Recovery from Anesthesia after Robotic-Assisted Radical Cystectomy: Two Different Reversals of Neuromuscular Blockade
Abstract
1. Introduction
2. Experimental Section
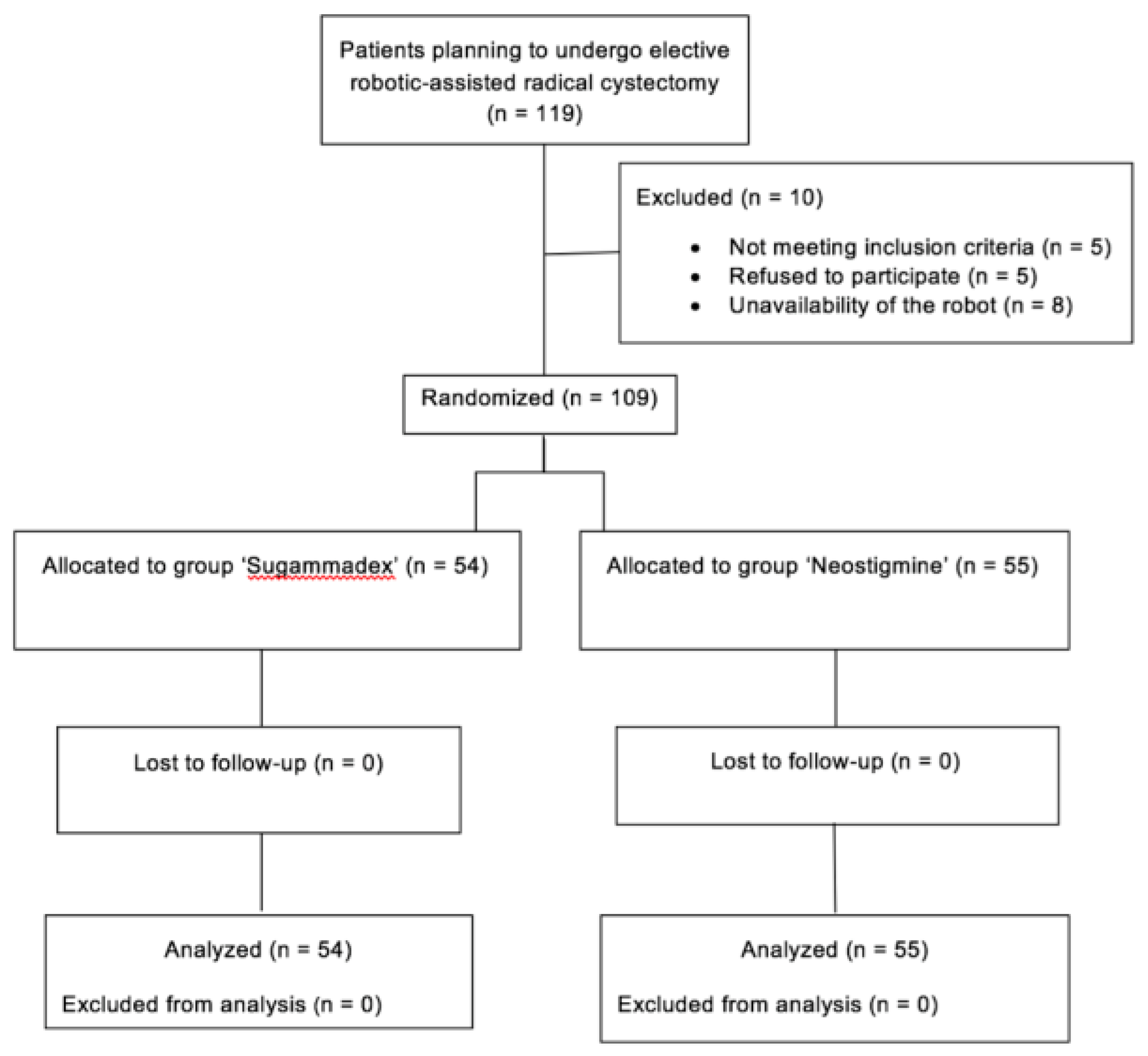
2.1. Patients and Procedures
2.2. Measurements
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Leow, J.J.; Chang, S.L.; Meyer, C.P.; Wang, Y.; Hanske, J.; Sammon, J.D.; Cole, A.P.; Preston, M.A.; Dasgupta, P.; Menon, M.; et al. Robot-assisted Versus Open Radical Prostatectomy: A Contemporary Analysis of an All-payer Discharge Database. Eur. Urol. 2016, 70, 837–845. [Google Scholar] [CrossRef]
- Trentman, T.L.; Fassett, S.L.; McGirr, D.; Anderson, B.; Chang, Y.H.H.; Nateras, R.N.; Castle, E.P.; Rosenfeld, D.M. Comparison of anesthetic management and outcomes of robot-assisted versus open radical cystectomy. J. Robot. Surg. 2013, 7, 273–279. [Google Scholar] [CrossRef]
- Oksar, M.; Akbulut, Z.; Ocal, H.; Balbay, M.D.; Kanbak, O. Anesthetic considerations for robotic cystectomy: A prospective study. Braz. J. Anesthesiol. 2014, 64, 109–115. [Google Scholar] [CrossRef]
- Cockcroft, J.O.; Berry, C.B.; McGrath, J.S.; Daugherty, M.O. Anesthesia for Major Urologic Surgery. Anesthesiol. Clin. 2015, 33, 165–172. [Google Scholar] [CrossRef]
- Martini, C.H.; Boon, M.; Bevers, R.F.; Aarts, L.P.; Dahan, A. Evaluation of Surgical Conditions During Laparoscopic Surgery in Patients with Moderate vs. Deep Neuromuscular Block. Surv. Anesthesiol. 2014, 58, 222–223. [Google Scholar] [CrossRef]
- Boon, M.; Martini, C.H.; Aarts, L.P.; Bevers, R.F.; Dahan, A. Effect of variations in depth of neuromuscular blockade on rating of surgical conditions by surgeon and anesthesiologist in patients undergoing laparoscopic renal or prostatic surgery (BLISS trial): Study protocol for a randomized controlled trial. Trials 2013, 14, 63. [Google Scholar] [CrossRef]
- Geldner, G.; Niskanen, M.; Laurila, P.; Mizikov, V.; Hübler, M.; Beck, G.; Rietbergen, H.; Nicolayenko, E. A randomized controlled trial comparing sugammadex and neostigmine at different depths of neuromuscular blockade in patients undergoing laparoscopic surgery. Anaesthesia 2012, 67, 991–998. [Google Scholar] [CrossRef]
- Paton, F.; Paulden, M.; Chambers, D.; Heirs, M.; Duffy, S.; Hunter, J.M.; Sculpher, M.; Woolacott, N. Sugammadex compared with neostigmine/glycopyrrolate for routine reversal of neuromuscular block: A systematic review and economic evaluation. Br. J. Anaesth. 2010, 105, 558–567. [Google Scholar] [CrossRef]
- Luo, J.; Chen, S.; Min, S.; Peng, L. Reevaluation and update on efficacy and safety of neostigmine for reversal of neuromuscular blockade. Ther. Clin. Risk Manag. 2018, 14, 2397–2406. [Google Scholar] [CrossRef]
- Kamine, T.H.; Papavassiliou, E.; Schneider, B.E. Effect of Abdominal Insufflation for Laparoscopy on Intracranial Pressure. JAMA Surg. 2014, 149, 380. [Google Scholar] [CrossRef]
- Cerantola, Y.; Valerio, M.; Persson, B.; Jichlinski, P.; Ljungqvist, O.; Hübner, M.; Kassouf, W.; Müller, S.; Baldini, G.; Carli, F.; et al. Guidelines for perioperative care after radical cystectomy for bladder cancer: Enhanced Recovery After Surgery (ERAS®) society recommendations. Clin. Nutr. 2013, 32, 879–887. [Google Scholar] [CrossRef]
- Simone, G.; Papalia, R.; Misuraca, L.; Tuderti, G.; Minisola, F.; Ferriero, M.; Vallati, G.E.; Guaglianone, S.; Gallucci, M. Robotic Intracorporeal Padua Ileal Bladder: Surgical Technique, Perioperative, Oncologic and Functional Outcomes. Eur. Urol. 2018, 73, 934–940. [Google Scholar] [CrossRef]
- Yağan, Ö.; Taş, N.; Mutlu, T.; Hancı, V.; Hanci, V. Comparison of the effects of sugammadex and neostigmine on postoperative nausea and vomiting. Braz. J. Anesthesiol. 2017, 67, 147–152. [Google Scholar]
- Løvstad, R.Z.; Thagaard, K.S.; Berner, N.S.; Raeder, J.C. Neostigmine 50 microg kg(−1) with glycopyrrolate increases postoperative nausea in women after laparoscopic gynecological surgery. Acta Anaesthesiol. Scand. 2001, 45, 495–500. [Google Scholar] [CrossRef]
- Law, N.-M.; Bharucha, A.E.; Undale, A.S.; Zinsmeister, A.R. Cholinergic stimulation enhances colonic motor activity, transit, and sensation in humans. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 281, G1228–G1237. [Google Scholar] [CrossRef]
- Lassen, K.; Soop, M.; Nygren, J.; Cox, P.B.W.; Hendry, P.O.; Spies, C.; von Meyenfeldt, M.F.; Fearon, K.C.; Revhaug, A.; Norderval, S.; et al. Enhanced Recovery After Surgery (ERAS) Group. Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS) Group recommendations. Arch. Surg. 2009, 144, 961–969. [Google Scholar] [CrossRef]
- Paech, M.J.; Kaye, R.; Baber, C.; Nathan, E.A. Recovery characteristics of patients receiving either sugammadex or neostigmine and glycopyrrolate for reversal of neuromuscular block: A randomised controlled trial. Anaesthesia 2018, 73, 340–347. [Google Scholar] [CrossRef]
- Koyuncu, O.; Turhanoglu, S.; Akkurt, C.O.; Karcıoğlu, M.; Ozkan, M.; Ozer, C.; Sessler, D.I.; Turan, A. Comparison of sugammadex and conventional reversal on postoperative nausea and vomiting: A randomized, blinded trial. J. Clin. Anesth. 2015, 27, 51–56. [Google Scholar] [CrossRef]
- Goetz, B.; Benhaqi, P.; Müller, M.H.; Kreis, M.E.; Kasparek, M.S. Changes in beta-adrenergic neurotransmission during postoperative ileus in ratcircular jejunal muscle. Neurogastroenterol. Motil. 2013, 25, 154-e84. [Google Scholar] [CrossRef]
- Neunlist, M.; Rolli-Derkinderen, M.; Latorre, R.; Van Landeghem, L.; Coron, E.; Derkinderen, P.; De Giorgio, R. Enteric Glial Cells: Recent Developments and Future Directions. Gastroenterology 2014, 147, 1230–1237. [Google Scholar] [CrossRef]
- Rasmussen, L.S.; Larsen, K.; Houx, P.; Skovgaard, L.T.; Hanning, C.D.; Moller, J.T. The assessment of postoperative cognitive function. Acta Anaesthesiol. Scand. 2001, 45, 275–289. [Google Scholar] [CrossRef] [PubMed]
- KUŞKU, A.; Demir, G.; Çukurova, Z.; Eren, G.; Hergünsel, O. Monitorization of the effects of spinal anaesthesia on cerebral oxygen saturation in elder patients using near-infrared spectroscopy. Braz. J. Anesthesiol. 2014, 64, 241–246. [Google Scholar] [PubMed]
- Adam, J.M.; Bennett, D.J.; Bom, A.; Clark, J.K.; Feilden, H.; Hutchinson, E.J.; Palin, R.; Prosser, A.; Rees, D.C.; Rosair, G.M.; et al. Cyclodextrin-Derived Host Molecules as Reversal Agents for the Neuromuscular Blocker Rocuronium Bromide: Synthesis and Structure-Activity Relationships. J. Med. Chem. 2002, 45, 1806–1816. [Google Scholar] [CrossRef] [PubMed]
- Sparr, H.J.; Vermeyen, K.M.; Beaufort, A.M.; Rietbergen, H.; Proost, J.H.; Saldien, V.; Velik-Salchner, C.; Wierda, J.M. Early reversal of profound rocuronium-induced neuromuscular blockade by sugammadex in a randomized multicenter study: Efficacy, safety, and pharmacokinetics. Anesthesiology 2007, 106, 935–943. [Google Scholar] [CrossRef]
- Amorim, P.; Lagarto, F.; Gomes, B.; Esteves, S.; Bismarck, J.; Rodrigues, N.; Nogueira, M. Neostigmine vs. sugammadex: Observational cohort study comparing the quality of recovery using the Postoperative Quality Recovery Scale. Acta Anaesthesiol. Scand. 2014, 58, 1101–1110. [Google Scholar] [CrossRef]
- Chazot, T.; Dumont, G.; Le Guen, M.; Hausser-Hauw, C.; Liu, N.; Fischler, M. Sugammadex administration results in arousal from intravenous anaesthesia: A clinical and electroencephalographic observation. Br. J. Anaesth. 2011, 106, 914–916. [Google Scholar] [CrossRef][Green Version]
- Lanier, W.L.; Laizzo, P.A.; Milde, J.H.; Sharbrough, F.W. The Cerebral and Systemic Effects of Movement in Response to a Noxious Stimulus in Lightly Anesthetized Dogs Possible Modulation of Cerebral Function by Muscle Afferents. Anesthesiology 1994, 80, 392–401. [Google Scholar] [CrossRef]
- Illman, H.; Antila, H.; Olkkola, K.T. Reversal of neuromuscular blockade by sugammadex does not affect EEG derived indices of depth of anesthesia. J. Clin. Monit. Comput. 2010, 24, 371–376. [Google Scholar] [CrossRef]
- Sadhasivam, S.; Ganesh, A.; Robison, A.; Kaye, R.; Watcha, M.F. Validation of the Bispectral Index Monitor for Measuring the Depth of Sedation in Children. Anesth. Analg. 2006, 102, 383–388. [Google Scholar] [CrossRef]
- Abulrob, A.; Tauskela, J.S.; Mealing, G.; Brunette, E.; Faid, K.; Stanimirovic, D. Protection by cholesterol-extracting cyclodextrins: A role for N-methyl-d-aspartate receptor redistribution. J. Neurochem. 2005, 92, 1477–1486. [Google Scholar] [CrossRef]

| S Group (n = 54) | N Group (n = 55) | |
|---|---|---|
| Age (years), mean (SD) | 62.8 (8.9) | 60.2 (9.4) |
| BMI (kg/m2), mean (SD) | 26.3 (3.5) | 26.2 (4) |
| Gender (n), male/female | 42/12 | 40/14 |
| ASA status (n): I/II/III | 5/40/9 | 9/41/5 |
| Apfel risk score (n): I/II/III/IV | 20/30/4/0 | 19/32/4/0 |
| Comorbidities, n (%) | ||
| Hypertension | 18 (33.3) | 11 (20) |
| Dysthyroidism | 3 (5.5) | 4 (7.2) |
| Previous MI | 5 (9.2) | 2 (3.6) |
| Diabetes | 6 (11.1) | 3 (5.4) |
| COPD | 3 (5.5) | 2 (3.6) |
| Neoadiuvant chemotherapy, n (%) | 14 (25.9) | 11 (20) |
| Tumor stage (pT), n (%) | ||
| Tis | 8 (14.8) | 7 (12.7) |
| Ta | 3 (5.5) | 3 (5.4) |
| T1 | 7 (13) | 8 (14.8) |
| T2 | 13 (24) | 14 (25.4) |
| T3 | 17 (31.4) | 16 (29) |
| T4 | 6 (11.1) | 7 (12.7) |
| HADS > 8, n (%) | 25 (46.2) | 27 (49) |
| S Group (n = 54) | N Group (n = 55) | p-Value | |
|---|---|---|---|
| EtCO2 (mmHg) | 28.9 (3) | 28.6 (3.4) | 0.603 |
| SpO2 (%) | 98.6 (1.3) | 98.5 (1.5) | 0.821 |
| HR (bpm) | 68.3 (15.1) | 68.9 (13.9) | 0.622 |
| MAP (mmHg) | 87 (15.3) | 88.2 (15.5) | 0.854 |
| Estimated blood loss (mL) | 209 (31) | 218 (37) | 0.200 |
| Surgery time (min) | 340.7(80) | 326.7 (81.9) | 0.437 |
| Anesthesia time (min) | 378 (83) | 361 (81) | 0.526 |
| Recovery time from TOF 2 to TOF Ratio > 0.9 (min) | 3.2 (1) | 8 (2.8) | <0.001 * |
| Early PONV 0–6 h, n (%) | |||
| Cumulative incidence | 14 (25.9) | 16 (29) | 0.711 |
| Nausea | 10 (18.5) | 9 (16.3) | 0.767 |
| Vomiting | 4 (7.4) | 5 (9) | 0.750 |
| Late PONV 6–24 h, n (%) | |||
| Cumulative incidence | 10 (18.5) | 11 (20) | 0.845 |
| Nausea | 7 (13) | 8 (14.5) | 0.810 |
| Vomiting | 3 (5.5) | 3 (5.4) | 0.982 |
| Antiemetics consumption (mg) | |||
| Ondansetron | 2.6 (3) | 3.8 (4.4) | 0.105 |
| Metoclopramide | 3.7 (4.9) | 4.7 (5.7) | 0.358 |
| Morphine consumption (mg) | |||
| 0–6 h | 3 (2.4) | 3.7 (2.6) | 0.154 |
| 0–24 h | 6.2 (3) | 5.5 (2.8) | 0.177 |
| Early postoperative pulmonary failure, n (%) | 3 (5.5) | 4 (7.2) | 0.715 |
| Time to resumption of intestinal transit, days (IQR) | 3 (3–5) | 3 (3–5) | 0.761 |
| Length of stay, days (IQR) | 8 (7.5–12.25) | 8 (6–12) | 0.682 |
| S Group (n = 54) | N Group (n = 55) | p-Value | |
|---|---|---|---|
| OASS ¢ | |||
| 15 min | 3 (3; 4) | 3 (3; 4) | 0.16 |
| 30 min | 5 (4; 5) | 4 (3; 5) | 0.06 |
| 60 min | 5 (5; 5) | 5 (4; 5) | 0.023 * |
| MMSt ♯ | |||
| Preop | 29.3 (30; 30) | 29.2 (29; 30) | 0.78 |
| 1 h | 29.3 (29; 30) | 27.6 (27; 30) | 0.007 * |
| 4 h | 29.5 (30; 30) | 28.4 (28; 30) | 0.048 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Claroni, C.; Covotta, M.; Torregiani, G.; Marcelli, M.E.; Tuderti, G.; Simone, G.; Scotto di Uccio, A.; Zinilli, A.; Forastiere, E. Recovery from Anesthesia after Robotic-Assisted Radical Cystectomy: Two Different Reversals of Neuromuscular Blockade. J. Clin. Med. 2019, 8, 1774. https://doi.org/10.3390/jcm8111774
Claroni C, Covotta M, Torregiani G, Marcelli ME, Tuderti G, Simone G, Scotto di Uccio A, Zinilli A, Forastiere E. Recovery from Anesthesia after Robotic-Assisted Radical Cystectomy: Two Different Reversals of Neuromuscular Blockade. Journal of Clinical Medicine. 2019; 8(11):1774. https://doi.org/10.3390/jcm8111774
Chicago/Turabian StyleClaroni, Claudia, Marco Covotta, Giulia Torregiani, Maria Elena Marcelli, Gabriele Tuderti, Giuseppe Simone, Alessandra Scotto di Uccio, Antonio Zinilli, and Ester Forastiere. 2019. "Recovery from Anesthesia after Robotic-Assisted Radical Cystectomy: Two Different Reversals of Neuromuscular Blockade" Journal of Clinical Medicine 8, no. 11: 1774. https://doi.org/10.3390/jcm8111774
APA StyleClaroni, C., Covotta, M., Torregiani, G., Marcelli, M. E., Tuderti, G., Simone, G., Scotto di Uccio, A., Zinilli, A., & Forastiere, E. (2019). Recovery from Anesthesia after Robotic-Assisted Radical Cystectomy: Two Different Reversals of Neuromuscular Blockade. Journal of Clinical Medicine, 8(11), 1774. https://doi.org/10.3390/jcm8111774





