 451–460. [Google Scholar] [CrossRef]
- Mehra, M.R.; Goldstein, D.J.; Uriel, N.; Cleveland, J.C., Jr.; Yuzefpolskaya, M.; Salerno, C.; Walsh, M.N.; Milano, C.A.; Patel, C.B.; Ewald, G.A.; et al. Two-Year Outcomes with a Magnetically Levitated Cardiac Pump in Heart Failure. N. Engl. J. Med. 2018, 378, 1386–1395. [Google Scholar] [CrossRef]
- Kiernan, M.S.; Joseph, S.M.; Katz, J.N.; Kilic, A.; Rich, J.D.; Tallman, M.P.; van Buren, P.; Lyons, J.J.; Bethea, B.; Eckman, P.; et al. Evolving Mechanical Support Research Group, Sharing the care of mechanical circulatory support: Collaborative efforts of patients/caregivers, shared-care sites, and left ventricular assist device implanting centers. Circ. Heart Fail. 2015, 8, 629–635. [Google Scholar] [CrossRef]
- Estep, J.D.; Trachtenberg, B.H.; Loza, L.P.; Bruckner, B.A. Continuous flow left ventricular assist devices: Shared care goals of monitoring and treating patients. Methodist Debakey Cardiovasc. J. 2015, 11, 33–44. [Google Scholar] [CrossRef]
- Bennett, M.K.; Roberts, C.A.; Dordunoo, D.; Shah, A.; Russell, S.D. Ideal methodology to assess systemic blood pressure in patients with continuous-flow left ventricular assist devices. J. Heart Lung Transpl. 2010, 29, 593–594. [Google Scholar] [CrossRef]
- Feldman, D.; Pamboukian, S.V.; Teuteberg, J.J.; Birks, E.; Lietz, K.; Moore, S.A.; Morgan, J.A.; Arabia, F.; Bauman, M.E.; Buchholz, H.W.; et al. The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: Executive summary. J. Heart Lung Transpl. 2013, 32, 157–187. [Google Scholar] [CrossRef] [PubMed]
- Tochikubo, O.; Nishijima, K.; Ohshige, K.; Kimura, K. Accuracy and applicability of the Terumo ES-H55 double-cuff sphygmomanometer for hospital use. Blood Press Monit. 2003, 8, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Lanier, G.M.; Orlanes, K.; Hayashi, Y.; Murphy, J.; Flannery, M.; Te-Frey, R.; Uriel, N.; Yuzefpolskaya, M.; Mancini, D.M.; Naka, Y.; et al. Validity and reliability of a novel slow cuff-deflation system for noninvasive blood pressure monitoring in patients with continuous-flow left ventricular assist device. Circ. Heart Fail. 2013, 6, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Milano, C.A.; Rogers, J.G.; Tatooles, A.J.; Bhat, G.; Slaughter, M.S.; Birks, E.J.; Mokadam, N.A.; Mahr, C.; Miller, J.S.; Markham, D.W.; et al. HVAD: The ENDURANCE Supplemental Trial. JACC Heart Fail. 2018, 6, 792–802. [Google Scholar] [CrossRef]
- Shah, P.; Mehta, V.M.; Cowger, J.A.; Aaronson, K.D.; Pagani, F.D. Diagnosis of hemolysis and device thrombosis with lactate dehydrogenase during left ventricular assist device support. J Heart Lung Transpl. 2014, 33, 102–104. [Google Scholar] [CrossRef]
- Uriel, N.; Morrison, K.A.; Garan, A.R.; Kato, T.S.; Yuzefpolskaya, M.; Latif, F.; Restaino, S.W.; Mancini, D.M.; Flannery, M.; Takayama, H.; et al. Development of a novel echocardiography ramp test for speed optimization and diagnosis of device thrombosis in continuous-flow left ventricular assist devices: The Columbia ramp study. J. Am. Coll. Cardiol. 2012, 60, 1764–1775. [Google Scholar] [CrossRef]
- Stainback, R.F.; Estep, J.D.; Agler, D.A.; Birks, E.J.; Bremer, M.; Hung, J.; Kirkpatrick, J.N.; Rogers, J.G.; Shah, N.R. American Society of, Echocardiography in the Management of Patients with Left Ventricular Assist Devices: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2015, 28, 853–909. [Google Scholar] [CrossRef]
- Vivo, R.P.; Kassi, M.; Estep, J.D.; Bhimaraj, A.; Trachtenberg, B.H.; Orrego, C.M.; Loebe, M.; Bruckner, B.A.; Nabi, F.; Mahmarian, J.J.; et al. MDCT assessment of mechanical circulatory support device complications. JACC Cardiovasc. Imaging 2015, 8, 100–102. [Google Scholar] [CrossRef]
- Akin, S.; Muslem, R.; Constantinescu, A.A.; Manintveld, O.C.; Birim, O.; Brugts, J.J.; Maat, A.; Froberg, A.C.; Bogers, A.; Caliskan, K. 18F-FDG PET/CT in the Diagnosis and Management of Continuous Flow Left Ventricular Assist Device Infections: A Case Series and Review of the Literature. ASAIO J. 2018, 64, e11–e19. [Google Scholar] [CrossRef]
- Clerkin, K.J.; Topkara, V.K.; Mancini, D.M.; Yuzefpolskaya, M.; Demmer, R.T.; Dizon, J.M.; Takeda, K.; Takayama, H.; Naka, Y.; Colombo, P.C.; et al. The role of implantable cardioverter defibrillators in patients bridged to transplantation with a continuous-flow left ventricular assist device: A propensity score matched analysis. J Heart Lung Transpl. 2017, 36, 633–639. [Google Scholar] [CrossRef]
- Garan, A.R.; Yuzefpolskaya, M.; Colombo, P.C.; Morrow, J.P.; Te-Frey, R.; Dano, D.; Takayama, H.; Naka, Y.; Garan, H.; Jorde, U.P.; et al. Ventricular arrhythmias and implantable cardioverter-defibrillator therapy in patients with continuous-flow left ventricular assist devices: Need for primary prevention? J. Am. Coll. Cardiol. 2013, 61, 2542–2550. [Google Scholar] [CrossRef] [PubMed]
- Epstein, A.E.; DiMarco, J.P.; Ellenbogen, K.A.; Estes, N.A., 3rd; Freedman, R.A.; Gettes, L.S.; Gillinov, A.M.; Gregoratos, G.; Hammill, S.C.; Hayes, D.L.; et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 2008, 51, e1–e62. [Google Scholar] [PubMed]
- Gopinathannair, R.; Roukoz, H.; Bhan, A.; Ravichandran, A.; Ahmed, M.M.; Familtsev, D.; Bhat, G.; Cowger, J.; Abdullah, M.; Sandesara, C.; et al. Cardiac Resynchronization Therapy and Clinical Outcomes in Continuous Flow Left Ventricular Assist Device Recipients. J. Am. Heart Assoc. 2018, 7, e009091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veenis, J.F.; Manintveld, O.C.; Constantinescu, A.A.; Caliskan, K.; Birim, O.; Bekkers, J.A.; van Mieghem, N.M.; den Uil, C.A.; Boersma, E.; Lenzen, M.J.; et al. Design and rationale of haemodynamic guidance with CardioMEMS in patients with a left ventricular assist device: The HEMO-VAD pilot study. ESC Heart Fail. 2019, 6, 194–201. [Google Scholar] [CrossRef]
- ClinicalTrials.gov [Internet]. Investigation to Optimize Hemodynamic Management of Left Ventricular Assist Devices Using the CardioMEMS™ (Intellect2); Identifier: NCT03247829; National Library of Medicine (US): Bethesda, MD, USA. Available online: https://clinicaltrials.gov/ct2/show/NCT03247829 (accessed on 14 August 2017).
- Feldman, D.S.; Moazami, N.; Adamson, P.B.; Vierecke, J.; Raval, N.; Shreenivas, S.; Cabuay, B.M.; Jimenez, J.; Abraham, W.T.; O’Connell, J.B.; et al. The Utility of a Wireless Implantable Hemodynamic Monitoring System in Patients Requiring Mechanical Circulatory Support. ASAIO J. 2018, 64, 301–308. [Google Scholar] [CrossRef]
- Netuka, I.; Ivak, P.; Tucanova, Z.; Gregor, S.; Szarszoi, O.; Sood, P.; Crandall, D.; Rimsans, J.; Connors, J.M.; Mehra, M.R. Evaluation of low-intensity anti-coagulation with a fully magnetically levitated centrifugal-flow circulatory pump-the MAGENTUM 1 study. J. Heart Lung Transpl. 2018, 37, 579–586. [Google Scholar] [CrossRef]
- Andreas, M.; Moayedifar, R.; Wieselthaler, G.; Wolzt, M.; Riebandt, J.; Haberl, T.; Angleitner, P.; Schloglhofer, T.; Wiedemann, D.; Schima, H.; et al. Increased Thromboembolic Events With Dabigatran Compared With Vitamin K Antagonism in Left Ventricular Assist Device Patients: A Randomized Controlled Pilot Trial. Circ. Heart Fail. 2017, 10. [Google Scholar] [CrossRef]
- Bhatia, A.; Juricek, C.; Sarswat, N.; Adatya, S.; Kim, G.; Sayer, G.; Ota, T.; Jeevanandam, V.; Uriel, N. Increased Risk of Bleeding in Left Ventricular Assist Device Patients Treated with Enoxaparin as Bridge to Therapeutic International Normalized Ratio. ASAIO J. 2018, 64, 140–146. [Google Scholar] [CrossRef]
- Hanke, J.S.; Riebandt, J.; Wahabzada, M.; Nur, F.; Wahabzada, A.; Dogan, G.; Feldmann, C.; Haverich, A.; Popov, A.F.; Zimpfer, D.; et al. Driving After Left Ventricular Assist Device Implantation. Artif. Organs 2018, 42, 695–699. [Google Scholar] [CrossRef]
- Eckman, P.M.; Dhungel, V.; Mandras, S.; Brisco, M.A.; Emani, S.; Duval, S.; Lindenfeld, J.; Sulemanjee, N.; Sokos, G.G.; Feldman, J. Sexual function after left ventricular assist device. J. Am. Coll. Cardiol. 2013, 61, 2021–2022. [Google Scholar] [CrossRef]
- Barbara, D.W.; Wetzel, D.R.; Pulido, J.N.; Pershing, B.S.; Park, S.J.; Stulak, J.M.; Zietlow, S.P.; Morris, D.S.; Boilson, B.A.; Mauermann, W.J. The perioperative management of patients with left ventricular assist devices undergoing noncardiac surgery. Mayo Clin. Proc. 2013, 88, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Findler, M.; Findler, M.; Rudis, E. Dental treatment of a patient with an implanted left ventricular assist device: Expanding the frontiers. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 111, e1–e4. [Google Scholar] [CrossRef] [PubMed]
- Pya, J.M.Y.; Bekbossynova, M.; Salov, R.; Schueler, S.; Meyns, B.; Kassif, Y.; Massetti, M.; Zilbershlag, M.; Netuka, I. First human use of a wireless coplanar energy transfer coupled with a continuous-flow left ventricular assist device. J Heart Lung Transpl. 2019, 38, 339–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hannan, M.M.; Husain, S.; Mattner, F.; Danziger-Isakov, L.; Drew, R.J.; Corey, G.R.; Schueler, S.; Holman, W.L.; Lawler, L.P.; Gordon, S.M.; et al. Working formulation for the standardization of definitions of infections in patients using ventricular assist devices. J. Heart Lung Transpl. 2011, 30, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Zierer, A.; Melby, S.J.; Voeller, R.K.; Guthrie, T.J.; Ewald, G.A.; Shelton, K.; Pasque, M.K.; Moon, M.R.; Damiano, R.J., Jr.; Moazami, N. Late-onset driveline infections: The Achilles’ heel of prolonged left ventricular assist device support. Ann. Thorac. Surg. 2007, 84, 515–520. [Google Scholar] [CrossRef] [PubMed]
- O’Horo, J.C.; Saleh, O.M.A.; Stulak, J.M.; Wilhelm, M.P.; Baddour, L.M.; Sohail, M.R. Left Ventricular Assist Device Infections: A Systematic Review. ASAIO J. 2018, 64, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Baddour, L.M.; Bettmann, M.A.; Bolger, A.F.; Epstein, A.E.; Ferrieri, P.; Gerber, M.A.; Gewitz, M.H.; Jacobs, A.K.; Levison, M.E.; Newburger, J.W.; et al. Nonvalvular cardiovascular device-related infections. Circulation 2003, 108, 2015–2031. [Google Scholar] [CrossRef]
- Goldstein, D.J.; Naftel, D.; Holman, W.; Bellumkonda, L.; Pamboukian, S.V.; Pagani, F.D.; Kirklin, J. Continuous-flow devices and percutaneous site infections: Clinical outcomes. J. Heart Lung Transpl. 2012, 31, 1151–1157. [Google Scholar] [CrossRef]
- Gordon, R.J.; Weinberg, A.D.; Pagani, F.D.; Slaughter, M.S.; Pappas, P.S.; Naka, Y.; Goldstein, D.J.; Dembitsky, W.P.; Giacalone, J.C.; Ferrante, J.; et al. Ventricular Assist Device Infection Study, Prospective, multicenter study of ventricular assist device infections. Circulation 2013, 127, 691–702. [Google Scholar] [CrossRef]
- Trachtenberg, B.H.; Cordero-Reyes, A.M.; Aldeiri, M.; Alvarez, P.; Bhimaraj, A.; Ashrith, G.; Elias, B.; Suarez, E.E.; Bruckner, B.; Loebe, M.; et al. Persistent blood stream infection in patients supported with a continuous-flow left ventricular assist device is associated with an increased risk of cerebrovascular accidents. J. Card Fail. 2015, 21, 119–125. [Google Scholar] [CrossRef]
- Tong, M.Z.; Smedira, N.G.; Soltesz, E.G.; Starling, R.C.; Koval, C.E.; Porepa, L.; Moazami, N. Outcomes of Heart Transplant After Left Ventricular Assist Device Specific and Related Infection. Ann. Thorac. Surg. 2015, 100, 1292–1297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suarez, J.; Patel, C.B.; Felker, G.M.; Becker, R.; Hernandez, A.F.; Rogers, J.G. Mechanisms of bleeding and approach to patients with axial-flow left ventricular assist devices. Circ. Heart Fail. 2011, 4, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Dakik, H.K.; McGhan, A.A.; Chiu, S.T.; Patel, C.B.; Milano, C.A.; Rogers, J.G.; Chow, S.C.; Wild, D.M. The Diagnostic Yield of Repeated Endoscopic Evaluation in Patients with Gastrointestinal Bleeding and Left Ventricular Assist Devices. Dig. Dis. Sci. 2016, 61, 1603–1610. [Google Scholar] [CrossRef] [PubMed]
- Tabit, C.E.; Chen, P.; Kim, G.H.; Fedson, S.E.; Sayer, G.; Coplan, M.J.; Jeevanandam, V.; Uriel, N.; Liao, J.K. Elevated Angiopoietin-2 Level in Patients With Continuous-Flow Left Ventricular Assist Devices Leads to Altered Angiogenesis and Is Associated With Higher Nonsurgical Bleeding. Circulation 2016, 134, 141–152. [Google Scholar] [CrossRef] [Green Version]
- Galand, V.; Flecher, E.; Auffret, V.; Boule, S.; Vincentelli, A.; Dambrin, C.; Mondoly, P.; Sacher, F.; Nubret, K.; Kindo, M.; et al. Predictors and Clinical Impact of Late Ventricular Arrhythmias in Patients With Continuous-Flow Left Ventricular Assist Devices. JACC Clin. Electrophysiol. 2018, 4, 1166–1175. [Google Scholar] [CrossRef]
- Vollkron, M.; Voitl, P.; Ta, J.; Wieselthaler, G.; Schima, H. Suction events during left ventricular support and ventricular arrhythmias. J Heart Lung Transpl. 2007, 26, 819–825. [Google Scholar] [CrossRef]
- Starling, R.C.; Moazami, N.; Silvestry, S.C.; Ewald, G.; Rogers, J.G.; Milano, C.A.; Rame, J.E.; Acker, M.A.; Blackstone, E.H.; Ehrlinger, J.; et al. Unexpected abrupt increase in left ventricular assist device thrombosis. N. Engl. J. Med. 2014, 370, 33–40. [Google Scholar] [CrossRef]
- Maltais, S.; Kilic, A.; Nathan, S.; Keebler, M.; Emani, S.; Ransom, J.; Katz, J.N.; Sheridan, B.; Brieke, A.; Egnaczyk, G.; et al. PREVENtion of HeartMate II Pump Thrombosis Through Clinical Management: The PREVENT multi-center study. J. Heart Lung Transpl. 2017, 36, 1–12. [Google Scholar] [CrossRef]
- Nair, N.; Schmitt, A.A.; Rau, E.M.; Anders, S.; Sandler, D.; Icenogle, T.B. Thrombolytics in VAD management-A single-center experience. Int. J. Cardiol. Heart Vasc. 2016, 11, 49–54. [Google Scholar] [CrossRef]
- Kato, T.S.; Ota, T.; Schulze, P.C.; Farr, M.; Jorde, U.; Takayama, H.; Naka, Y.; Yamashita, T.; Mancini, D.M. Asymmetric pattern of cerebrovascular lesions in patients after left ventricular assist device implantation. Stroke 2012, 43, 872–874. [Google Scholar] [CrossRef]
- Rich, J.D.; Gosev, I.; Patel, C.B.; Joseph, S.; Katz, J.N.; Eckman, P.M.; Lee, S.; Sundareswaran, K.; Kilic, A.; Bethea, B.; et al. Evolving Mechanical Support Research Group, The incidence, risk factors, and outcomes associated with late right-sided heart failure in patients supported with an axial-flow left ventricular assist device. J. Heart Lung Transpl. 2017, 36, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Drakos, S.G.; Janicki, L.; Horne, B.D.; Kfoury, A.G.; Reid, B.B.; Clayson, S.; Horton, K.; Haddad, F.; Li, D.Y.; Renlund, D.G.; et al. Risk factors predictive of right ventricular failure after left ventricular assist device implantation. Am. J. Cardiol. 2010, 105, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Kormos, R.L.; Teuteberg, J.J.; Pagani, F.D.; Russell, S.D.; John, R.; Miller, L.W.; Massey, T.; Milano, C.A.; Moazami, N.; Sundareswaran, K.S.; et al. Right ventricular failure in patients with the HeartMate II continuous-flow left ventricular assist device: Incidence, risk factors, and effect on outcomes. J. Thorac. Cardiovasc. Surg. 2010, 139, 1316–1324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peberdy, M.A.; Gluck, J.A.; Ornato, J.P.; Bermudez, C.A.; Griffin, R.E.; Kasirajan, V.; Kerber, R.E.; Lewis, E.F.; Link, M.S.; Miller, C.; et al. Cardiopulmonary Resuscitation in Adults and Children With Mechanical Circulatory Support: A Scientific Statement From the American Heart Association. Circulation 2017, 135, e1115–e1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Pump Parameter | HeartMate II | HeartMate 3 | HVAD |
|---|---|---|---|
| Typical speed, rpm | 8800–10,000 | 5000–6000 | 2400–3200 |
| Speed adjustment increment, rpm/increment | 200 | 100 | 20 |
| Flow, L/min | 4–7 | 4–6 | 4–6 |
| Power, Watts | 5–8 | 4.5–6.5 | 3–7 |
| Pulsatility index (or HVAD, peak to trough) | 5–8 | 3.5–5.5 | 2–4 L/min/beat |
| Feature | Heartmate II | HVAD and HeartMate 3 |
|---|---|---|
| Pump design | Axial flow | Centrifugal flow |
| Size and surgical implant |
|
|
| Blood flow and power consumption |
| |
| Hydrodynamic performance (determined by the relation between the flow rate and pressure head i.e., the differential pressure between the inlet in the left ventricle and the outlet in the aorta [13,14]) |
|
|
| Additional feature |
|
| Study, Year (Reference) | N | Device Tested | Indication | Design | Patient Population | Outcome |
|---|---|---|---|---|---|---|
| REMATCH, 2001 [6] | 129 | HeartMate XVE | DT | Prospective 1:1 HeartMate XVE vs. medical therapy | NYHA functional class IV for 60 days, LVEF < 25%, and peak VO2 < 14 mL/min/kg (unless on balloon pump, IV inotropes, or physically unable to perform exercise test), or intra-aortic balloon pump (IABP) or IV inotrope dependent for 14 days | 1- and 2-yr HeartMate XVE survival of 52% and 23% vs. 25% and 8% on medical therapy |
| INTREPID, 2007 [20] | 55 | Novacor | DT | Prospective nonrandomized | Inotrope-dependent patients | 1-yr Novacor survival of 27% vs. 11% on medical therapy |
| HeartMate II, 2007 [21] | 133 | HeartMate II | BTT | Prospective nonrandomized | Transplant candidates with systolic HF and NYHA functional class IV and inotrope dependence or need for IABP support | 75% survival to transplant, recovery, or ongoing support although remaining eligible for transplant at 6 months |
| HeartMate II, 2009 [22] | 192 | HeartMate II | DT | Prospective randomized 2:1 HeartMate II vs. HeartMate XVE | NYHA functional class IIIB or IV symptoms for >45 of the last 60 days, LVEF<25%, and peak VO2 <14 mL/min/kg (unless on IABP, IV inotropes, or physically unable to perform exercise test), or IABP dependent for 7 days or IV inotrope dependent for 14 days | 1- and 2-yr HeartMate II survival of 68% and 58% vs. 55% and 24% with HeartMate XVE |
| HeartMate II post-approval, 2011 [23] | 169 | HeartMate II | BTT | Prospective nonrandomized | Consecutive patients eligible for transplant in INTERMACS | 90% survival to transplant, recovery, or ongoing support at 6 months |
| HeartMate II post-approval, 2014 [24] | 247 | HeartMate II | DT | Prospective nonrandomized | Consecutive patients eligible for DT in INTERMACS | 1- and 2-yr survival of 74% and 61% |
| ADVANCE, 2012 [25] | 137 | HVAD | BTT | Prospective nonrandomized. HVAD compared with 499 patients who received FDA-approved LVADs in INTERMACS | Transplant candidates | 90.7% survival to transplant, recovery, or ongoing support on the original device vs. 90.1% in control group at 6 months |
| ENDURANCE, 2017 [26] | 446 | HVAD | DT | Prospective, DT patients randomized 2:1 HVAD vs. HeartMate II | Chronic, advanced HF, NYHA functional class IIIB or IV despite recommended medical therapy, EF< 25%, and ineligible for transplantation at the time of enrollment |
|
| MOMENTUM 3 long-term cohort, 2018 [27] | 366 | HeartMate 3 | BTT, DT and bridge to candidacy | Prospective, randomized, 1:1 HeartMate 3 vs. HeartMate II. Pre-specified interim analysis at 2 years | Advanced heart failure requiring LVAD. 60% ineligible for transplantation. 85% on IV inotropic therapy | Survival free from disabling stroke or reoperation to replace/remove a malfunctioning device at 24 months, in 79.5% of HeartMate 3 vs. 60.2% of HeartMate II (p < 0.001 for superiority). |
| MOMENTUM 3 full cohort, 2019 [19] | 1028 | HeartMate 3 | BTT, DT and bridge to candidacy | Prospective, randomized, 1:1 HeartMate 3 vs. HeartMate II. Adaptive trial design. Follow up period 2 years. | Advanced heart failure requiring LVAD. 61% were ineligible for transplantation. 86% were on intravenous inotrope therapy. |
|
| Complication | Management Strategy |
|---|---|
| LVAD infections |
|
| Bleeding (non-surgical) |
|
| Ventricular arrhythmias |
|
| LVAD malfunction |
|
| Pump thrombosis |
|
| Neurologic complications (stroke, intracranial hemorrhage) |
|
| Heart failure |
|
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singhvi, A.; Trachtenberg, B. Left Ventricular Assist Devices 101: Shared Care for General Cardiologists and Primary Care. J. Clin. Med. 2019, 8, 1720. https://doi.org/10.3390/jcm8101720
Singhvi A, Trachtenberg B. Left Ventricular Assist Devices 101: Shared Care for General Cardiologists and Primary Care. Journal of Clinical Medicine. 2019; 8(10):1720. https://doi.org/10.3390/jcm8101720
Chicago/Turabian StyleSinghvi, Aditi, and Barry Trachtenberg. 2019. "Left Ventricular Assist Devices 101: Shared Care for General Cardiologists and Primary Care" Journal of Clinical Medicine 8, no. 10: 1720. https://doi.org/10.3390/jcm8101720
APA StyleSinghvi, A., & Trachtenberg, B. (2019). Left Ventricular Assist Devices 101: Shared Care for General Cardiologists and Primary Care. Journal of Clinical Medicine, 8(10), 1720. https://doi.org/10.3390/jcm8101720





