Nuclear Factor-κB Overexpression is Correlated with Poor Outcomes after Multimodality Bladder-Preserving Therapy in Patients with Muscle-Invasive Bladder Cancer
Abstract
:1. Introduction
2. Methods and Materials
2.1. Patient Sample Collection
2.2. Immunohistochemical (IHC) Staining Analysis
2.3. Interpretation of IHC Staining
2.4. Bladder Tumor Tissue Collection
2.5. Cell Culture
2.6. Reagents
2.7. Western Blot Assay
2.8. Immunofluorescence Staining
2.9. Colony Formation Assay
2.10. Cell Invasion Assay
3. Results
3.1. Patient Characteristics
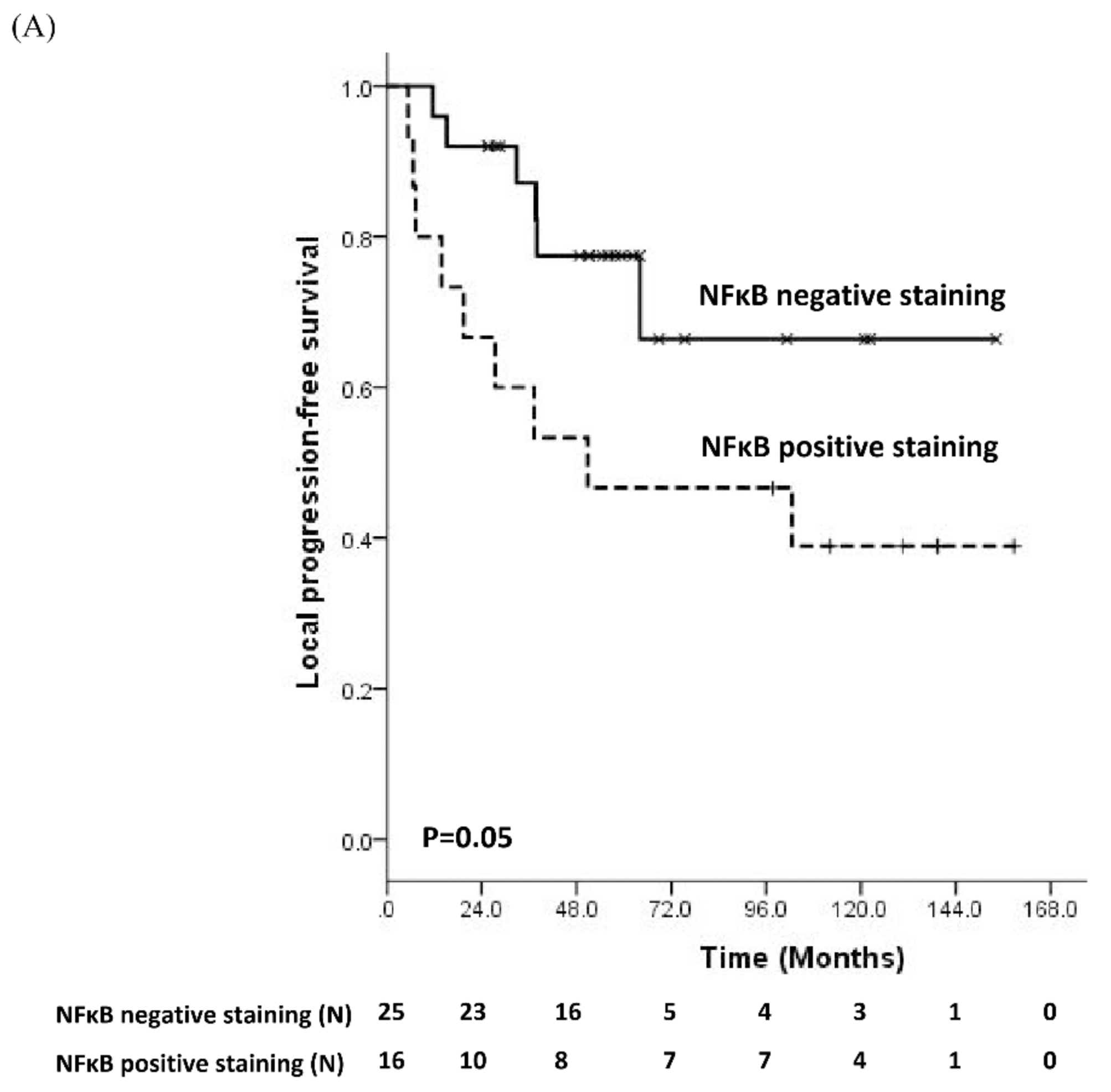
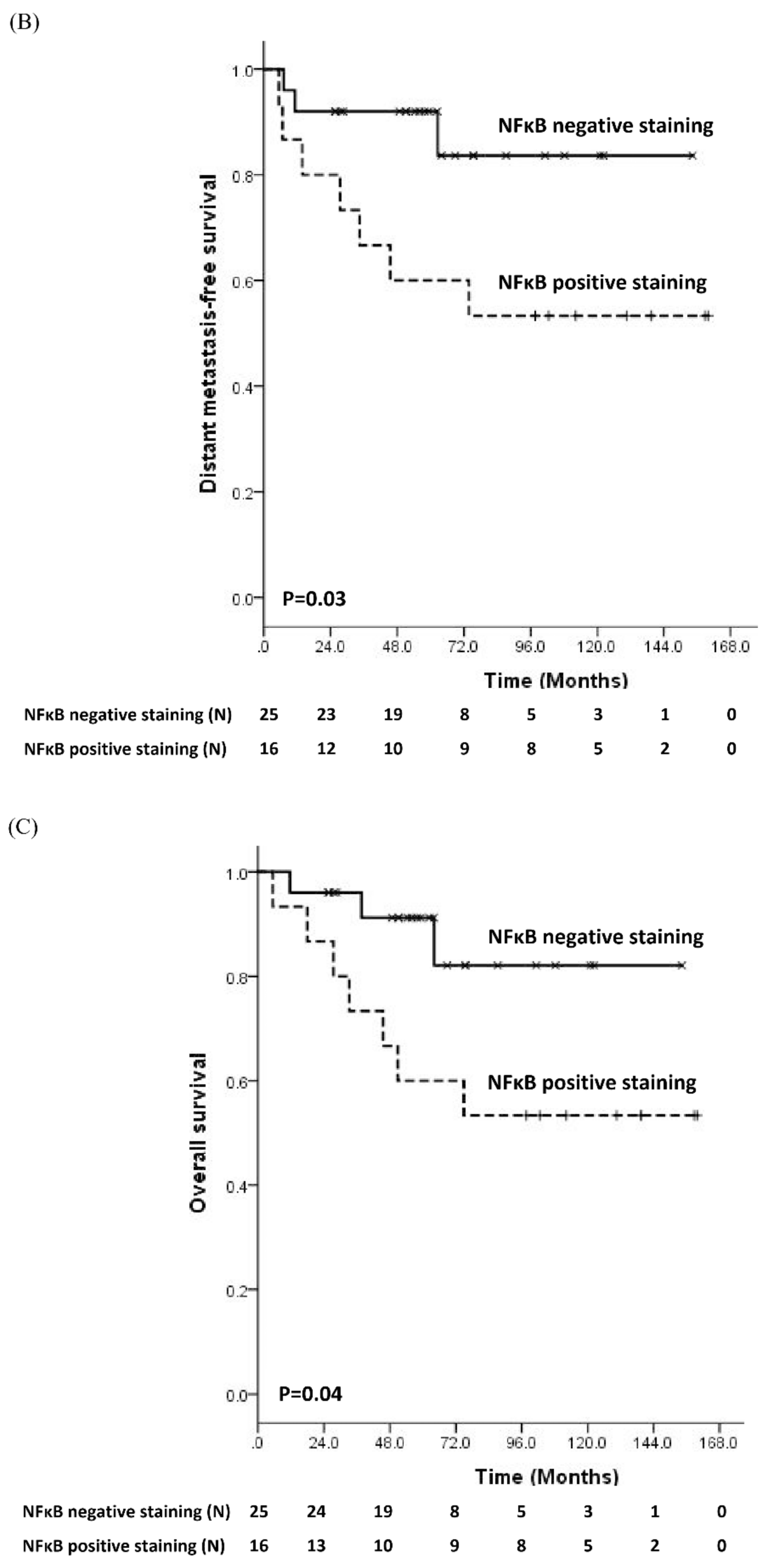
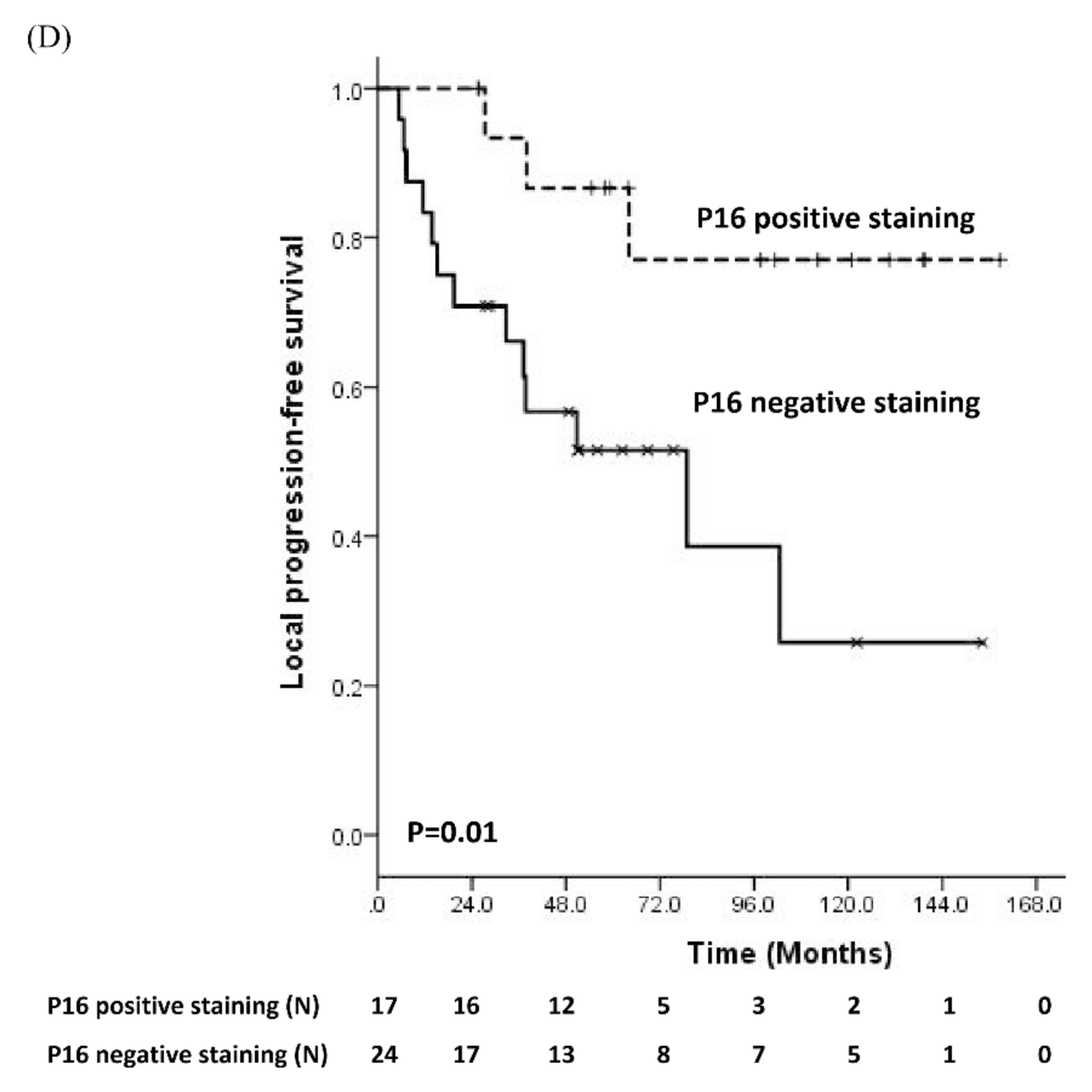
3.2. Prognostic Significance of Molecular Biomarker
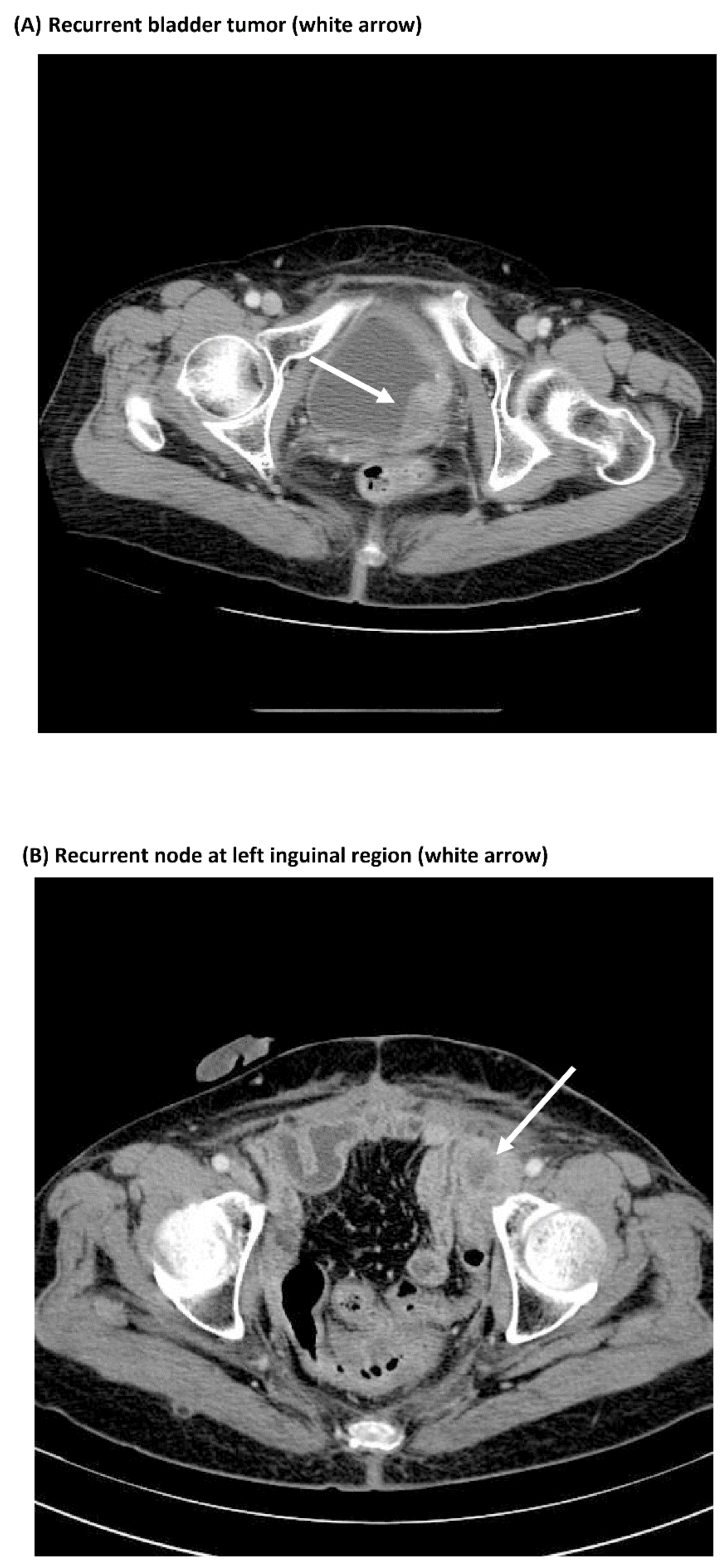
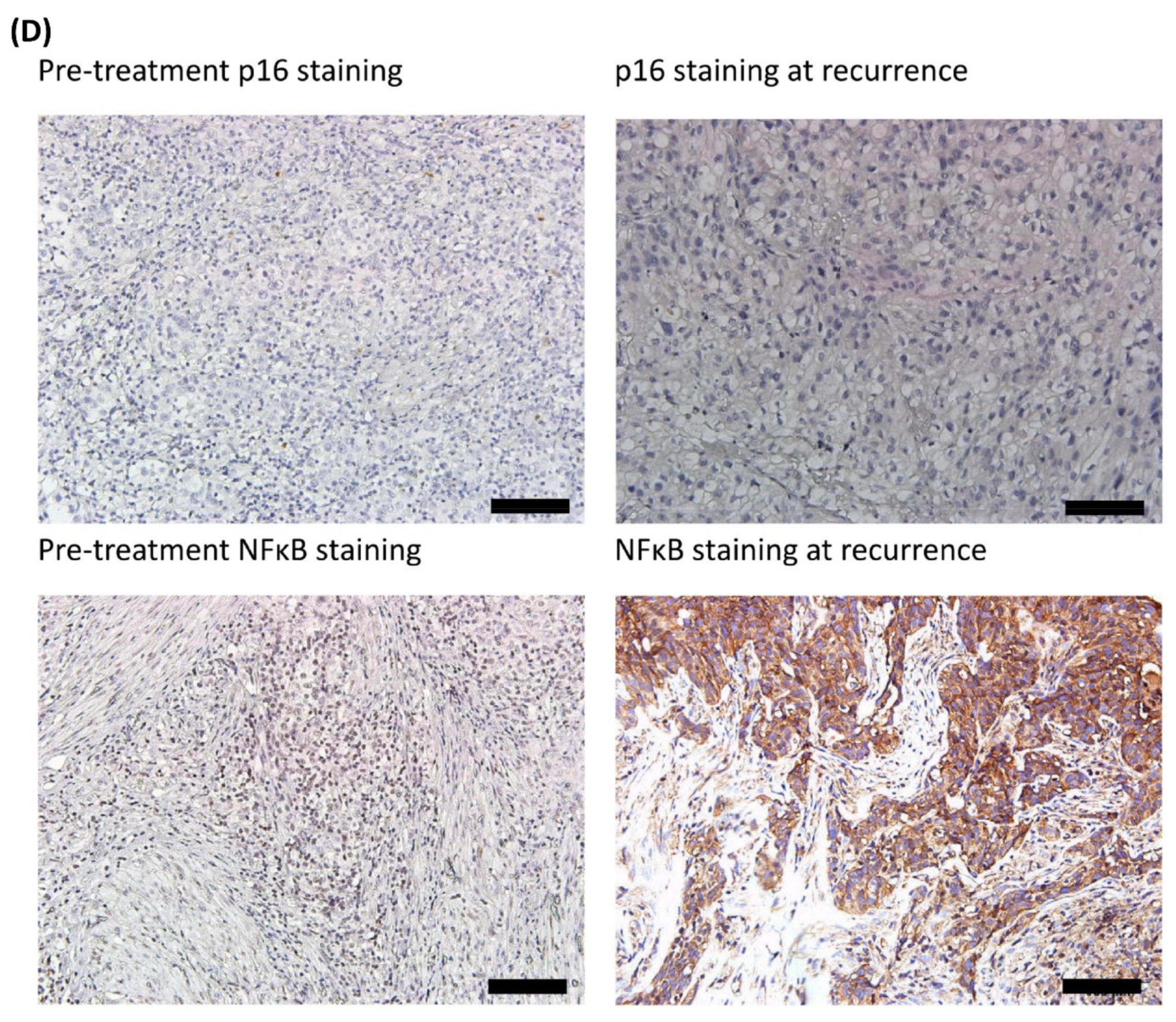
3.3. Comparisons of Immunostaining of NFκB between Pre-Treatment Samples and Recurrent Samples
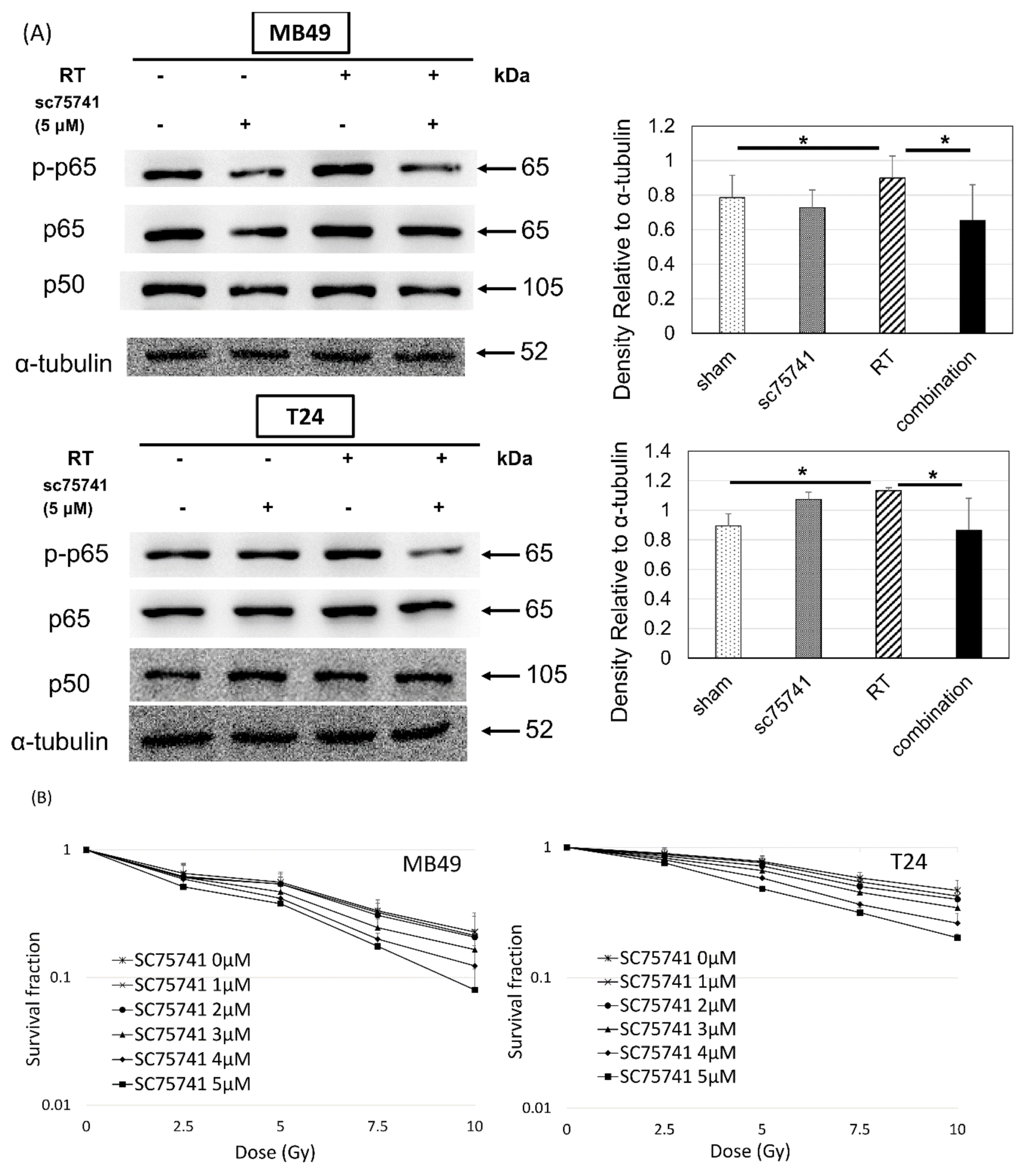
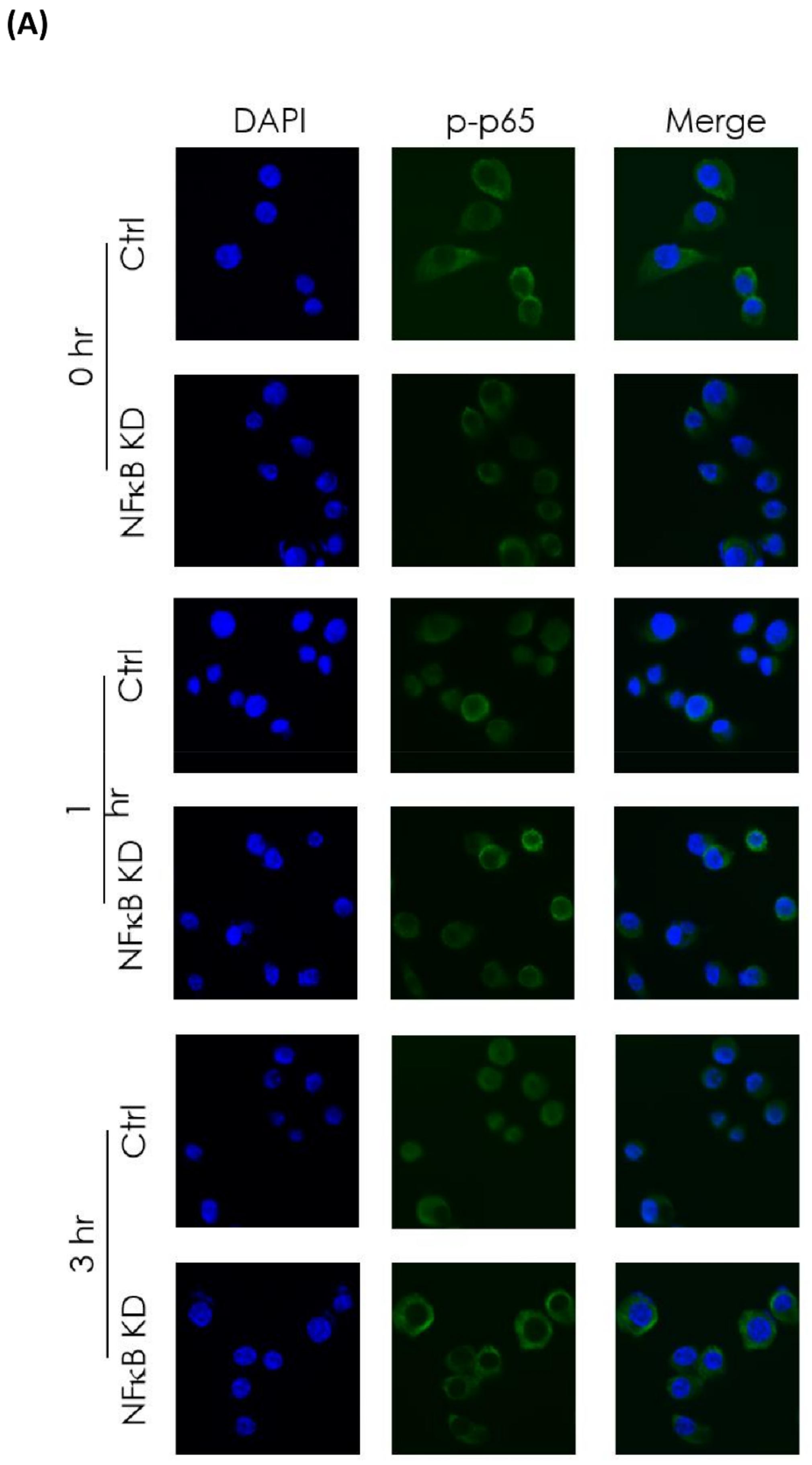
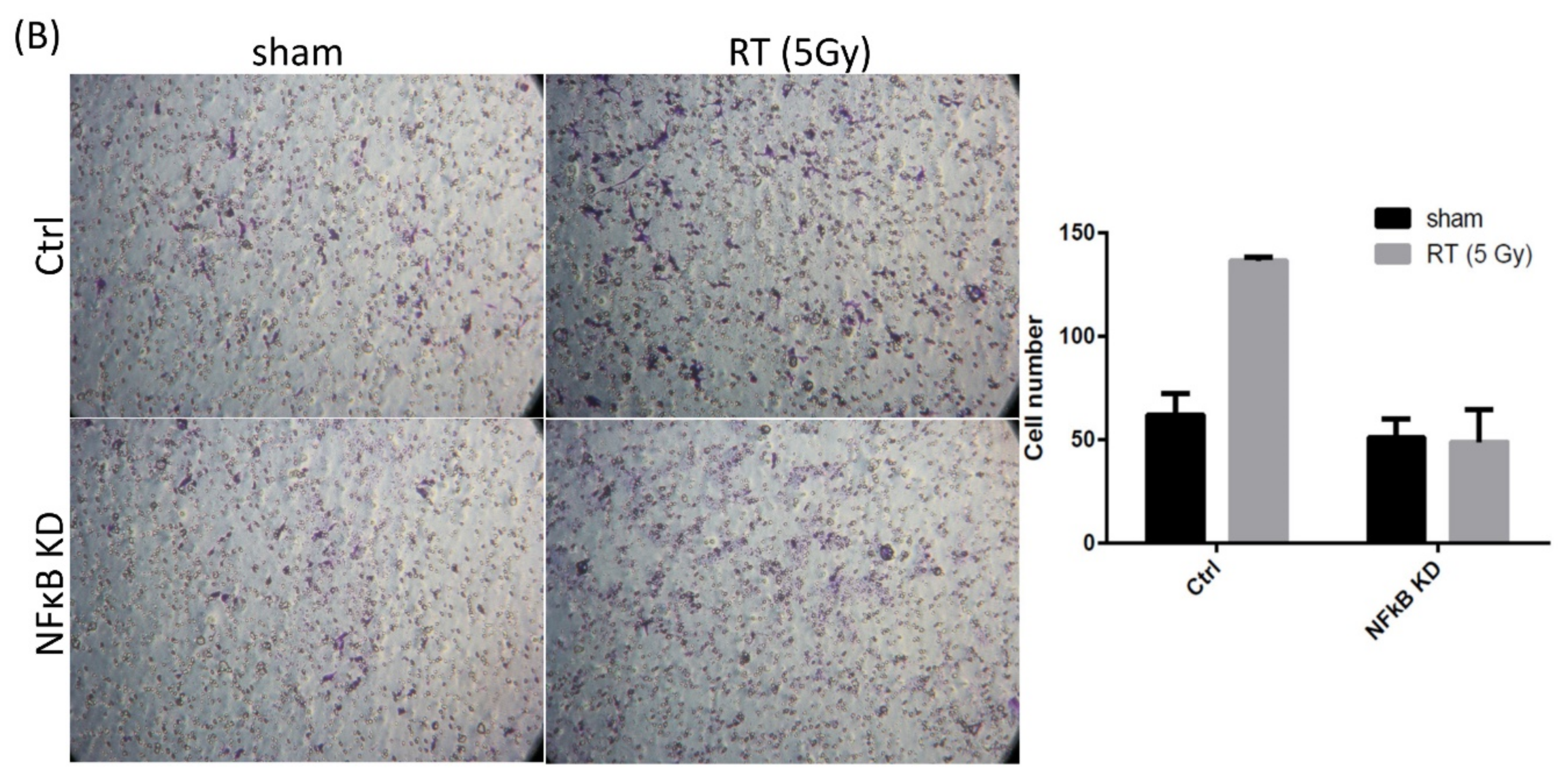
3.4. In Vitro Investigation of NFκB Expression of Irradiated Bladder Cancer Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sternberg, C.N.; Calabro, F. Neo-adjuvant chemotherapy in invasive bladder cancer. World J. Urol. 2001, 19, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Dalbagni, G.; Genega, E.; Hashibe, M.; Zang, Z.F.; Russo, P.; Herr, H.; Reuter, V. Cystectomy for bladder cancer: A contemporary series. J. Urol. 2001, 165, 1111–1116. [Google Scholar] [CrossRef]
- Goossens-Laan, C.A.; Kil, P.J.; Bosch, J.L.; De Vries, J. Patient-reported outcomes for patients undergoing radical cystectomy: A prospective case-control study. Supportive Care Cancer 2014, 22, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Rodel, C.; Grabenbauer, G.G.; Kuhn, R.; Papadopoulos, T.; Dunst, J.; Meyer, M.; Schrott, K.M.; Sauer, R. Combined-modality treatment and selective organ preservation in invasive bladder cancer: Long-term results. J. Clin. Oncol. 2002, 20, 3061–3071. [Google Scholar] [CrossRef] [PubMed]
- Chung, P.W.; Bristow, R.G.; Milosevic, M.F.; Yi, Q.L.; Jewett, M.A.; Warde, P.R.; Catton, C.N.; McLean, M.; Moore, M.; Tannock, I.F.; et al. Long-term outcome of radiation-based conservation therapy for invasive bladder cancer. Urol. Oncol. 2007, 25, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Efstathiou, J.A.; Spiegel, D.Y.; Shipley, W.U.; Heney, N.M.; Kaufman, D.S.; Niemierko, A.; Coen, J.J.; Skowronski, R.Y.; Paly, J.J.; McGovern, F.J.; et al. Long-term outcomes of selective bladder preservation by combined-modality therapy for invasive bladder cancer: The MGH experience. Eur. Urol. 2012, 61, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Mak, R.H.; Hunt, D.; Shipley, W.U.; Efstathiou, J.A.; Tester, W.J.; Hagan, M.P.; Kaufman, D.S.; Heney, N.M.; Zietman, A.L. Long-term outcomes in patients with muscle-invasive bladder cancer after selective bladder-preserving combined-modality therapy: A pooled analysis of Radiation Therapy Oncology Group protocols 8802, 8903, 9506, 9706, 9906, and 0233. J. Clin. Oncol. 2014, 32, 3801–3809. [Google Scholar] [CrossRef] [PubMed]
- Krause, F.S.; Walter, B.; Ott, O.J.; Haberle, L.; Weiss, C.; Rodel, C.; Wullich, B.; Sauer, R. 15-year survival rates after transurethral resection and radiochemotherapy or radiation in bladder cancer treatment. Anticancer Res. 2011, 31, 985–990. [Google Scholar] [PubMed]
- Stein, J.P.; Lieskovsky, G.; Cote, R.; Groshen, S.; Feng, A.C.; Boyd, S.; Skinner, E.; Bochner, B.; Thangathurai, D.; Mikhail, M.; et al. Radical cystectomy in the treatment of invasive bladder cancer: Long-term results in 1054 patients. J. Clin. Oncol. 2001, 19, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, G.C.; Kuefer, R.; Gschwend, J.E.; de Petriconi, R.; Hautmann, R.E.; Volkmer, B.G. Hydronephrosis as a prognostic marker in bladder cancer in a cystectomy-only series. Eur. Urol. 2007, 51, 690–697; discussion 697–698. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.; Cheng, J.C.; Huang, C.Y.; Tsai, Y.C.; Lin, C.C.; Hsu, C.H.; Cheng, A.L.; Pu, Y.S. A role of multimodality bladder-preserving therapy in patients with muscle-invasive bladder cancer plus hydronephrosis with or without pelvic nodal involvement. J. Formos. Med. Assoc. 2017, 116, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Rodel, C.; Grabenbauer, G.G.; Rodel, F.; Birkenhake, S.; Kühn, R.; Martus, P.; Zorcher, T.; Fursich, D.; Papadopoulos, T.; Dunst, J.; et al. Apoptosis, p53, bcl-2, and Ki-67 in invasive bladder carcinoma: Possible predictors for response to radiochemotherapy and successful bladder preservation. Int. J. Radiat. Oncol. Biol. Phys. 2000, 46, 1213–1221. [Google Scholar] [CrossRef]
- Del Muro, X.G.; Condom, E.; Vigues, F.; Castellsague, X.; Figueras, A.; Muñoz, J.; Sola, J.; Soler, T.; Capellà, G.; Germa, J.R. p53 and p21 Expression levels predict organ preservation and survival in invasive bladder carcinoma treated with a combined-modality approach. Cancer 2004, 100, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Malats, N.; Bustos, A.; Nascimento, C.M.; Fernandez, F.; Rivas, M.; Puente, D.; Kogevinas, M.; Real, F.X. P53 as a prognostic marker for bladder cancer: A meta-analysis and review. Lancet Oncol. 2005, 6, 678–686. [Google Scholar] [CrossRef]
- Puzio-Kuter, A.M.; Castillo-Martin, M.; Kinkade, C.W.; Wang, X.; Shen, T.H.; Matos, T.; Shen, M.M.; Cordon-Cardo, C.; Abate-Shen, C. Inactivation of p53 and Pten promotes invasive bladder cancer. Genes Dev. 2009, 23, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Esrig, D.; Elmajian, D.; Groshen, S.; Freeman, J.A.; Stein, J.P.; Chen, S.C.; Nichols, P.W.; Skinner, D.G.; Jones, P.A.; Cote, R.J. Accumulation of nuclear p53 and tumor progression in bladder cancer. N. Engl. J. Med. 1994, 331, 1259–1264. [Google Scholar] [CrossRef] [PubMed]
- Ong, F.; Moonen, L.M.; Gallee, M.P.; ten Bosch, C.; Zerp, S.F.; Hart, A.A.; Bartelink, H.; Verheij, M. Prognostic factors in transitional cell cancer of the bladder: An emerging role for Bcl-2 and p53. Radiother. Oncol. 2001, 61, 169–175. [Google Scholar] [CrossRef]
- Shariat, S.F.; Tokunaga, H.; Zhou, J.; Kim, J.; Ayala, G.E.; Benedict, W.F.; Lerner, S.P. p53, p21, pRB, and p16 expression predict clinical outcome in cystectomy with bladder cancer. J. Clin. Oncol. 2004, 22, 1014–1024. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.C.; Chu, K.C.; Chen, H.Y.; Chen, W.C. Expression of p16 and cyclin D1 in bladder cancer and correlation in cancer progression. Urol. Int. 2002, 69, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Gan, X.; Lin, X.; He, R.; Lin, X.; Wang, H.; Yan, L.; Zhou, H.; Qin, H.; Chen, G.; et al. Prognostic and Clinicopathological Significance of Downregulated p16 Expression in Patients with Bladder Cancer: A Systematic Review and Meta-Analysis. Dis. Markers 2016, 2016, 5259602. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, J.; Wester, K.; De La Torre, M.; Malmstrom, P.U.; Gardmark, T. EGFR-expression in primary urinary bladder cancer and corresponding metastases and the relation to HER2-expression. On the possibility to target these receptors with radionuclides. Radiol. Oncol. 2015, 49, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Chakravarti, A.; Winter, K.; Wu, C.L.; Kaufman, D.; Hammond, E.; Parliament, M.; Tester, W.; Hagan, M.; Grignon, D.; Heney, N.; et al. Expression of the epidermal growth factor receptor and Her-2 are predictors of favorable outcome and reduced complete response rates, respectively, in patients with muscle-invading bladder cancers treated by concurrent radiation and cisplatin-based chemotherapy: A report from the Radiation Therapy Oncology Group. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 309–317. [Google Scholar] [PubMed]
- Koga, F.; Yoshida, S.; Tatokoro, M.; Kawakami, S.; Fujii, Y.; Kumagai, J.; Neckers, L.; Kihara, K. ErbB2 and NFkappaB overexpression as predictors of chemoradiation resistance and putative targets to overcome resistance in muscle-invasive bladder cancer. PLoS ONE 2011, 6, e27616. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Kikuchi, E.; Tanaka, N.; Kosaka, T.; Suzuki, E.; Mizuno, R.; Shinojima, T.; Miyajima, A.; Umezawa, K.; Oya, M. Down-regulation of NF kappa B activation is an effective therapeutic modality in acquired platinum-resistant bladder cancer. BMC Cancer 2015, 15, 324. [Google Scholar] [CrossRef] [PubMed]
- Muramaki, M.; Miyake, H.; Terakawa, T.; Kumano, M.; Sakai, I.; Fujisawa, M. Expression profile of E-cadherin and N-cadherin in non-muscle-invasive bladder cancer as a novel predictor of intravesical recurrence following transurethral resection. Urol. Oncol. 2012, 30, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Reis, S.T.; Leite, K.R.; Piovesan, L.F.; Pontes-Junior, J.; Viana, N.I.; Abe, D.K.; Crippa, A.; Moura, C.M.; Adonias, S.P.; Srougi, M.; et al. Increased expression of MMP-9 and IL-8 are correlated with poor prognosis of Bladder Cancer. BMC Urol. 2012, 12, 18. [Google Scholar] [CrossRef] [PubMed]
- Teo, M.T.; Dyrskjot, L.; Nsengimana, J.; Buchwald, C.; Snowden, H.; Morgan, J.; Jensen, J.B.; Knowles, M.A.; Taylor, G.; Barrett, J.H.; et al. Next-generation sequencing identifies germline MRE11A variants as markers of radiotherapy outcomes in muscle-invasive bladder cancer. Ann. Oncol. 2014, 25, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, A.; Nelson, L.D.; Teo, M.T.; Chilka, S.; Bhattarai, S.; Johnston, C.F.; Elliott, F.; Lowery, J.; Taylor, C.F.; Churchman, M.; et al. MRE11 expression is predictive of cause-specific survival following radical radiotherapy for muscle-invasive bladder cancer. Cancer Res. 2010, 70, 7017–7026. [Google Scholar] [CrossRef] [PubMed]
- Laurberg, J.R.; Brems-Eskildsen, A.S.; Nordentoft, I.; Fristrup, N.; Schepeler, T.; Ulhøi, B.P.; Agerbaek, M.; Hartmann, A.; Bertz, S.; Wittlinger, M.; et al. Expression of TIP60 (tat-interactive protein) and MRE11 (meiotic recombination 11 homolog) predict treatment-specific outcome of localised invasive bladder cancer. BJU Int. 2012, 110, E1228–E1236. [Google Scholar] [CrossRef] [PubMed]
- Bellmunt, J.; Mullane, S.A.; Werner, L.; Fay, A.P.; Callea, M.; Leow, J.J.; Taplin, M.E.; Choueiri, T.K.; Hodi, F.S.; Freeman, G.J.; et al. Association of PD-L1 expression on tumor-infiltrating mononuclear cells and overall survival in patients with urothelial carcinoma. Ann. Oncol. 2015, 26, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Durham, J.N. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corso, C.D.; Ali, A.N.; Diaz, R. Radiation-induced tumor neoantigens: Imaging and therapeutic implications. Am. J. Cancer Res. 2011, 1, 390–412. [Google Scholar] [PubMed]
- Peng, C.C.; Chen, K.C.; Peng, R.Y.; Su, C.H. Human urinary bladder cancer T24 cells are susceptible to the Antrodia camphorate extracts. Cancer Lett. 2006, 243, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Summerhayes, I.C.; Franks, L.M. Effects of donor age on neoplastic transformation of adult mouse bladder epithelium in vitro. J. Natl. Cancer Inst. 1979, 62, 1017–1023. [Google Scholar] [PubMed]
- Loskog, A.; Dzojic, H.; Vikman, S.; Ninalga, C.; Essand, M.; Korsgren, O.; Totterman, T.H. Adenovirus CD40 ligand gene therapy counteracts immune escape mechanisms in the tumor microenvironment. J. Immunol. 2004, 172, 7200–7205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mumtaz, S.; Hashmi, A.A.; Hasan, S.H.; Edhi, M.M.; Khan, M. Diagnostic utility of p53 and CK20 immunohistochemical expression grading urothelial malignancies. Int. Arch. Med. 2014, 7, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.H.; Wu, C.C.; Chen, W.T.; Chai, C.Y.; Yang, S.F. Expressions of p16 and p27 in urothelial carcinoma and their prognostic value. Kaohsiung J. Med. Sci. 2014, 30, 453–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolff, A.C.; Hammond, M.E.; Hicks, D.G.; Dowsett, M.; McShane, L.M.; Allison, K.H.; Allred, D.C.; Bartlett, J.M.; Bilous, M.; Fitzgibbons, P.; et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J. Clin. Oncol. 2013, 31, 3997–4013. [Google Scholar] [CrossRef] [PubMed]
- Fehrenbacher, L.; Spira, A.; Ballinger, M.; Kowanetz, M.; Vansteenkiste, J.; Mazieres, J.; Park, K.; Smith, D.; Artal-Cortes, A.; Lewanski, C.; et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): A multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016, 387, 1837–1846. [Google Scholar] [CrossRef]
- Hissong, E.; Crowe, E.P.; Yantiss, R.K.; Chen, Y.T. Assessing colorectal cancer mismatch repair status in the modern era: A survey of current practices and re-evaluation of the role of microsatellite instability testing. Mod. Pathol. 2018, 31, 1756–1766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehrhardt, C.; Rückle, A.; Hrincius, E.R.; Haasbach, E. The NFκB inhibitor SC75741 efficiently blocks influenza virus propagation and confers a high barrier for development of viral resistance. Cell. Microbiol. 2013, 15, 1198–1211. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Cao, Y.; Greten, F.R.; Li, Z.W. NF-kappaB in cancer: From innocent bystander to major culprit. Nat. Rev. Cancer 2002, 2, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Shen, S.; Verma, I.M. NF-kappaB, an active player in human cancers. Cancer Immunol. Res. 2014, 2, 823–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolcet, X.; Llobet, D.; Pallares, J.; Matias-Guiu, X. NF-kB in development and progression of human cancer. Virchows Arch. 2005, 446, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Christian, F.; Smith, E.L.; Carmody, R.J. The Regulation of NF-kappaB Subunits by Phosphorylation. Cells 2016, 5, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karashima, T.; Sweeney, P.; Kamat, A.; Huang, S.; Kim, S.J.; Bar-Eli, M.; McConkey, D.J.; Dinney, C.P. Nuclear factor-kappaB mediates angiogenesis and metastasis of human bladder cancer through the regulation of interleukin-8. Clin. Cancer Res. 2003, 9, 2786–2797. [Google Scholar] [PubMed]
- Zhu, J.; Li, Y.; Chen, C.; Ma, J.; Sun, W.; Tian, Z.; Li, J.; Xu, J.; Liu, C.S.; Zhang, D.; et al. NF-kappaB p65 Overexpression Promotes Bladder Cancer Cell Migration via FBW7-Mediated Degradation of RhoGDIalpha Protein. Neoplasia 2017, 19, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Brach, M.A.; Hass, R.; Sherman, M.L.; Gunji, H.; Weichselbaum, R.; Kufe, D. Ionizing radiation induces expression and binding activity of the nuclear factor kappa B. J. Clin. Investig. 1991, 88, 691–695. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Brown, S.A.; Yu, T.; Chen, G.; Barve, S.; Kang, B.C.; Thompson, J.S. A high dose of ionizing radiation induces tissue-specific activation of nuclear factor-kappaB in vivo. Radiat. Res. 1999, 151, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Lindholm, P.F.; Bub, J.; Kaul, S.; Shidham, V.B.; Kajdacsy-Balla, A. The role of constitutive NF-kappaB activity in PC-3 human prostate cancer cell invasive behavior. Clin. Exp. Metastasis 2000, 18, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.P.; Hansel, D.E.; Cote, R.J. Prognostic value of cell-cycle regulation biomarkers in bladder cancer. Semin. Oncol. 2012, 39, 524–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veena, M.S.; Wilken, R.; Zheng, J.Y.; Gholkar, A.; Venkatesan, N.; Vira, D.; Ahmed, S.; Basak, S.K.; Dalgard, C.L.; Ravichandran, S.; et al. p16 Protein and gigaxonin are associated with the ubiquitination of NFkappaB in cisplatin-induced senescence of cancer cells. J. Biol. Chem. 2014, 289, 34921–34937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellmunt, J.; de Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenberg, J.E.; Hoffman-Censits, J.; Powles, T.; van der Heijden, M.S.; Balar, A.V.; Necchi, A.; Dawson, N.; O’Donnell, P.H.; Balmanoukian, A.; Loriot, Y.; et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet 2016, 387, 1909–1920. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Retz, M.; Siefker-Radtke, A.; Baron, A.; Necchi, A.; Bedke, J.; Plimack, E.R.; Vaena, D.; Grimm, M.O.; Bracarda, S.; et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017, 18, 312–322. [Google Scholar] [CrossRef]
- Massard, C.; Gordon, M.S.; Sharma, S.; Rafii, S.; Wainberg, Z.A.; Luke, J.; Curiel, T.J.; Colon-Otero, G.; Hamid, O.; Sanborn, R.E.; et al. Safety and Efficacy of Durvalumab (MEDI4736), an Anti-Programmed Cell Death Ligand-1 Immune Checkpoint Inhibitor, in Patients with Advanced Urothelial Bladder Cancer. J. Clin. Oncol. 2016, 34, 3119–3125. [Google Scholar] [CrossRef] [PubMed]
- Ben-Neriah, Y.; Karin, M. Inflammation meets cancer, with NF-kappaB as the matchmaker. Nat. Immunol. 2011, 12, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Garlanda, C.; Dinarello, C.A.; Mantovani, A. The interleukin-1 family: back to the future. Immunity 2013, 39, 1003–1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajek, E.; Krebs, F.; Bent, R.; Haas, K.; Bast, A.; Steinmetz, I.; Tuettenberg, A.; Grabbe, S.; Bros, M. BRAF inhibitors stimulate inflammasome activation and interleukin 1 beta production in dendritic cells. Oncotarget 2018, 9, 28294–28308. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | No of Patients (%) | Fisher’s Exact Test or χ2 Test p-Value | ||
|---|---|---|---|---|
| Non-IHC Group (n = 20) | IHC Group (n = 41) | |||
| Sex | Male | 14 (70) | 25 (61) | 0.35 |
| Female | 6 (30) | 16 (39) | ||
| Age (years) | <70 | 16 (80) | 24 (59) | 0.084 |
| ≥70 | 4 (20) | 17 (41) | ||
| Clinical T stage | T2 | 19 (95) | 30 (73) | 0.083 |
| T3–T4a | 1 (5) | 11 (27) | ||
| Induction chemotherapy | CF | 4 (20) | 12 (29) | 0.78 |
| PCF | 5 (25) | 11 (27) | ||
| GC | 11 (55) | 18 (44) | ||
| Prognostic factors | Favorable group | 16 (80) | 33 (80) | 0.61 |
| Unfavorable group | 4 (20) | 8 (20) | ||
| Characteristics | No. of Patients (%) | |
|---|---|---|
| Sex | Male | 25 (61) |
| Female | 16 (39) | |
| Age (years) | <70 | 24 (59) |
| ≥70 | 17 (41) | |
| Clinical T stage | T2 | 30 (73) |
| T3–T4a | 11 (27) | |
| Induction chemotherapy | CF | 12 (29) |
| PCF | 11 (27) | |
| GC | 18 (44) | |
| Risk factors | Favorable group | 33 (80) |
| Unfavorable group | 8 (20) | |
| NFκB (p65) | Negative (≤0) | 25 (61) |
| Positive (>0) | 16 (39) | |
| p16 | Negative (score 0–1) | 24 (59) |
| Positive (score 2–3) | 17 (41) | |
| p53 | Negative (score 0 or 4) | 25 (61) |
| Positive (score 1–3) | 16 (39) | |
| EGFR | Negative (score 0–1) | 26 (63) |
| Positive (score 2–3) | 15 (37) | |
| Her-2 | Negative (score 0–1) | 29 (71) |
| Positive (score 2–3) | 12 (29) | |
| E-cadherin | Negative (score 0–1) | 6 (15) |
| Positive (score 2–3) | 35 (85) | |
| MMP9 | Negative (≤0) | 30 (73) |
| Positive (>0) | 11 (27) | |
| MRE11 | Low (≤median) | 21 (51) |
| High (>median) | 20 (49) | |
| IC of PD-L1 | Negative (≤0) | 24 (59) |
| Positive (>0) | 17 (41) | |
| TC of PD-L1 | Negative (≤5%) | 29 (71) |
| Positive (>5%) | 12 (29) | |
| MMR proteins (PMS2/MLH1/MSH2/MSH6) | Negative | 0 |
| Positive | 41 (100) | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiang, Y.; Wang, C.-C.; Tsai, Y.-C.; Huang, C.-Y.; Pu, Y.-S.; Lin, C.-C.; Cheng, J.C.-H. Nuclear Factor-κB Overexpression is Correlated with Poor Outcomes after Multimodality Bladder-Preserving Therapy in Patients with Muscle-Invasive Bladder Cancer. J. Clin. Med. 2019, 8, 1954. https://doi.org/10.3390/jcm8111954
Chiang Y, Wang C-C, Tsai Y-C, Huang C-Y, Pu Y-S, Lin C-C, Cheng JC-H. Nuclear Factor-κB Overexpression is Correlated with Poor Outcomes after Multimodality Bladder-Preserving Therapy in Patients with Muscle-Invasive Bladder Cancer. Journal of Clinical Medicine. 2019; 8(11):1954. https://doi.org/10.3390/jcm8111954
Chicago/Turabian StyleChiang, Yun, Chung-Chieh Wang, Yu-Chieh Tsai, Chao-Yuan Huang, Yeong-Shiau Pu, Chia-Chi Lin, and Jason Chia-Hsien Cheng. 2019. "Nuclear Factor-κB Overexpression is Correlated with Poor Outcomes after Multimodality Bladder-Preserving Therapy in Patients with Muscle-Invasive Bladder Cancer" Journal of Clinical Medicine 8, no. 11: 1954. https://doi.org/10.3390/jcm8111954




