Nitric Oxide and Biological Mediators in Pediatric Chronic Rhinosinusitis and Asthma
Abstract
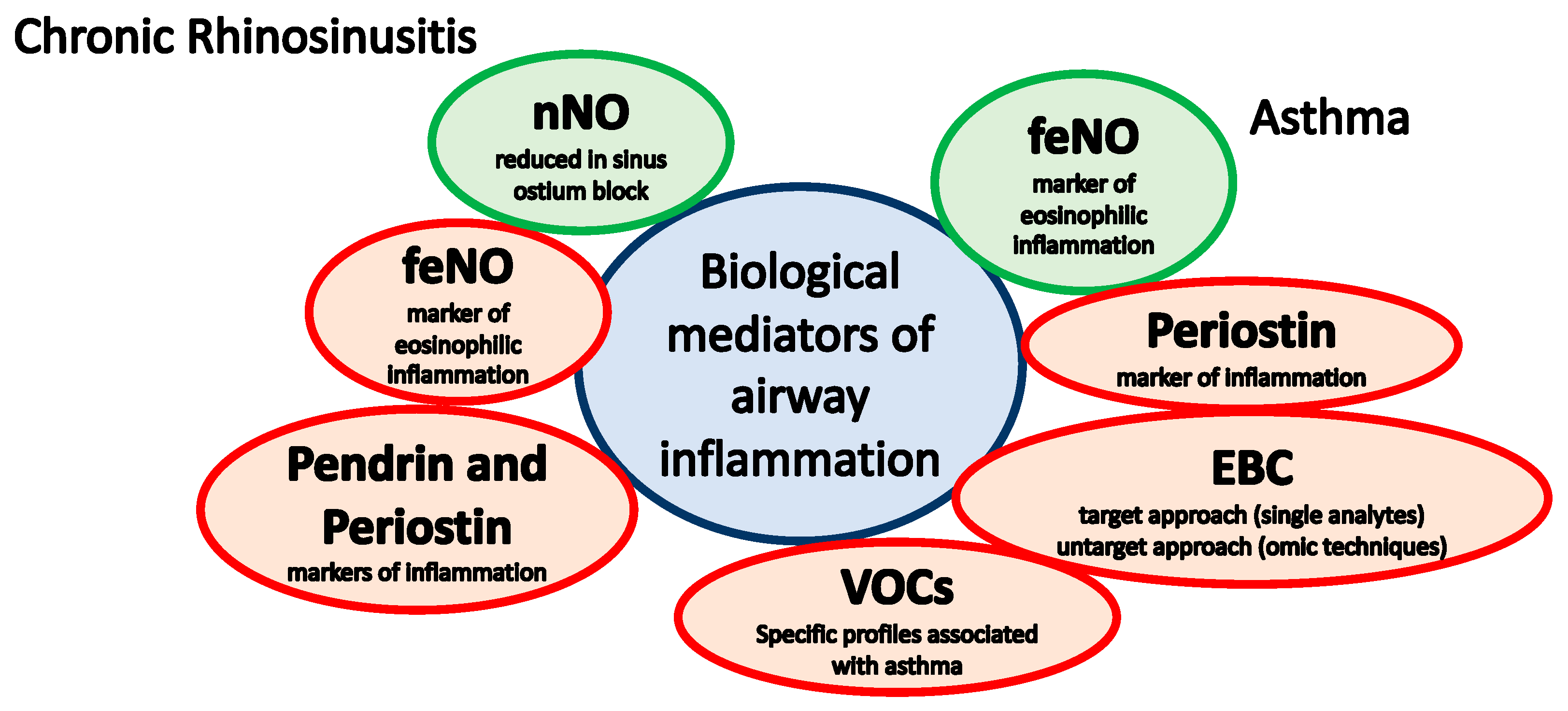
:1. Introduction
2. Chronic Rhinosinusitis
2.1. Nitric Oxide
2.2. Pendrin and Periostin
3. Asthma
3.1. Fractional Concentration of Exhaled Nitric Oxide
- The National Institute for Health and Care Excellence (NICE, 2017) recommends measuring feNO (described as positive test when more than or equal to 35 ppb) in children (aged 5 to 16 years) with symptoms suggestive of asthma, if there is diagnostic uncertainty after initial assessment (e.g., normal spirometry or obstructive spirometry with a negative bronchodilator reversibility test) [86]. Furthermore, using feNO to monitor asthma control is not routinely recommended [86].
- The 2019 British Thoracic Society guidelines recommend to use feNO (if available) to find evidence of eosinophilic inflammation (regard a feNO level of 35 ppb or more as a positive test), keeping in mind that a positive test increases the probability of asthma, but a negative test does not exclude asthma [87]. Also, except in specialist asthma clinics, the routine use of feNO testing to monitor asthma in children is not recommended [87].
- The 2019 Global Initiative of Asthma (GINA) guidelines report that feNO is not useful for ruling in or ruling out a diagnosis of asthma nor for guiding asthma treatment in the general population. Among alternative strategies for adjusting asthma treatment in children, GINA guidelines report that feNO-guided treatment significantly reduces exacerbation rates compared with guidelines-based treatment (Evidence A). Furthermore, feNO seems to be a useful adjunct in diagnosing asthma in pre-school children with recurrent wheezing, in whom an elevated feNO (recorded 4 weeks from any URTI) predicts asthma at school age [88].
3.2. Periostin
3.3. Exhaled Breath Condensate (EBC)
- Leukotrienes (LT): LTB4, a potent inflammatory mediator and a chemoattractant for neutrophils, was increased in the EBC of asthmatic children, being twice as high in steroid-naïve patients with asthma as in healthy subjects [104,105]; Cysteinyl leukotrienes (LTC4, LTD4, and LTE4), powerful constrictors and proinflammatory mediators, were increased in particular in unstable or severe asthma [106,107,108];
3.4. Volatile Organic Compounds (VOCs)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rosati, M.G.; Peters, A.T. Relationships among allergic rhinitis, asthma, and chronic rhinosinusitis. Am. J. Rhinol. Allergy 2016, 30, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Massoth, L.; Anderson, C.; McKinney, K.A. Asthma and Chronic Rhinosinusitis: Diagnosis and Medical Management. Med. Sci. 2019, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Licari, A.; Castagnoli, R.; Denicolò, C.F.; Rossini, L.; Marseglia, A.; Marseglia, G.L. The Nose and the Lung: United Airway Disease? Front. Pediatr. 2017, 5, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stachler, R.J. Comorbidities of asthma and the unified airway. Int. Forum Allergy Rhinol. 2015, 5 (Suppl. 1), S17–S22. [Google Scholar] [CrossRef] [PubMed]
- Grossman, J. One airway, one disease. Chest 1997, 111 (Suppl. 2), 11S–16S. [Google Scholar] [CrossRef]
- Licari, A.; Caimmi, S.; Bosa, L.; Marseglia, A.; Marseglia, G.L.; Caimmi, D. Rhinosinusitis and asthma: A very long engagement. Int. J. Immunopathol. Pharmacol. 2014, 27, 499–508. [Google Scholar] [CrossRef]
- Maniscalco, M.; Sofia, M.; Pelaia, G. Nitric oxide in upper airways inflammatory diseases. Inflamm. Res. 2007, 56, 58–69. [Google Scholar] [CrossRef]
- Maniscalco, M.; Bianco, A.; Mazzarella, G.; Motta, A. Recent Advances on Nitric Oxide in the Upper Airways. Curr. Med. Chem. 2016, 23, 2736–2745. [Google Scholar] [CrossRef]
- Kim, H.B.; Eckel, S.P.; Kim, J.H.; Gilliland, F.D. Exhaled NO: Determinants and Clinical Application in Children with Allergic Airway Disease. Allergy Asthma Immunol. Res. 2016, 8, 12–21. [Google Scholar] [CrossRef]
- Lucas, J.S.; Walker, W.T. NO way! Nasal nitric oxide measurement in infants. Eur. Respir. J. 2018, 51, 1800958. [Google Scholar] [CrossRef] [Green Version]
- American Thoracic Society; European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide. Am. J. Respir. Crit. Care Med. 2005, 171, 912–930. [Google Scholar] [CrossRef] [PubMed]
- Marthin, J.K.; Nielsen, K.G. Hand-held tidal breathing nasal nitric oxide measurement—A promising targeted case-finding tool for the diagnosis of primary ciliary dyskinesia. PLoS ONE 2013, 8, e57262. [Google Scholar] [CrossRef] [PubMed]
- Mateos-Corral, D.; Coombs, R.; Grasemann, H.; Ratjen, F.; Dell, S.D. Diagnostic value of nasal nitric oxide measured with non-velum closure techniques for children with primary ciliary dyskinesia. J. Pediatr. 2011, 159, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.; Bhullar, E.; Gove, K.; Joslin, R.; Pelling, J.; Evans, H.J.; Walker, W.T.; Lucas, J.S. Validation of a portable nitric oxide analyzer for screening in primary ciliary dyskinesias. BMC Pulm. Med. 2014, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Beydon, N.; Chambellan, A.; Alberti, C.; de Blic, J.; Clément, A.; Escudier, E.; Le Bourgeois, M. Technical and practical issues for tidal breathing measurements of nasal nitric oxide in children. Pediatr. Pulmonol. 2015, 50, 1374–1382. [Google Scholar] [CrossRef] [PubMed]
- Dweik, R.A.; Boggs, P.B.; Erzurum, S.C.; Irvin, C.G.; Leigh, M.W.; Lundberg, J.O.; Olin, A.C.; Plummer, A.L.; Taylor, D.R.; on behalf of the American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels (FeNO) for Clinical Applications. An official ATS clinical practice guideline: Interpretation of exhaled nitric oxide levels (FeNO) for clinical applications. Am. J. Respir. Crit. Care Med. 2011, 184, 602–615. [Google Scholar] [CrossRef] [PubMed]
- Baraldi, E.; Scollo, M.; Zaramella, C.; Zanconato, S.; Zacchello, F. A simple flow-driven method for online measurement of exhaled NO starting at the age of 4 to 5 years. Am. J. Respir. Crit. Care Med. 2000, 162, 1828–1832. [Google Scholar] [CrossRef]
- Baraldi, E.; de Jongste, J.C. European Respiratory Society/American Thoracic Society (ERS/ATS) Task Force. Measurement of exhaled nitric oxide in children, 2001. Eur. Respir. J. 2002, 20, 223–237. [Google Scholar]
- Franklin, P.J.; Turner, S.W.; Mutch, R.C.; Stick, S.M. Comparison of single-breath and tidal breathing exhaled nitric oxide levels in infants. Eur. Respir. J. 2004, 23, 369–372. [Google Scholar] [CrossRef] [Green Version]
- Silkoff, P.E.; Stevens, A.; Pak, J.; Bucher-Bartelson, B.; Martin, R.J. A method for the standardized offline collection of exhaled nitric oxide. Chest 1999, 116, 754–759. [Google Scholar] [CrossRef]
- Van der Heijden, H.H.; Brouwer, M.L.; Hoekstra, F.; van der Pol, P.; Merkus, P.J. Reference values of exhaled nitric oxide in healthy children 1–5 years using off-line tidal breathing. Pediatr. Pulmonol. 2014, 49, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Pijnenburg, M.W. The Role of FeNO in Predicting Asthma. Front. Pediatr. 2019, 7, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heijkenskjöld-Rentzhog, C.; Kalm-Stephens, P.; Nordvall, L.; Malinovschi, A.; Alving, K. New method for single-breath fraction of exhaled nitric oxide measurement with improved feasibility in preschool children with asthma. Pediatr. Allergy Immunol. 2015, 26, 662–667. [Google Scholar] [CrossRef]
- Van Mastrigt, E.; de Groot, R.C.A.; van Kesteren, H.W.; Vink, A.T.J.; de Jongste, J.C.; Pijnenburg, M.W.H. Tidal breathing FeNO measurements: A new algorithm. Pediatr. Pulmonol. 2014, 49, 15–20. [Google Scholar] [CrossRef]
- Magit, A. Pediatric rhinosinusitis. Otolaryngol. Clin. N. Am. 2014, 47, 733–746. [Google Scholar] [CrossRef]
- Alving, K.; Weitzberg, E.; Lundberg, J.M. Increased amount of nitric oxide in exhaled air of asthmatics. Eur. Respir. J. 1993, 6, 1368–1370. [Google Scholar]
- Lundberg, J.O.; Weitzberg, E. Nasal nitric oxide in man. Thorax 1999, 54, 947–952. [Google Scholar] [CrossRef] [Green Version]
- Lundberg, J.O.; Farkas-Szallasi, T.; Weitzberg, E.; Rinder, J.; Lidholm, J.; Anggåard, A.; Hökfelt, T.; Lundberg, J.M.; Alving, K. High nitric oxide production in human paranasal sinuses. Nat. Med. 1995, 1, 370–373. [Google Scholar] [CrossRef]
- Schairer, D.O.; Chouake, J.S.; Nosanchuk, J.D.; Friedman, A.J. The potential of nitric oxide releasing therapies as antimicrobial agents. Virulence 2012, 3, 271–279. [Google Scholar] [CrossRef] [Green Version]
- Lindberg, S.; Cervin, A.; Runer, T. Low levels of nasal nitric oxide (NO) correlate to impaired mucociliary function in the upper airways. Acta Oto-Laryngol. 1997, 117, 728–734. [Google Scholar] [CrossRef]
- Holden, W.E.; Wilkins, J.P.; Harris, M.; Milczuk, H.A.; Giraud, G.D. Temperature conditioning of nasal air: Effects of vasoactive agents and involvement of nitric oxide. J. Appl. Physiol. 1999, 87, 1260–1265. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, H.; Rossaint, R.; Pappert, D.; Knorr, M.; Falke, K.J. Autoinhalation of nitric oxide after endogenous synthesis in nasopharynx. Lancet 1994, 343, 518–519. [Google Scholar] [CrossRef]
- Lundberg, J. Airborne nitric oxide: Inflammatory marker and aerocrine messenger in man. Acta Physiol. Scand. 1996, 157, 4–27. [Google Scholar] [CrossRef]
- Baraldi, E.; Azzolin, N.M.; Biban, P.; Zacchello, F. Effect of antibiotic therapy on nasal nitric oxide concentration in children with acute sinusitis. Am. J. Respir. Crit. Care Med. 1997, 155, 1680–1683. [Google Scholar] [CrossRef]
- Colantonio, D.; Brouillette, L.; Parikh, A.; Scadding, G.K. Paradoxical low nasal nitric oxide in nasal polyposis. Clin. Exp. Allergy 2002, 32, 698–701. [Google Scholar] [CrossRef]
- Ragab, S.M.; Lund, V.J.; Saleh, H.A.; Scadding, G. Nasal nitric oxide in objective evaluation of chronic rhinosinusitis therapy. Allergy 2006, 61, 717–724. [Google Scholar] [CrossRef]
- Arnal, J.F.; Flores, P.; Rami, J.; Murris-Espin, M.; Bremont, F.; Pasto, I.; Aguilla, M.; Serrano, E.; Didier, A. Nasal nitric oxide concentration in paranasal sinus inflammatory diseases. Eur. Respir. J. 1999, 13, 307–312. [Google Scholar] [CrossRef]
- Rouby, J.-J. The nose, nitric oxide, and paranasal sinuses: The outpost of pulmonary antiinfectious defenses? Am. J. Respir. Crit. Care Med. 2003, 168, 265–266. [Google Scholar] [CrossRef]
- Phillips, P.S.; Sacks, R.; Marcells, G.N.; Cohen, N.A.; Harvey, R.J. Nasal nitric oxide and sinonasal disease: A systematic review of published evidence. Otolaryngol. Head Neck Surg. 2011, 144, 159–169. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Asako, M.; Ooka, H.; Kanda, A.; Tomoda, K.; Yasuba, H. Residual exhaled nitric oxide elevation in asthmatics is associated with eosinophilic chronic rhinosinusitis. J. Asthma 2015, 52, 1060–1064. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, Y.; Liu, M.; Sun, C.; Tian, L. Predictive and Diagnostic Value of Fractional Exhaled Nitric Oxide in Patients with Chronic Rhinosinusitis. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; Yoo, H.S.; Lee, S.H.; Kim, K.R.; Yoon, H.J.; Kim, S.H. Nasal and exhaled nitric oxide in chronic rhinosinusitis with polyps. Am. J. Rhinol. Allergy 2014, 28, e11–e16. [Google Scholar] [CrossRef] [PubMed]
- Takeno, S.; Taruya, T.; Ueda, T.; Noda, N.; Hirakawa, K. Increased exhaled nitric oxide and its oxidation metabolism in eosinophilic chronic rhinosinusitis. Auris Nasus Larynx 2013, 40, 458–464. [Google Scholar] [CrossRef]
- Kambara, R.; Minami, T.; Akazawa, H.; Tsuji, F.; Sasaki, T.; Inohara, H.; Horii, A. Lower Airway Inflammation in Eosinophilic Chronic Rhinosinusitis as Determined by Exhaled Nitric Oxide. Int. Arch. Allergy Immunol. 2017, 173, 225–232. [Google Scholar] [CrossRef]
- Nakagami, Y.; Favoreto, S.; Zhen, G.; Park, S.-W.; Nguyenvu, L.T.; Kuperman, D.A.; Dolganov, G.M.; Huang, X.; Boushey, H.A.; Avila, P.C.; et al. The epithelial anion transporter pendrin is induced by allergy and rhinovirus infection, regulates airway surface liquid, and increases airway reactivity and inflammation in an asthma model. J. Immunol. 2008, 181, 2203–2210. [Google Scholar] [CrossRef]
- Nakao, I.; Kanaji, S.; Ohta, S.; Matsushita, H.; Arima, K.; Yuyama, N.; Yamaya, M.; Nakayama, K.; Kubo, H.; Watanabe, M.; et al. Identification of pendrin as a common mediator for mucus production in bronchial asthma and chronic obstructive pulmonary disease. J. Immunol. 2008, 180, 6262–6269. [Google Scholar] [CrossRef]
- Garnett, J.P.; Hickman, E.; Burrows, R.; Hegyi, P.; Tiszlavicz, L.; Cuthbert, A.W.; Fong, P.; Gray, M.A. Novel role for pendrin in orchestrating bicarbonate secretion in cystic fibrosis transmembrane conductance regulator (CFTR)-expressing airway serous cells. J. Biol. Chem. 2011, 286, 41069–41082. [Google Scholar] [CrossRef]
- Ishida, A.; Ohta, N.; Suzuki, Y.; Kakehata, S.; Okubo, K.; Ikeda, H.; Shiraishi, H.; Izuhara, K. Expression of pendrin and periostin in allergic rhinitis and chronic rhinosinusitis. Allergol. Int. 2012, 61, 589–595. [Google Scholar] [CrossRef]
- Seshadri, S.; Lu, X.; Purkey, M.R.; Homma, T.; Choi, A.W.; Carter, R.; Suh, L.; Norton, J.; Harris, K.E.; Conley, D.B.; et al. Increased expression of the epithelial anion transporter pendrin/SLC26A4 in nasal polyps of patients with chronic rhinosinusitis. J. Allergy Clin. Immunol. 2015, 136, 1548–1558.e7. [Google Scholar] [CrossRef] [Green Version]
- Jia, G.; Erickson, R.W.; Choy, D.F.; Mosesova, S.; Wu, L.C.; Solberg, O.D.; Shikotra, A.; Carter, R.; Audusseau, S.; Hamid, Q.; et al. Periostin is a systemic biomarker of eosinophilic airway inflammation in asthmatic patients. J. Allergy Clin. Immunol. 2012, 130, 647–654.e10. [Google Scholar] [CrossRef] [Green Version]
- Loutsios, C.; Farahi, N.; Porter, L.; Lok, L.S.C.; Peters, A.M.; Condliffe, A.M.; Chilvers, E.R. Biomarkers of eosinophilic inflammation in asthma. Expert Rev. Respir. Med. 2014, 8, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Takayama, G.; Arima, K.; Kanaji, T.; Toda, S.; Tanaka, H.; Shoji, S.; McKenzie, A.N.; Nagai, H.; Hotokebuchi, T.; Izuhara, K. Periostin: A novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J. Allergy Clin. Immunol. 2006, 118, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Ebenezer, J.A.; Christensen, J.M.; Oliver, B.G.; Oliver, R.A.; Tjin, G.; Ho, J.; Habib, A.R.; Rimmer, J.; Sacks, R.; Harvey, R.J. Periostin as a marker of mucosal remodelling in chronic rhinosinusitis. Rhinology 2017, 55, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Stankovic, K.M.; Goldsztein, H.; Reh, D.D.; Platt, M.P.; Metson, R. Gene expression profiling of nasal polyps associated with chronic sinusitis and aspirin-sensitive asthma. Laryngoscope 2008, 118, 881–889. [Google Scholar] [CrossRef]
- Maxfield, A.Z.; Landegger, L.D.; Brook, C.D.; Lehmann, A.E.; Campbell, A.P.; Bergmark, R.W.; Stankovic, K.M.; Metson, R. Periostin as a Biomarker for Nasal Polyps in Chronic Rhinosinusitis. Otolaryngol. Head Neck Surg. 2018, 158, 181–186. [Google Scholar] [CrossRef]
- Xu, M.; Chen, D.; Zhou, H.; Zhang, W.; Xu, J.; Chen, L. The Role of Periostin in the Occurrence and Progression of Eosinophilic Chronic Sinusitis with Nasal Polyps. Sci. Rep. 2017, 7, 9479. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, W.; Chen, D.; Zhou, H.; Chen, L. Diagnostic significance of serum periostin in eosinophilic chronic sinusitis with nasal polyps. Acta Otolaryngol. 2018, 138, 387–391. [Google Scholar] [CrossRef]
- Jonstam, K.; Westman, M.; Holtappels, G.; Holweg, C.T.J.; Bachert, C. Serum periostin, IgE, and SE-IgE can be used as biomarkers to identify moderate to severe chronic rhinosinusitis with nasal polyps. J. Allergy Clin. Immunol. 2017, 140, 1705–1708.e3. [Google Scholar] [CrossRef] [Green Version]
- Papi, A.; Brightling, C.; Pedersen, S.E.; Reddel, H.K. Asthma. Lancet 2018, 391, 783–800. [Google Scholar] [CrossRef]
- Landgraf-Rauf, K.; Anselm, B.; Schaub, B. The puzzle of immune phenotypes of childhood asthma. Mol. Cell Pediatr. 2016, 3, 27. [Google Scholar] [CrossRef]
- Pavord, I.D.; Beasley, R.; Agusti, A.; Anderson, G.P.; Bel, E.; Brusselle, G.; Cullinan, P.; Custovic, A.; Ducharme, F.M.; Fahy, J.V.; et al. After asthma: Redefining airways diseases. Lancet 2018, 391, 350–400. [Google Scholar] [CrossRef]
- Borland, C.; Cox, Y.; Higenbottam, T. Measurement of exhaled nitric oxide in man. Thorax 1993, 48, 1160–1162. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.V.; Sears, S.; Woods, J.; Ling, C.Y.; Hunt, J.; Clapper, L.M.; Gaston, B. Expired nitric oxide as a marker for childhood asthma. J. Pediatr. 1997, 130, 423–427. [Google Scholar] [CrossRef]
- Baraldi, E.; Azzolin, N.M.; Zanconato, S.; Dario, C.; Zacchello, F. Corticosteroids decrease exhaled nitric oxide in children with acute asthma. J. Pediatr. 1997, 131, 381–385. [Google Scholar] [CrossRef]
- Mahr, T.A.; Malka, J.; Spahn, J.D. Inflammometry in pediatric asthma: A review of fractional exhaled nitric oxide in clinical practice. Allergy Asthma Proc. 2013, 34, 210–219. [Google Scholar] [CrossRef]
- Pijnenburg, M.W.H.; De Jongste, J.C. Exhaled nitric oxide in childhood asthma: A review. Clin. Exp. Allergy 2008, 38, 246–259. [Google Scholar] [CrossRef]
- Fleming, L.; Tsartsali, L.; Wilson, N.; Regamey, N.; Bush, A. Longitudinal relationship between sputum eosinophils and exhaled nitric oxide in children with asthma. Am. J. Respir. Crit. Care Med. 2013, 188, 400–402. [Google Scholar] [CrossRef]
- Karrasch, S.; Linde, K.; Rücker, G.; Sommer, H.; Karsch-Völk, M.; Kleijnen, J.; Jörres, R.A.; Schneider, A. Accuracy of FeNO for diagnosing asthma: A systematic review. Thorax 2017, 72, 109–116. [Google Scholar] [CrossRef]
- Taylor, D.R.; Pijnenburg, M.W.; Smith, A.D.; De Jongste, J.C. Exhaled nitric oxide measurements: Clinical application and interpretation. Thorax 2006, 61, 817–827. [Google Scholar] [CrossRef]
- Moeller, A.; Carlsen, K.-H.; Sly, P.D.; Baraldi, E.; Piacentini, G.; Pavord, I.; Lex, C.; Saglani, S.; on behalf of the ERS Task Force Monitoring Asthma in Children. Monitoring asthma in childhood: Lung function, bronchial responsiveness and inflammation. Eur. Respir. Rev. 2015, 24, 204–215. [Google Scholar] [CrossRef]
- Baraldi, E.; Dario, C.; Ongaro, R.; Scollo, M.; Azzolin, N.M.; Panza, N.; Paganini, N.; Zacchello, F. Exhaled nitric oxide concentrations during treatment of wheezing exacerbation in infants and young children. Am. J. Respir. Crit. Care Med. 1999, 159, 1284–1288. [Google Scholar] [CrossRef] [PubMed]
- Sayão, L.B.; de Britto, M.C.A.; Burity, E.; Rattes, C.; Reinaux, C.M.A.; Fink, J.; Dornelas de Andrade, A. Exhaled nitric oxide as a diagnostic tool for wheezing in preschool children: A diagnostic accuracy study. Respir. Med. 2016, 113, 15–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soh, J.E.; Kim, K.-M.; Kwon, J.-W.; Kim, H.Y.; Seo, J.-H.; Kim, H.-B.; Lee, S.Y.; Jang, G.C.; Song, D.J.; Kim, W.K.; et al. Recurrent wheeze and its relationship with lung function and airway inflammation in preschool children: A cross-sectional study in South Korea. BMJ Open 2017, 7, e018010. [Google Scholar] [CrossRef] [PubMed]
- Moeller, A.; Diefenbacher, C.; Lehmann, A.; Rochat, M.; Brooks-Wildhaber, J.; Hall, G.L.; Wildhaber, J.H. Exhaled nitric oxide distinguishes between subgroups of preschool children with respiratory symptoms. J. Allergy Clin. Immunol. 2008, 121, 705–709. [Google Scholar] [CrossRef]
- Castro-Rodriguez, J.A.; Sardón, O.; Pérez-Yarza, E.G.; Korta, J.; Aldasoro, A.; Corcuera, P.; Mintegui, J. Young infants with recurrent wheezing and positive asthma predictive index have higher levels of exhaled nitric oxide. J. Asthma 2013, 50, 162–165. [Google Scholar] [CrossRef]
- Oh, M.-A.; Shim, J.Y.; Jung, Y.-H.; Seo, J.-H.; Young Kim, H.; Kwon, J.-W.; Kim, B.J.; Kim, H.B.; Kim, W.K.; Lee, S.Y.; et al. Fraction of exhaled nitric oxide and wheezing phenotypes in preschool children. Pediatr. Pulmonol. 2013, 48, 563–570. [Google Scholar] [CrossRef]
- Singer, F.; Luchsinger, I.; Inci, D.; Knauer, N.; Latzin, P.; Wildhaber, J.H.; Moeller, A. Exhaled nitric oxide in symptomatic children at preschool age predicts later asthma. Allergy 2013, 68, 531–538. [Google Scholar] [CrossRef]
- Caudri, D.; Wijga, A.H.; Hoekstra, M.O.; Kerkhof, M.; Koppelman, G.H.; Brunekreef, B.; Smit, H.A.; de Jongste, J.C. Prediction of asthma in symptomatic preschool children using exhaled nitric oxide, Rint and specific IgE. Thorax 2010, 65, 801–807. [Google Scholar] [CrossRef] [Green Version]
- Elliott, M.; Heltshe, S.L.; Stamey, D.C.; Cochrane, E.S.; Redding, G.J.; Debley, J.S. Exhaled nitric oxide predicts persistence of wheezing, exacerbations, and decline in lung function in wheezy infants and toddlers. Clin. Exp. Allergy 2013, 43, 1351–1361. [Google Scholar] [CrossRef] [Green Version]
- Debley, J.S.; Stamey, D.C.; Cochrane, E.S.; Gama, K.L.; Redding, G.J. Exhaled nitric oxide, lung function, and exacerbations in wheezy infants and toddlers. J. Allergy Clin. Immunol. 2010, 125, 1228–1234.e13. [Google Scholar] [CrossRef] [Green Version]
- Linn, W.S.; Rappaport, E.B.; Berhane, K.T.; Bastain, T.M.; Avol, E.L.; Gilliland, F.D. Exhaled nitric oxide in a population-based study of southern California schoolchildren. Respir. Res. 2009, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Di Cara, G.; Marcucci, F.; Palomba, A.; Milioni, M.; Pecoraro, L.; Ciprandi, G.; Buttafava, S.; Frati, F.; Verrotti, A. Exhaled nitric oxide in children with allergic rhinitis: A potential biomarker of asthma development. Pediatr. Allergy Immunol. 2015, 26, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Horváth, I.; Barnes, P.J.; Loukides, S.; Sterk, P.J.; Högman, M.; Olin, A.-C.; Amann, A.; Antus, B.; Baraldi, E.; Bikov, A.; et al. A European Respiratory Society technical standard: Exhaled biomarkers in lung disease. Eur. Respir. J. 2017, 49, 1600965. [Google Scholar] [CrossRef]
- Moeller, A.; Franklin, P.; Hall, G.L.; Turner, S.; Straub, D.; Wildhaber, J.H.; Stick, S.M. Inhaled fluticasone dipropionate decreases levels of nitric oxide in recurrenty wheezy infants. Pediatr. Pulmonol. 2004, 38, 250–255. [Google Scholar] [CrossRef]
- Petsky, H.L.; Cates, C.J.; Kew, K.M.; Chang, A.B. Tailoring asthma treatment on eosinophilic markers (exhaled nitric oxide or sputum eosinophils): A systematic review and meta-analysis. Thorax 2018, 73, 1110–1119. [Google Scholar] [CrossRef]
- NICE. Asthma: Diagnosis, Monitoring and Chronic Asthma Management. Guidance and Guidelines. Available online: https://www.nice.org.uk/guidance/ng80 (accessed on 3 July 2018).
- SIGN 158 British Guideline on the Management of Asthma. Available online: https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma.html (accessed on 19 August 2019).
- 2019 GINA Main Report. Global Initiative for Asthma—GINA. Available online: https://ginasthma.org/gina-reports/ (accessed on 23 July 2019).
- Fingleton, J.; Braithwaite, I.; Travers, J.; Bowles, D.; Strik, R.; Siebers, R.; Holweg, C.; Matthews, J.; Weatherall, M.; Beasley, R. Serum periostin in obstructive airways disease. Eur. Respir. J. 2016, 47, 1383–1391. [Google Scholar] [CrossRef] [Green Version]
- Kanemitsu, Y.; Matsumoto, H.; Izuhara, K.; Tohda, Y.; Kita, H.; Horiguchi, T.; Kuwabara, K.; Tomii, K.; Otsuka, K.; Fujimura, M.; et al. Increased periostin associates with greater airflow limitation in patients receiving inhaled corticosteroids. J. Allergy Clin. Immunol. 2013, 132, 305–312.e3. [Google Scholar] [CrossRef] [Green Version]
- Matsusaka, M.; Kabata, H.; Fukunaga, K.; Suzuki, Y.; Masaki, K.; Mochimaru, T.; Sakamaki, F.; Oyamada, Y.; Inoue, T.; Oguma, T.; et al. Phenotype of asthma related with high serum periostin levels. Allergol. Int. 2015, 64, 175–180. [Google Scholar] [CrossRef]
- Sidhu, S.S.; Yuan, S.; Innes, A.L.; Kerr, S.; Woodruff, P.G.; Hou, L.; Muller, S.J.; Fahy, J.V. Roles of epithelial cell-derived periostin in TGF-beta activation, collagen production, and collagen gel elasticity in asthma. Proc. Natl. Acad. Sci. USA 2010, 107, 14170–14175. [Google Scholar] [CrossRef]
- James, A.; Hedlin, G. Biomarkers for the Phenotyping and Monitoring of Asthma in Children. Curr. Treat. Options Allergy 2016, 3, 439–452. [Google Scholar] [CrossRef] [Green Version]
- Anderson, H.M.; Lemanske, R.F.; Arron, J.R.; Holweg, C.T.J.; Rajamanickam, V.; Gangnon, R.E.; Gern, J.E.; Jackson, D.J. Relationships among aeroallergen sensitization, peripheral blood eosinophils, and periostin in pediatric asthma development. J. Allergy Clin. Immunol. 2017, 139, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Akashi, K.; Watanabe, M.; Ikeda, Y.; Ashizuka, S.; Motoki, T.; Suzuki, R.; Sagara, N.; Yanagida, N.; Sato, S.; et al. Periostin as a biomarker for the diagnosis of pediatric asthma. Pediatr. Allergy Immunol. 2016, 27, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Licari, A.; Castagnoli, R.; Brambilla, I.; Marseglia, A.; Tosca, M.A.; Marseglia, G.L.; Ciprandi, G. Asthma Endotyping and Biomarkers in Childhood Asthma. Pediatr. Allergy Immunol. Pulmonol. 2018, 1, 3144–3155. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.D.; Montpetit, A.J. Exhaled Breath Condensate: An Update. Immunol. Allergy Clin. N. Am. 2018, 38, 667–678. [Google Scholar] [CrossRef]
- Moschino, L.; Zanconato, S.; Bozzetto, S.; Baraldi, E.; Carraro, S. Childhood asthma biomarkers: Present knowledge and future steps. Paediatr. Respir. Rev. 2015, 16, 205–212. [Google Scholar] [CrossRef]
- Ferraro, V.; Carraro, S.; Bozzetto, S.; Zanconato, S.; Baraldi, E. Exhaled biomarkers in childhood asthma: Old and new approaches. Asthma Res. Pract. 2018, 4, 9. [Google Scholar] [CrossRef]
- Horváth, I.; Hunt, J.; Barnes, P.J.; Alving, K.; Antczak, A.; Baraldi, E.; Becher, G.; van Beurden, W.J.; Corradi, M.; Dekhuijzen, R.; et al. Exhaled breath condensate: Methodological recommendations and unresolved questions. Eur. Respir. J. 2005, 26, 523–548. [Google Scholar] [CrossRef]
- Thomas, P.S.; Lowe, A.J.; Samarasinghe, P.; Lodge, C.J.; Huang, Y.; Abramson, M.J.; Dharmage, S.C.; Jaffe, A. Exhaled breath condensate in pediatric asthma: Promising new advance or pouring cold water on a lot of hot air? A systematic review. Pediatr. Pulmonol. 2013, 48, 419–442. [Google Scholar] [CrossRef]
- Morton, J.; Henry, R.L.; Thomas, P.S. Exhaled breath condensate nitrite/nitrate and pH in relation to pediatric asthma control and exhaled nitric oxide. Pediatr. Pulmonol. 2006, 41, 929–936. [Google Scholar]
- Brunetti, L.; Francavilla, R.; Tesse, R.; Strippoli, A.; Polimeno, L.; Loforese, A.; Miniello, V.L.; Armenio, L. Exhaled breath condensate pH measurement in children with asthma, allergic rhinitis and atopic dermatitis. Pediatr. Allergy Immunol. 2006, 17, 422–427. [Google Scholar] [CrossRef]
- Montuschi, P. LC/MS/MS analysis of leukotriene B4 and other eicosanoids in exhaled breath condensate for assessing lung inflammation. J. Chromatogr. B 2009, 877, 1272–1280. [Google Scholar] [CrossRef] [PubMed]
- Montuschi, P.; Barnes, P.J. Exhaled leukotrienes and prostaglandins in asthma. J. Allergy Clin. Immunol. 2002, 109, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Wan, G.-H.; Yan, D.-C.; Tseng, H.-Y.; Tung, T.-H.; Lin, S.-J.; Lin, Y.-W. Cysteinyl leukotriene levels correlate with 8-isoprostane levels in exhaled breath condensates of atopic and healthy children. Pediatr. Res. 2013, 74, 584–591. [Google Scholar] [CrossRef]
- Baraldi, E.; Carraro, S.; Alinovi, R.; Pesci, A.; Ghiro, L.; Bodini, A.; Piacentini, G.; Zacchello, F.; Zanconato, S. Cysteinyl leukotrienes and 8-isoprostane in exhaled breath condensate of children with asthma exacerbations. Thorax 2003, 58, 505–509. [Google Scholar] [CrossRef] [Green Version]
- Samitas, K.; Chorianopoulos, D.; Vittorakis, S.; Zervas, E.; Economidou, E.; Papatheodorou, G.; Loukides, S.; Gaga, M. Exhaled cysteinyl-leukotrienes and 8-isoprostane in patients with asthma and their relation to clinical severity. Respir. Med. 2009, 103, 750–756. [Google Scholar] [CrossRef] [Green Version]
- Bodini, A.; Peroni, D.; Vicentini, L.; Loiacono, A.; Baraldi, E.; Ghiro, L.; Corradi, M.; Alinovi, R.; Boner, A.L.; Piacentini, G.L. Exhaled breath condensate eicosanoids and sputum eosinophils in asthmatic children: A pilot study. Pediatr. Allergy Immunol. 2004, 15, 26–31. [Google Scholar] [CrossRef]
- Teng, Y.; Sun, P.; Zhang, J.; Yu, R.; Bai, J.; Yao, X.; Huang, M.; Adcock, I.M.; Barnes, P.J. Hydrogen peroxide in exhaled breath condensate in patients with asthma: A promising biomarker? Chest 2011, 140, 108–116. [Google Scholar] [CrossRef]
- Jöbsis, Q.; Raatgeep, H.C.; Hermans, P.W.; de Jongste, J.C. Hydrogen peroxide in exhaled air is increased in stable asthmatic children. Eur. Respir. J. 1997, 10, 519–521. [Google Scholar]
- Baraldi, E.; Giordano, G.; Pasquale, M.F.; Carraro, S.; Mardegan, A.; Bonetto, G.; Bastardo, C.; Zacchello, F.; Zanconato, S. 3-Nitrotyrosine, a marker of nitrosative stress, is increased in breath condensate of allergic asthmatic children. Allergy 2006, 61, 90–96. [Google Scholar] [CrossRef]
- Formanek, W.; Inci, D.; Lauener, R.P.; Wildhaber, J.H.; Frey, U.; Hall, G.L. Elevated nitrite in breath condensates of children with respiratory disease. Eur. Respir. J. 2002, 19, 487–491. [Google Scholar] [CrossRef] [Green Version]
- Wheelock, C.E.; Goss, V.M.; Balgoma, D.; Nicholas, B.; Brandsma, J.; Skipp, P.J.; Snowden, S.; Burg, D.; D’Amico, A.; Horvath, I.; et al. Application of’omics technologies to biomarker discovery in inflammatory lung diseases. Eur. Respir. J. 2013, 42, 802–825. [Google Scholar] [CrossRef] [PubMed]
- Bush, A. Translating Asthma: Dissecting the Role of Metabolomics, Genomics and Personalized Medicine. Indian J. Pediatr. 2018, 85, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-L.; Bonnichsen, M.H.; Nogeh, E.U.; Raftery, M.J.; Thomas, P.S. Proteomics in detection and monitoring of asthma and smoking-related lung diseases. Expert Rev. Proteom. 2010, 7, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Wiktorowicz, J.E.; Jamaluddin, M. Proteomic analysis of the asthmatic airway. Adv. Exp. Med. Biol. 2014, 795, 221–232. [Google Scholar]
- Lacombe, M.; Marie-Desvergne, C.; Combes, F.; Kraut, A.; Bruley, C.; Vandenbrouck, Y.; Chamel Mossuz, V.; Couté, Y.; Brun, V. Proteomic characterization of human exhaled breath condensate. J. Breath Res. 2018, 12, 021001. [Google Scholar] [CrossRef]
- Muccilli, V.; Saletti, R.; Cunsolo, V.; Ho, J.; Gili, E.; Conte, E.; Sichili, S.; Vancheri, C.; Foti, S. Protein profile of exhaled breath condensate determined by high resolution mass spectrometry. J. Pharm. Biomed. Anal. 2015, 105, 134–149. [Google Scholar] [CrossRef]
- Bloemen, K.; Van Den Heuvel, R.; Govarts, E.; Hooyberghs, J.; Nelen, V.; Witters, E.; Desager, K.; Schoeters, G. A new approach to study exhaled proteins as potential biomarkers for asthma. Clin. Exp. Allergy 2011, 41, 346–356. [Google Scholar] [CrossRef]
- Turi, K.N.; Romick-Rosendale, L.; Ryckman, K.K.; Hartert, T.V. A review of metabolomics approaches and their application in identifying causal pathways of childhood asthma. J. Allergy Clin. Immunol. 2018, 141, 1191–1201. [Google Scholar] [CrossRef]
- Carraro, S.; Giordano, G.; Reniero, F.; Perilongo, G.; Baraldi, E. Metabolomics: A new frontier for research in pediatrics. J. Pediatr. 2009, 154, 638–644. [Google Scholar] [CrossRef]
- Carraro, S.; Rezzi, S.; Reniero, F.; Héberger, K.; Giordano, G.; Zanconato, S.; Guillou, C.; Baraldi, E. Metabolomics applied to exhaled breath condensate in childhood asthma. Am. J. Respir. Crit. Care Med. 2007, 175, 986–990. [Google Scholar] [CrossRef]
- Carraro, S.; Giordano, G.; Reniero, F.; Carpi, D.; Stocchero, M.; Sterk, P.J.; Baraldi, E. Asthma severity in childhood and metabolomic profiling of breath condensate. Allergy 2013, 68, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidi, E.M.; Lappas, A.S.; Tzortzi, A.S.; Behrakis, P.K. Exhaled Breath Condensate: Technical and Diagnostic Aspects. Sci. World J. 2015, 2015, 435160. [Google Scholar] [CrossRef] [PubMed]
- Bannier, M.A.; Rosias, P.P.; Jöbsis, Q.; Dompeling, E. Exhaled Breath Condensate in Childhood Asthma: A Review and Current Perspective. Front. Pediatr. 2019, 7, 150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neerincx, A.H.; Vijverberg, S.J.H.; Bos, L.D.J.; Brinkman, P.; van der Schee, M.P.; de Vries, R.; Sterk, P.J.; Maitland-van der Zee, A.H. Breathomics from exhaled volatile organic compounds in pediatric asthma. Pediatr. Pulmonol. 2017, 52, 1616–1627. [Google Scholar] [CrossRef] [PubMed]
- Van Mastrigt, E.; de Jongste, J.C.; Pijnenburg, M.W. The analysis of volatile organic compounds in exhaled breath and biomarkers in exhaled breath condensate in children—Clinical tools or scientific toys? Clin. Exp. Allergy 2015, 45, 1170–1188. [Google Scholar] [CrossRef] [PubMed]
- Van der Schee, M.P.; Paff, T.; Brinkman, P.; van Aalderen, W.M.C.; Haarman, E.G.; Sterk, P.J. Breathomics in lung disease. Chest 2015, 147, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Fens, N.; van der Schee, M.P.; Brinkman, P.; Sterk, P.J. Exhaled breath analysis by electronic nose in airways disease. Established issues and key questions. Clin. Exp. Allergy 2013, 43, 705–715. [Google Scholar] [CrossRef]
- Van Mastrigt, E.; Reyes-Reyes, A.; Brand, K.; Bhattacharya, N.; Urbach, H.P.; Stubbs, A.P.; de Jongste, J.C.; Pijnenburg, M.W. Exhaled breath profiling using broadband quantum cascade laser-based spectroscopy in healthy children and children with asthma and cystic fibrosis. J. Breath Res. 2016, 10, 026003. [Google Scholar] [CrossRef]
- Gahleitner, F.; Guallar-Hoyas, C.; Beardsmore, C.S.; Pandya, H.C.; Thomas, C.P. Metabolomics pilot study to identify volatile organic compound markers of childhood asthma in exhaled breath. Bioanalysis 2013, 5, 2239–2247. [Google Scholar] [CrossRef]
- Caldeira, M.; Barros, A.S.; Bilelo, M.J.; Parada, A.; Câmara, J.S.; Rocha, S.M. Profiling allergic asthma volatile metabolic patterns using a headspace-solid phase microextraction/gas chromatography based methodology. J. Chromatogr. A 2011, 1218, 3771–3780. [Google Scholar] [CrossRef]
- Dallinga, J.W.; Robroeks, C.M.H.H.T.; van Berkel, J.J.B.N.; Moonen, E.J.C.; Godschalk, R.W.L.; Jöbsis, Q.; Dompeling, E.; Wouters, E.F.; van Schooten, F.J. Volatile organic compounds in exhaled breath as a diagnostic tool for asthma in children. Clin. Exp. Allergy 2010, 40, 68–76. [Google Scholar]
- Caldeira, M.; Perestrelo, R.; Barros, A.S.; Bilelo, M.J.; Morête, A.; Câmara, J.S.; Rocha, S.M. Allergic asthma exhaled breath metabolome: A challenge for comprehensive two-dimensional gas chromatography. J. Chromatogr. A 2012, 1254, 87–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klaassen, E.M.M.; van de Kant, K.D.G.; Jöbsis, Q.; van Schayck, O.C.P.; Smolinska, A.; Dallinga, J.W.; van Schooten, F.J.; den Hartog, G.J.; de Jongste, J.C.; Rijkers, G.T.; et al. Exhaled biomarkers and gene expression at preschool age improve asthma prediction at 6 years of age. Am. J. Respir. Crit. Care Med. 2015, 191, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Smolinska, A.; Klaassen, E.M.M.; Dallinga, J.W.; van de Kant, K.D.G.; Jobsis, Q.; Moonen, E.J.C.; van Schayck, O.C.; Dompeling, E.; van Schooten, F.J. Profiling of volatile organic compounds in exhaled breath as a strategy to find early predictive signatures of asthma in children. PLoS ONE 2014, 9, e95668. [Google Scholar] [CrossRef]
- Van Vliet, D.; Smolinska, A.; Jöbsis, Q.; Rosias, P.; Muris, J.; Dallinga, J.; Dompeling, E.; van Schooten, F.J. Can exhaled volatile organic compounds predict asthma exacerbations in children? J. Breath Res. 2017, 11, 016016. [Google Scholar] [CrossRef]
- Robroeks, C.M.; van Berkel, J.J.; Jöbsis, Q.; van Schooten, F.-J.; Dallinga, J.W.; Wouters, E.F.; Dompeling, E. Exhaled volatile organic compounds predict exacerbations of childhood asthma in a 1-year prospective study. Eur. Respir. J. 2013, 42, 98–106. [Google Scholar] [CrossRef] [Green Version]
- Van Vliet, D.; Smolinska, A.; Jöbsis, Q.; Rosias, P.P.R.; Muris, J.W.M.; Dallinga, J.W.; van Schooten, F.J.; Dompeling, E. Association between exhaled inflammatory markers and asthma control in children. J. Breath Res. 2016, 10, 016014. [Google Scholar] [CrossRef]
- Bannier, M.A.; van de Kant, K.D.; Jöbsis, Q.; Dompeling, E. Feasibility and diagnostic accuracy of an electronic nose in children with asthma and cystic fibrosis. J. Breath Res. 2019, 13, 036009. [Google Scholar] [CrossRef]
| Study | Aims | Population | Results (mean ± SD) | Conclusions |
|---|---|---|---|---|
| [34] | To evaluate nNO in children with acute maxillary sinusitis before and after treatment with antibiotic therapy | 16 children (4–13 years) with acute maxillary sinusitis; 16 age- and sex -matched healthy control subjects |
| During acute maxillary sinusitis, nNO is decreased; nNO returns to normal after antibiotic therapy |
| [37] | To examine if nNO is affected by paranasal sinus inflammatory diseases | 20 patients with nonallergic nasal polyposis (age 48 ± 3 years); 42 control subjects (age 42 ± 3 years) |
| nNO in patients with nasal polyposis is decreased compared to controls, and it depends on the degree of obstruction of the paranasal sinuses |
| [35] | To evaluate nNO in patients with nasal polyposis compared with allergic rhinitis and to analyze the effect of polyp treatment on nNO | 44 patients with rhinitis without polyps (age = 39 ± 13.6 years) and 38 with polyps (age = 45.6 ± 4.5 years); 20 normal controls (age = 36.9 ± 11.6 years); 23 patients with polyposis pre- and post-treatment (age = 48.8 ± 4.2 years) |
| nNO levels are low in nasal polyps. A rise in nNO is seen with successful polyp treatment |
| [36] | To study the effect of CRS therapy on nNO and to see whether nNO changes correlate with other assessments. | 90 patients (mean age 43 ± 13 years) with CRS who still had troublesome symptoms after initial therapy with dexarhinaspray and nasal douching |
| nNO provides a valuable non-invasive objective measure of the response of CRS to therapy |
| Guideline | Cut-off Value | How to Use feNO in Clinical Practice |
|---|---|---|
| [86] | feNO positive if more than or equal to 35 ppb |
|
| [87] | feNO positive if more than or equal to 35 ppb |
|
| [88] | No clear cut-off value |
|
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferraro, V.A.; Zanconato, S.; Baraldi, E.; Carraro, S. Nitric Oxide and Biological Mediators in Pediatric Chronic Rhinosinusitis and Asthma. J. Clin. Med. 2019, 8, 1783. https://doi.org/10.3390/jcm8111783
Ferraro VA, Zanconato S, Baraldi E, Carraro S. Nitric Oxide and Biological Mediators in Pediatric Chronic Rhinosinusitis and Asthma. Journal of Clinical Medicine. 2019; 8(11):1783. https://doi.org/10.3390/jcm8111783
Chicago/Turabian StyleFerraro, Valentina Agnese, Stefania Zanconato, Eugenio Baraldi, and Silvia Carraro. 2019. "Nitric Oxide and Biological Mediators in Pediatric Chronic Rhinosinusitis and Asthma" Journal of Clinical Medicine 8, no. 11: 1783. https://doi.org/10.3390/jcm8111783
APA StyleFerraro, V. A., Zanconato, S., Baraldi, E., & Carraro, S. (2019). Nitric Oxide and Biological Mediators in Pediatric Chronic Rhinosinusitis and Asthma. Journal of Clinical Medicine, 8(11), 1783. https://doi.org/10.3390/jcm8111783




