Texture Analysis of Multi-Shot Echo-Planar Diffusion-Weighted Imaging in Head and Neck Squamous Cell Carcinoma: The Diagnostic Value for Nodal Metastasis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. MR Imaging Protocol
2.3. Reference Standards
2.4. 18F-FDG PET-CT Protocol
2.5. Texture Analysis
2.6. Statistical Analysis
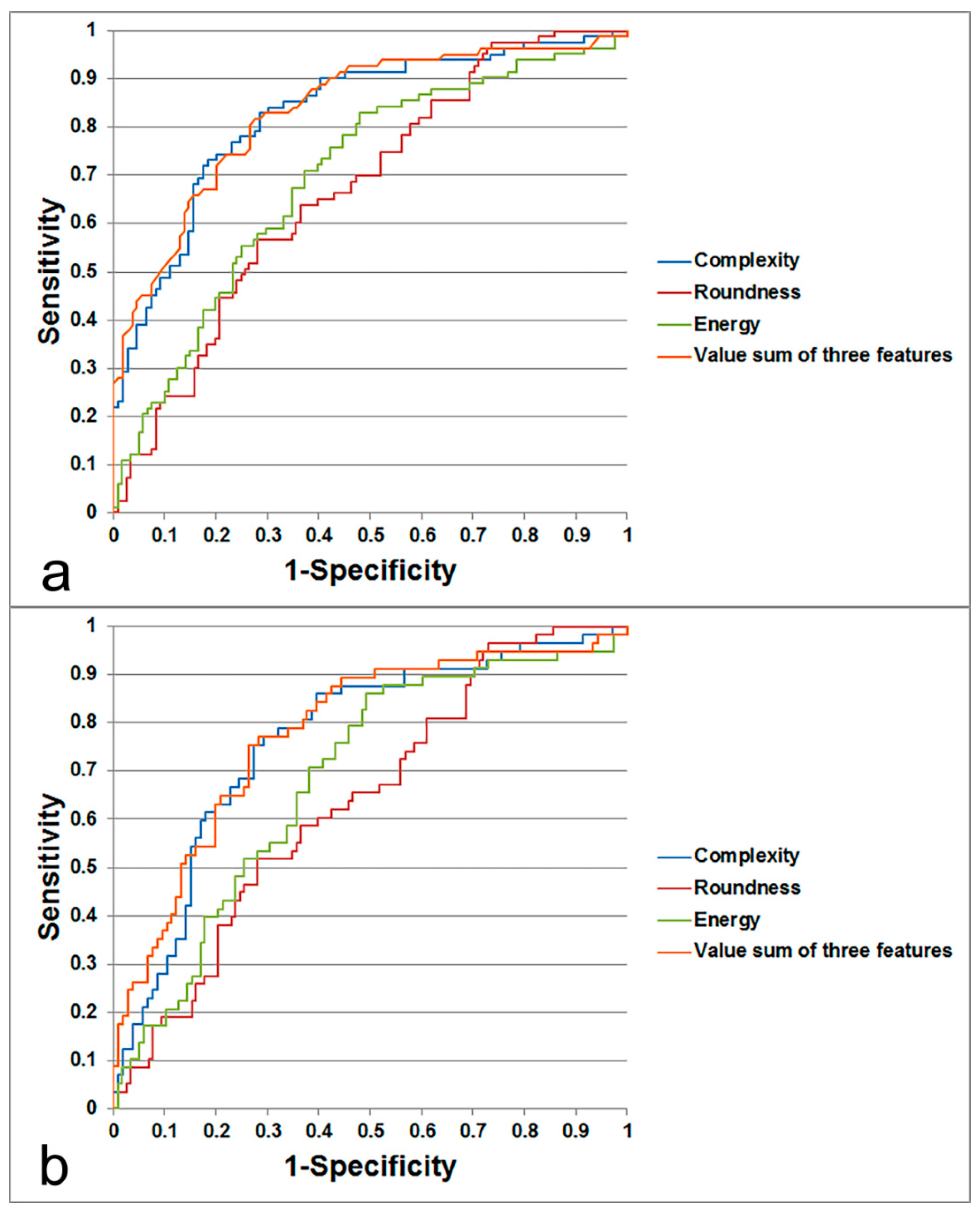
3. Results
3.1. Patients
3.2. MRI Characteristics of LNs
3.3. ADC Texture Analysis
3.4. Prediction of Metastatic LN by Texture Feature
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thoeny, H.C. Diffusion-weighted MRI in head and neck radiology: Applications in oncology. Cancer Imaging 2011, 10, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Vandecaveye, V.; De Keyzer, F.; Vander Poorten, V.; Dirix, P.; Verbeken, E.; Nuyts, S.; Hermans, R. Head and neck squamous cell carcinoma: Value of diffusion-weighted MR imaging for nodal staging. Radiology 2009, 251, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Layland, M.K.; Sessions, D.G.; Lenox, J. The influence of lymph node metastasis in the treatment of squamous cell carcinoma of the oral cavity, oropharynx, larynx and hypopharynx: N0 versus n+. Laryngoscope 2005, 115, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Van den Brekel, M.; Stel, H.; Castelijns, J.; Nauta, J.; Van der Waal, I.; Valk, J.; Meyer, C.; Snow, G. Cervical lymph node metastasis: Assessment of radiologic criteria. Radiology 1990, 177, 379–384. [Google Scholar] [CrossRef]
- Surov, A.; Meyer, H.J.; Wienke, A. Correlation between apparent diffusion coefficient (ADC) and cellularity is different in several tumors: A meta-analysis. Oncotarget 2017, 10, 59492–59499. [Google Scholar] [CrossRef]
- Surov, A.; Meyer, H.J.; Wienke, A. Associations between apparent diffusion coefficient (ADC) and KI 67 in different tumors: A meta-analysis. Part 1: ADCmean. Oncotarget 2017, 24, 75434–75444. [Google Scholar] [CrossRef]
- Ravanelli, M.; Grammatica, A.; Tononcelli, E.; Morello, R.; Leali, M.; Battocchio, S.; Agazzi, G.M.; e Bastia, M.B.D.M.; Maroldi, R.; Nicolai, P.; et al. Correlation between human papillomavirus status and quantitative MR imaging parameters including diffusion-weighted imaging and texture features in oropharyngeal carcinoma. AJNR Am. J. Neuroradiol. 2018, 39, 1878–1883. [Google Scholar] [CrossRef]
- Surov, A.; Meyer, H.J.; Wienke, A. Can imaging parameters provide information regarding histopathology in head and neck squamous cell carcinoma? A meta-analysis. Transl. Oncol. 2018, 11, 498–503. [Google Scholar] [CrossRef]
- Le Bihan, D.; Breton, E.; Lallemand, D.; Aubin, M.L.; Vignaud, J.; Laval-Jeantet, M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 1988, 168, 497–505. [Google Scholar] [CrossRef]
- Thoeny, H.C.; De Keyzer, F.; King, A.D. Diffusion-weighted MR imaging in the head and neck. Radiology 2012, 263, 19–32. [Google Scholar] [CrossRef]
- Payabvash, S.; Brackett, A.; Forghani, R.; Malhotra, A. Differentiation of lymphomatous, metastatic and non-malignant lymphadenopathy in the neck with quantitative diffusion-weighted imaging: Systematic review and meta-analysis. Neuroradiology 2019, 61, 897–910. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.M.; Xu, J.R.; Hua, J.; Gu, H.Y.; Zhu, J.; Hu, J. Value of diffusion-weighted MR imaging performed with quantitative apparent diffusion coefficient values for cervical lymphadenopathy. J. Magn. Reson. Imaging 2013, 38, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Sumi, M.; Sakihama, N.; Sumi, T.; Morikawa, M.; Uetani, M.; Kabasawa, H.; Shigeno, K.; Hayashi, K.; Takahashi, H.; Nakamura, T. Discrimination of metastatic cervical lymph nodes with diffusion-weighted MR imaging in patients with head and neck cancer. AJNR Am. J. Neuroradiol. 2003, 24, 1627–1634. [Google Scholar]
- King, A.D.; Ahuja, A.T.; Yeung, D.K.; Fong, D.K.; Lee, Y.Y.; Lei, K.I.; Tse, G.M. Malignant cervical lymphadenopathy: Diagnostic accuracy of diffusion-weighted MR imaging. Radiology 2007, 245, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Holzapfel, K.; Duetsch, S.; Fauser, C.; Eiber, M.; Rummeny, E.J.; Gaa, J. Value of diffusion-weighted MR imaging in the differentiation between benign and malignant cervical lymph nodes. Eur. J. Radiol. 2009, 72, 381–387. [Google Scholar] [CrossRef]
- De Bondt, R.B.; Hoeberigs, M.C.; Nelemans, P.J.; Deserno, W.M.; Peutz-Kootstra, C.; Kremer, B.; Beets-Tan, R.G. Diagnostic accuracy and additional value of diffusion-weighted imaging for discrimination of malignant cervical lymph nodes in head and neck squamous cell carcinoma. Neuroradiology 2009, 51, 183–192. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Zhu, L.; Huang, X.; Niu, G.; Chen, X. Differentiation of reactive and tumor metastatic lymph nodes with diffusion-weighted and SPIO-enhanced MRI. Mol. Imaging Biol. 2013, 15, 40–47. [Google Scholar] [CrossRef]
- Thoeny, H.C.; Froehlich, J.M.; Triantafyllou, M.; Huesler, J.; Bains, L.J.; Vermathen, P.; Fleischmann, A.; Studer, U.E. Metastases in normal-sized pelvic lymph nodes: Detection with diffusion-weighted MR imaging. Radiology 2014, 273, 125–135. [Google Scholar] [CrossRef]
- Lim, H.K.; Lee, J.H.; Baek, H.J.; Kim, N.; Lee, H.; Park, J.W.; Kim, S.Y.; Cho, K.J.; Baek, J.H. Is diffusion-weighted MRI useful for differentiation of small non-necrotic cervical lymph nodes in patients with head and neck malignancies? Korean J. Radiol. 2014, 15, 810–816. [Google Scholar] [CrossRef]
- Fong, D.; Bhatia, K.S.; Yeung, D.; King, A.D. Diagnostic accuracy of diffusion-weighted MR imaging for nasopharyngeal carcinoma, head and neck lymphoma and squamous cell carcinoma at the primary site. Oral Oncol. 2010, 46, 603–606. [Google Scholar] [CrossRef]
- Yan, D.F.; Zhang, W.B.; Ke, S.B.; Zhao, F.; Yan, S.X.; Wang, Q.D.; Teng, L.S. The prognostic value of pretreatment tumor apparent diffusion coefficient values in nasopharyngeal carcinoma. BMC Cancer 2017, 17, 678. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.J.; Choi, B.S.; Jeong, H.K.; Sunwoo, L.; Jung, C.; Kim, J.H. Diffusion-weighted imaging of the head and neck: Influence of fat-suppression technique and multishot 2d navigated interleaved acquisitions. AJNR Am. J. Neuroradiol. 2018, 39, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Reischauer, C.; Patzwahl, R.; Koh, D.-M.; Froehlich, J.M.; Gutzeit, A. Texture analysis of apparent diffusion coefficient maps for treatment response assessment in prostate cancer bone metastases—A pilot study. Eur. J. Radiol. 2018, 101, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Edge, S.B. AJCC Cancer Staging Manual; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Zhang, L.; Fried, D.V.; Fave, X.J.; Hunter, L.A.; Yang, J.; Court, L.E. Ibex: An open infrastructure software platform to facilitate collaborative work in radiomics. Med. Phys. 2015, 42, 1341–1353. [Google Scholar] [CrossRef] [PubMed]
- Fave, X.; Zhang, L.; Yang, J.; Mackin, D.; Balter, P.; Gomez, D.; Followill, D.; Jones, A.K.; Stingo, F. Impact of image preprocessing on the volume dependence and prognostic potential of radiomics features in non-small cell lung cancer. Transl. Cancer Res. 2016, 5, 349–363. [Google Scholar] [CrossRef] [Green Version]
- Harrell, F.E., Jr.; Lee, K.L.; Mark, D.B. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy and measuring and reducing errors. Stat. Med. 1996, 15, 361–387. [Google Scholar] [CrossRef]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Vandecaveye, V.; De Keyzer, F.; Hermans, R. Diffusion-weighted magnetic resonance imaging in neck lymph adenopathy. Cancer Imaging 2008, 8, 173. [Google Scholar] [CrossRef]
- Choi, Y.J.; Lee, J.H.; Kim, H.O.; Kim, D.Y.; Yoon, R.G.; Cho, S.H.; Koh, M.J.; Kim, N.; Kim, S.Y.; Baek, J.H. Histogram analysis of apparent diffusion coefficients for occult tonsil cancer in patients with cervical nodal metastasis from an unknown primary site at presentation. Radiology 2016, 278, 146–155. [Google Scholar] [CrossRef]
- Elmore, S.A. Enhanced histopathology of mucosa-associated lymphoid tissue. Toxicol. Pathol. 2006, 34, 687–696. [Google Scholar] [CrossRef]
- Aerts, H.J.; Velazquez, E.R.; Leijenaar, R.T.; Parmar, C.; Grossmann, P.; Carvalho, S.; Bussink, J.; Monshouwer, R.; Haibe-Kains, B.; Rietveld, D. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat. Commun. 2014, 5, 4006. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.S.; Ghafoor, S.; Marcon, M.; Perucho, J.A.; Wurnig, M.C.; Wagner, M.W.; Khong, P.-L.; Lee, E.Y.; Boss, A. Mri texture features may predict differentiation and nodal stage of cervical cancer: A pilot study. Acta Radiol. Open 2017, 6, 2058460117729574. [Google Scholar] [CrossRef] [PubMed]
- Meyer, H.J.; Leifels, L.; Hamerla, G.; Höhn, A.K.; Surov, A. ADC-histogram analysis in head and neck squamous cell carcinoma. Associations with different histopathological features including expression of EGFR, VEGF, HIF-1α, Her 2 and p53. A preliminary study. Magn. Reson. Imaging 2018, 54, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Surov, A.; Meyer, H.J.; Leifels, L.; Höhn, A.K.; Richter, C.; Winter, K. Associations between histogram analysis parameters of dynamic contrast-enhanced magnetic resonance imaging and histopathological findings including proliferation potential, cellularity and nucleic areas in head and neck squamous cell carcinoma. Oncotarget 2018, 9, 21070–21077. [Google Scholar] [CrossRef] [PubMed]
- Ganeshan, B.; Abaleke, S.; Young, R.C.; Chatwin, C.R.; Miles, K.A. Texture analysis of non-small cell lung cancer on unenhanced computed tomography: Initial evidence for a relationship with tumour glucose metabolism and stage. Cancer Imaging 2010, 10, 137. [Google Scholar] [CrossRef] [PubMed]
- Rose, C.J.; Mills, S.J.; O’Connor, J.P.; Buonaccorsi, G.A.; Roberts, C.; Watson, Y.; Cheung, S.; Zhao, S.; Whitcher, B.; Jackson, A. Quantifying spatial heterogeneity in dynamic contrast-enhanced MRI parameter maps. Magn. Reson. Med. 2009, 62, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Amadasun, M.; King, R. Textural features corresponding to textural properties. IEEE Trans. Syst. Man Cybern. 1989, 19, 1264–1274. [Google Scholar] [CrossRef]
- Davnall, F.; Yip, C.S.; Ljungqvist, G.; Selmi, M.; Ng, F.; Sanghera, B.; Ganeshan, B.; Miles, K.A.; Cook, G.J.; Goh, V. Assessment of tumor heterogeneity: An emerging imaging tool for clinical practice? Insights Imaging 2012, 3, 573–589. [Google Scholar] [CrossRef]
- Schacht, D.V.; Drukker, K.; Pak, I.; Abe, H.; Giger, M.L. Using quantitative image analysis to classify axillary lymph nodes on breast MRI: A new application for the z 0011 era. Eur. J. Radiol. 2015, 84, 392–397. [Google Scholar] [CrossRef]
- Bayanati, H.; Thornhill, R.E.; Souza, C.A.; Sethi-Virmani, V.; Gupta, A.; Maziak, D.; Amjadi, K.; Dennie, C. Quantitative CT texture and shape analysis: Can it differentiate benign and malignant mediastinal lymph nodes in patients with primary lung cancer? Eur. Radiol. 2015, 25, 480–487. [Google Scholar] [CrossRef]
- Malik, F.; Baharudin, B. The statistical quantized histogram texture features analysis for image retrieval based on median and laplacian filters in the DCT domain. Int. Arab J. Inf. Technol. 2013, 10, 1–9. [Google Scholar]
- Kim, S.G.; Friedman, K.; Patel, S.; Hagiwara, M. Potential role of PET/MRI for imaging metastatic lymph nodes in head and neck cancer. AJR Am. J. Roentgenol. 2016, 207, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Scalco, E.; Marzi, S.; Sanguineti, G.; Vidiri, A.; Rizzo, G. Characterization of cervical lymph-nodes using a multi-parametric and multi-modal approach for an early prediction of tumor response to chemo-radiotherapy. Phys. Med. 2016, 32, 1672–1680. [Google Scholar] [CrossRef] [PubMed]



| Group | Texture Feature | Coefficient | p Value | OR | 95% CI of OR |
|---|---|---|---|---|---|
| All-sized LNs | Complexity | 0.22 (×10−5) | <0.001 | 1.000002 | 1.000001–1.000004 |
| Energy | 0.19 (×10−5) | 0.014 | 1.000002 | 1.0000004–1.000004 | |
| Roundness | 4.64 | 0.008 | 103.56 | 3.35–3675.25 | |
| Subcentimeter-sized LNs | Complexity | 0.20 (×10−5) | 0.001 | 1.0000020 | 1.0000008–1.000004 |
| Energy | 0.23 (×10−5) | 0.001 | 1.000002 | 1.0000009–1.000004 | |
| Roundness | 4.76 | 0.008 | 116.88 | 3.39–4675.74 |
| Diagnostic Performance | All-Sized LNs | Subcentimeter-Sized LNs | ||||
|---|---|---|---|---|---|---|
| Complexity | Energy | Roundness | Complexity | Energy | Roundness | |
| AUC | 0.829 | 0.699 | 0.671 | 0.767 | 0.685 | 0.638 |
| Ben mean (SD) | 224278.15 (291381.92) | 675113.33 (239553.38) | 0.24 (0.11) | 213192.64 (266248.09) | 679498.11 (240590.86) | 0.24 (0.11) |
| Met mean (SD) | 1522108.84 (3090971.29) | 850806.46 (303868.16) | 0.30 (0.10) | 506720.89 (449132.79) | 825260.67 (288103.42) | 0.28 (0.10) |
| Cutoff value | 245196 | 667805 | 0.2473978 | 211147 | 677054.9 | 0.2473978 |
| Sensitivity (%) | 82.93 | 72.29 | 65.06 | 78.95 | 70.69 | 60.34 |
| Specificity (%) | 71.56 | 60.33 | 60.33 | 67.92 | 61.86 | 60.17 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.H.; Bae, Y.J.; Choi, B.S.; Jung, Y.H.; Jeong, W.-J.; Kim, H.; Sunwoo, L.; Jung, C.; Kim, J.H. Texture Analysis of Multi-Shot Echo-Planar Diffusion-Weighted Imaging in Head and Neck Squamous Cell Carcinoma: The Diagnostic Value for Nodal Metastasis. J. Clin. Med. 2019, 8, 1767. https://doi.org/10.3390/jcm8111767
Park JH, Bae YJ, Choi BS, Jung YH, Jeong W-J, Kim H, Sunwoo L, Jung C, Kim JH. Texture Analysis of Multi-Shot Echo-Planar Diffusion-Weighted Imaging in Head and Neck Squamous Cell Carcinoma: The Diagnostic Value for Nodal Metastasis. Journal of Clinical Medicine. 2019; 8(11):1767. https://doi.org/10.3390/jcm8111767
Chicago/Turabian StylePark, Jung Hyun, Yun Jung Bae, Byung Se Choi, Young Ho Jung, Woo-Jin Jeong, Hyojin Kim, Leonard Sunwoo, Cheolkyu Jung, and Jae Hyoung Kim. 2019. "Texture Analysis of Multi-Shot Echo-Planar Diffusion-Weighted Imaging in Head and Neck Squamous Cell Carcinoma: The Diagnostic Value for Nodal Metastasis" Journal of Clinical Medicine 8, no. 11: 1767. https://doi.org/10.3390/jcm8111767
APA StylePark, J. H., Bae, Y. J., Choi, B. S., Jung, Y. H., Jeong, W.-J., Kim, H., Sunwoo, L., Jung, C., & Kim, J. H. (2019). Texture Analysis of Multi-Shot Echo-Planar Diffusion-Weighted Imaging in Head and Neck Squamous Cell Carcinoma: The Diagnostic Value for Nodal Metastasis. Journal of Clinical Medicine, 8(11), 1767. https://doi.org/10.3390/jcm8111767






