Interaction of the p.Q141K Variant of the ABCG2 Gene with Clinical Data and Cytokine Levels in Primary Hyperuricemia and Gout
Abstract
:1. Introduction
2. Experimental Section
2.1. Cohort and Subcohorts
2.2. Cytokine Detection
2.3. Statistical Analysis
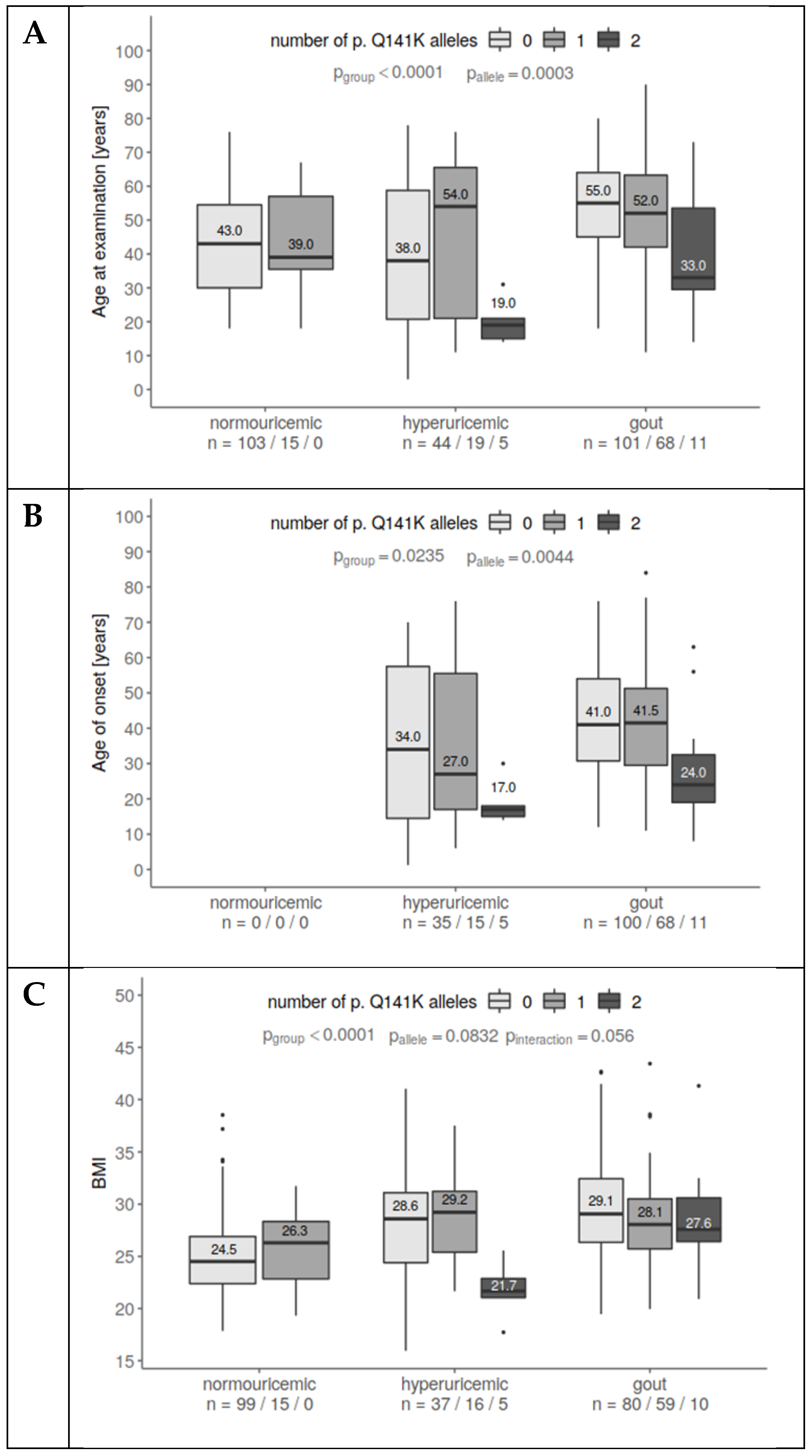
3. Results
3.1. Analysis of the p.Q141K Variant in Normouricemic and Patient Cohort
3.2. Analysis of the p.Q141K Variant and Its Relationship with Risk Factors for Gout
3.3. Analysis of the p.Q141K Variant in Association with Cytokine Plasma Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Major, T.J.; Dalbeth, N.; Stahl, E.A.; Merriman, T.R. An update on the genetics of hyperuricaemia and gout. Nat. Rev. Rheumatol. 2018, 14, 351–353. [Google Scholar] [CrossRef] [PubMed]
- Cadzow, M.; Merriman, T.R.; Dalbeth, N. Performance of gout definitions for genetic epidemiological studies: Analysis of UK Biobank. Arthritis Res. Ther. 2017, 19, 181. [Google Scholar] [CrossRef] [PubMed]
- So, A.K.; Martinon, F. Inflammation in gout: Mechanisms and therapeutic targets. Nat. Rev. Rheumatol. 2017, 13, 639–647. [Google Scholar] [CrossRef] [PubMed]
- El Ridi, R.; Tallima, H. Physiological functions and pathogenic potential of uric acid: A review. J. Adv. Res. 2017, 8, 487–493. [Google Scholar] [CrossRef]
- Deuteraiou, K.; Kitas, G.; Garyfallos, A.; Dimitroulas, T. Novel insights into the role of inflammasomes in autoimmune and metabolic rheumatic diseases. Rheumatol. Int. 2018, 38, 1345–1354. [Google Scholar] [CrossRef]
- Martinon, F.; Pétrilli, V.; Mayor, A.; Tardivel, A.; Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006, 440, 237–241. [Google Scholar] [CrossRef]
- Schlesinger, N.; Thiele, R.G. The pathogenesis of bone erosions in gouty arthritis. Ann. Rheum. Dis. 2010, 69, 1907–1912. [Google Scholar] [CrossRef]
- Cavalcanti, N.G.; Marques, C.D.L.; Lins e Lins, T.U.; Pereira, M.C.; Rêgo, M.J.B.D.M.; Duarte, A.L.B.P.; Pitta, I.D.R.; Pitta, M.G.D.R. Cytokine profile in gout: Inflammation driven by IL-6 and IL-18? Immunol. Investig. 2016, 45, 383–395. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Q.; Yin, Y.; McNutt, M.A.; Zhang, T.; Cao, Y. Serum levels of IL-17 are elevated in patients with acute gouty arthritis. Biochem. Biophys. Res. Commun. 2018, 497, 897–902. [Google Scholar] [CrossRef]
- Mills, K.H.G.; Dungan, L.S.; Jones, S.A.; Harris, J. The role of inflammasome-derived IL-1 in driving IL-17 responses. J. Leukoc. Biol. 2013, 93, 489–497. [Google Scholar] [CrossRef]
- Matsuo, H.; Ichida, K.; Takada, T.; Nakayama, A.; Nakashima, H.; Nakamura, T.; Kawamura, Y.; Takada, Y.; Yamamoto, K.; Inoue, H.; et al. Common dysfunctional variants in ABCG2 are a major cause of early-onset gout. Sci. Rep. 2013, 3, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Stiburkova, B.; Miyata, H.; Závada, J.; Tomčík, M.; Pavelka, K.; Storkanova, G.; Toyoda, Y.; Takada, T.; Suzuki, H. Novel dysfunctional variant in ABCG2 as a cause of severe tophaceous gout: Biochemical, molecular genetics and functional analysis. Rheumatology 2016, 55, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Stiburkova, B.; Pavelcova, K.; Zavada, J.; Petru, L.; Simek, P.; Cepek, P.; Pavlikova, M.; Matsuo, H.; Merriman, T.R.; Pavelka, K. Functional non-synonymous variants of ABCG2 and gout risk. Rheumatology 2017, 56, 1982–1992. [Google Scholar] [CrossRef] [PubMed]
- Higashino, T.; Takada, T.; Nakaoka, H.; Toyoda, Y.; Stiburkova, B.; Miyata, H.; Ikebuchi, Y.; Nakashima, H.; Shimizu, S.; Kawaguchi, M.; et al. Multiple common and rare variants of ABCG2 cause gout. RMD Open 2017, 3, e000464. [Google Scholar] [CrossRef]
- Woodward, O.M.; Tukaye, D.N.; Cui, J.; Greenwell, P.; Constantoulakis, L.M.; Parker, B.S.; Rao, A.; Kottgen, M.; Maloney, P.C.; Guggino, W.B. Gout-causing Q141K mutation in ABCG2 leads to instability of the nucleotide-binding domain and can be corrected with small molecules. Proc. Natl. Acad. Sci. USA 2013, 110, 5223–5228. [Google Scholar] [CrossRef]
- Mizuarai, S.; Aozasa, N.; Kotani, H. Single nucleotide polymorphisms result in impaired membrane localization and reduced atpase activity in multidrug transporter ABCG2. Int. J. Cancer 2004, 109, 238–246. [Google Scholar] [CrossRef]
- Cleophas, M.C.; Joosten, L.A.; Stamp, L.K.; Dalbeth, N.; Woodward, O.M.; Merriman, T.R. ABCG2 polymorphisms in gout: Insights into disease susceptibility and treatment approaches. Pharmgenom. Pers. Med. 2017, 10, 129–142. [Google Scholar] [CrossRef]
- Dehghan, A.; Köttgen, A.; Yang, Q.; Hwang, S.J.; Kao, W.L.; Rivadeneira, F.; Boerwinkle, E.; Levy, D.; Hofman, A.; Astor, B.C.; et al. Association of three genetic loci with uric acid concentration and risk of gout: A genome-wide association study. Lancet 2008, 372, 1953–1961. [Google Scholar] [CrossRef]
- Li, R.; Miao, L.; Qin, L.; Xiang, Y.; Zhang, X.; Peng, H.; Mailamuguli; Sun, Y.; Yao, H. A meta-analysis of the associations between the Q141K and Q126X ABCG2 gene variants and gout risk. Int. J. Clin. Exp. Pathol. 2015, 8, 9812–9823. [Google Scholar]
- Roberts, R.L.; Wallace, M.C.; Phipps-Green, A.J.; Topless, R.; Drake, J.M.; Tan, P.; Dalbeth, N.; Merriman, T.R.; Stamp, L.K. ABCG2 loss-of-function polymorphism predicts poor response to allopurinol in patients with gout. Pharm. J. 2017, 17, 201–203. [Google Scholar] [CrossRef]
- Mosaffa, F.; Lage, H.; Afshari, J.T.; Behravan, J. Interleukin-1 beta and tumor necrosis factor-alpha increase ABCG2 expression in MCF-7 breast carcinoma cell line and its mitoxantrone-resistant derivative, MCF-7/MX. Inflamm. Res. 2009, 58, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Evseenko, D.A.; Paxton, J.W.; Keelan, J.A. Independent regulation of apical and basolateral drug transporter expression and function in placental trophoblasts by cytokines, steroids, and growth factors. Drug Metab. Dispos. 2007, 35, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, M.; Bembinster, L.A.; Baumgarten, S.C.; Frasor, J. Proinflammatory cytokines enhance estrogen-dependent expression of the multidrug transporter gene ABCG2 through estrogen receptor and NFκB cooperativity at adjacent response elements. J. Biol. Chem. 2010, 285, 31100–31106. [Google Scholar] [CrossRef] [PubMed]
- Stiburkova, B.; Pavelcova, K.; Pavlikova, M.; Ješina, P.; Pavelka, K. The impact of dysfunctional variants of ABCG2 on hyperuricemia and gout in pediatric-onset patients. Arthritis Res. Ther. 2019, 21, 77. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, Y.; Mančíková, A.; Krylov, V.; Morimoto, K.; Pavelcová, K.; Bohatá, J.; Pavelka, K.; Pavlíková, M.; Suzuki, H.; Matsuo, H.; et al. Functional characterization of clinically-relevant rare variants in ABCG2 identified in a gout and hyperuricemia cohort. Cells 2019, 8, 363. [Google Scholar] [CrossRef] [PubMed]
- Wallace, S.L.; Robinson, H.; Masi, A.T.; Decker, J.L.; McCarty, D.J.; Yü, T.F. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977, 20, 895–900. [Google Scholar] [CrossRef]
- Takada, T.; Ichida, K.; Matsuo, H.; Nakayama, A.; Murakami, K.; Yamanashi, Y.; Kasuga, H.; Shinomiya, N.; Suzuki, H. ABCG2 dysfunction increases serum uric acid by decreased intestinal urate excretion. Nucleosides Nucleotides Nucleic Acids 2014, 33, 275–281. [Google Scholar] [CrossRef]
- Woodward, O.M.; Köttgen, A.; Coresh, J.; Boerwinkle, E.; Guggino, W.B.; Köttgen, M. Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout. Proc. Natl. Acad. Sci. USA 2009, 106, 10338–10342. [Google Scholar] [CrossRef]
- Nakayama, A.; Matsuo, H.; Nakaoka, H.; Nakamura, T.; Nakashima, H.; Takada, Y.; Oikawa, Y.; Takada, T.; Sakiyama, M.; Shimizu, S.; et al. Common dysfunctional variants of ABCG2 have stronger impact on hyperuricemia progression than typical environmental risk factors. Sci. Rep. 2014, 4, 5227. [Google Scholar] [CrossRef]
- Aune, D.; Norat, T.; Vatten, L.J. Body mass index and the risk of gout: A systematic review and dose–response meta-analysis of prospective studies. Eur. J. Nutr. 2014, 53, 1591–1601. [Google Scholar] [CrossRef]
- Evrin, P.-E.; Nilsson, S.E.; Öberg, T.; Malmberg, B. Serum C-reactive protein in elderly men and women: Association with mortality, morbidity and various biochemical values. Scand. J. Clin. Lab. Investig. 2005, 65, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Michaud, M.; Balardy, L.; Moulis, G.; Gaudin, C.; Peyrot, C.; Vellas, B.; Cesari, M.; Nourhashemi, F. Proinflammatory cytokines, aging, and age-related diseases. J. Am. Med. Dir. Assoc. 2013, 14, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Wyczalkowska-Tomasik, A.; Czarkowska-Paczek, B.; Zielenkiewicz, M.; Paczek, L. Inflammatory markers change with age, but do not fall beyond reported normal ranges. Arch. Immunol. Ther. Exp. 2016, 64, 249–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnan, E. Reduced glomerular function and prevalence of gout: NHANES 2009–10. PLoS ONE 2012, 7, e50046. [Google Scholar] [CrossRef] [Green Version]
- Rothenbacher, D.; Primatesta, P.; Ferreira, A.; Cea-Soriano, L.; Rodriguez, L.A.G. Frequency and risk factors of gout flares in a large population-based cohort of incident gout. Rheumatology 2011, 50, 973–981. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wu, X.; Wang, C.; Zhang, W.; Ouyang, Y.; Yu, Y.; He, Z. Transcriptional suppression of breast cancer resistance protein (BCRP) by wild-type p53 through the NF-κB pathway in MCF-7 cells. FEBS Lett. 2010, 584, 3392–3397. [Google Scholar] [CrossRef] [Green Version]
- Shen, S.; Callaghan, D.; Juzwik, C.; Xiong, H.; Huang, P.; Zhang, W. ABCG2 reduces ROS-mediated toxicity and inflammation: A potential role in Alzheimer’s disease. J. Neurochem. 2010, 114, 1590–1604. [Google Scholar] [CrossRef]
- Von Wedel-Parlow, M.; Wölte, P.; Galla, H.-J. Regulation of major efflux transporters under inflammatory conditions at the blood-brain barrier in vitro. J. Neurochem. 2009, 111, 111–118. [Google Scholar] [CrossRef]
- Mosaffa, F.; Kalalinia, F.; Lage, H.; Afshari, J.T.; Behravan, J. Pro-inflammatory cytokines interleukin-1 beta, interleukin 6, and tumor necrosis factor-alpha alter the expression and function of ABCG2 in cervix and gastric cancer cells. Mol. Cell. Biochem. 2012, 363, 385–393. [Google Scholar] [CrossRef]
- Deuring, J.J.; de Haar, C.; Koelewijn, C.L.; Kuipers, E.J.; Peppelenbosch, M.P.; van der Woude, C.J. Absence of ABCG2-mediated mucosal detoxification in patients with active inflammatory bowel disease is due to impeded protein folding. Biochem. J. 2012, 441, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Wen, T.; Rothenberg, M.E. The regulatory function of eosinophils. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef] [Green Version]
- Kienhorst, L.B.E.; van Lochem, E.; Kievit, W.; Dalbeth, N.; Merriman, M.E.; Phipps-Green, A.; Loof, A.; van Heerde, W.; Vermeulen, S.; Stamp, L.K.; et al. Gout is a chronic inflammatory disease in which high levels of interleukin-8 (CXCL8), myeloid-related protein 8/myeloid-related protein 14 complex, and an altered proteome are associated with diabetes mellitus and cardiovascular disease. Arthritis Rheumatol. 2015, 67, 3303–3313. [Google Scholar] [CrossRef]
- Estevez-Garcia, I.O.; Gallegos-Nava, S.; Vera-Pérez, E.; Silveira, L.H.; Ventura-Ríos, L.; Vancini, G.; Hernández-Díaz, C.; Sánchez-Muñoz, F.; Ballinas-Verdugo, M.A.; Gutierrez, M.; et al. Levels of cytokines and microRNAs in individuals with asymptomatic hyperuricemia and ultrasonographic findings of gout: A bench-to-bedside approach. Arthritis Care Res. 2018, 70, 1814–1821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenberg-Hasson, Y.; Hansmann, L.; Liedtke, M.; Herschmann, I.; Maecker, H.T. Effects of serum and plasma matrices on multiplex immunoassays. Immunol. Res. 2014, 58, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, A.R.; Wu, H.; Xydakis, A.M.; Jones, P.H.; Smith, E.O.; Sweeney, J.F.; Corry, D.B.; Ballantyne, C.M. Eotaxin and obesity. J. Clin. Endocrinol. Metab. 2006, 91, 256–261. [Google Scholar] [CrossRef] [Green Version]
- Grainger, R.; McLaughlin, R.J.; Harrison, A.A.; Harper, J.L. Hyperuricaemia elevates circulating CCL2 levels and primes monocyte trafficking in subjects with inter-critical gout. Rheumatology 2013, 52, 1018–1021. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Fang, L.; Jiang, L.; Wen, P.; Cao, H.; He, W.; Dai, C.; Yang, J. Uric acid induces renal inflammation via activating tubular NF-κB signaling pathway. PLoS ONE 2012, 7, e39738. [Google Scholar] [CrossRef] [Green Version]
- Mcnearney, T.; Baethge, B.A.; Cao, S.; Alam, R.; Lisse, J.R.; Westlund, K.N. Excitatory amino acids, TNF-alpha, and chemokine levels in synovial fluids of patients with active arthropathies. Clin. Exp. Immunol. 2004, 137, 621–627. [Google Scholar] [CrossRef]


| Normouricemic Subjects (N = 132) | Hyperuricemic Patients (N = 69) | Gout Patients (N = 177) | Fisher Test p-Value | ||||
| n | % | n | % | n | % | ||
| Sex M/F | 54/78 | 40.9/59.1 | 50/20 | 71.4/28.6 | 166/16 | 91.2/8.8 | <0.0001 |
| Familial occurrence | Not collected | 31 | 50.0 | 66 | 63.5 | 0.0717 | |
| No treatment | 132 | 100.0 | 31 | 44.3 | 28 | 15.4 | <0.0001 |
| Allopurinol treatment | Not applicable | 39 | 55.7 | 137 | 75.3 | ||
| Febuxostat treatment | 0 | 0.0 | 17 | 9.3 | |||
| p.Q141K, GG | 103 | 86.6/78.0 | 44 | 64.7/62.9 | 100 | 57.5/56.5 | <0.0001 |
| GT | 15 | 12.6/11.4 | 19 | 27.9/27.1 | 66 | 37.9/37.3 | |
| TT | 1 | 0.8/0.8 | 5 | 7.4/7.1 | 8 | 4.6/4.5 | |
| no data | 13 | 9.8 | 2 | 2.9 | 2 | 1.7 | |
| p.Q141K, MAF | 17 | 7.1 | 29 | 21.3 | 90 | 25 | <0.0001 |
| Data on interleukins subjects/measurements | 132/132 | 44/53 | 132/149/23 during attack | ||||
| Median (IQR) | Range | Median (IQR) | Range | Median (IQR) | Range | KW Test p-Value | |
| Age of onset, years | Not applicable | 28.5 (39.2) | 1.2–76 | 42.0 (24.0) | 11–84 | 0.0035 | |
| Age now, years | 41.0 (25.0) | 18–76 | 38.0 (42.0) | 3–78 | 54.0 (21.0) | 11–90 | <0.0001 |
| BMI (N = 127/59/146) # | 25.5 (4.9) | 17.9–38.5 | 28.1 (6.5) | 16–41 | 28.4 (5.4) | 19.5–50 | <0.0001 |
| SUA off treatment, µmol/L (N = 132/46/112) # | 337 (118.2) | 140–617 | 448 (116.8) | 253–608 | 462 (124.5) | 181–683 | <0.0001 |
| SUA on treatment, µmol/L (N = 0/42/155) # | Not applicable | 424 (140) | 240–628 | 372 (126.0) | 163–725 | 0.0352 | |
| FE-UA * | Not collected | 3.7 (2.0) | 1.6–20 | 3.6 (1.6) | 0.8–14.3 | 0.6959 | |
| eGFR-MDRD, mL/min/1.73 m2 * | Not collected | 88.0 (36.0) | 27.6–426 | 86.0 (26.0) | 24–154 | 0.2965 | |
| Serum creatinine, µmol/L * | 75.5 (22.2) | 49–121 | 79.0 (20.5) | 26–132 | 82.0 (20.5) | 47–226 | 0.0016 |
| Max CRP ** (N = 132/54/146) # | 1.3 (1.9) | 0.1–17.9 | 1.9 (4.6) | 0.2–153.1 | 4.0 (6.4) | 0.2–222.4 | <0.0001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horváthová, V.; Bohatá, J.; Pavlíková, M.; Pavelcová, K.; Pavelka, K.; Šenolt, L.; Stibůrková, B. Interaction of the p.Q141K Variant of the ABCG2 Gene with Clinical Data and Cytokine Levels in Primary Hyperuricemia and Gout. J. Clin. Med. 2019, 8, 1965. https://doi.org/10.3390/jcm8111965
Horváthová V, Bohatá J, Pavlíková M, Pavelcová K, Pavelka K, Šenolt L, Stibůrková B. Interaction of the p.Q141K Variant of the ABCG2 Gene with Clinical Data and Cytokine Levels in Primary Hyperuricemia and Gout. Journal of Clinical Medicine. 2019; 8(11):1965. https://doi.org/10.3390/jcm8111965
Chicago/Turabian StyleHorváthová, Veronika, Jana Bohatá, Markéta Pavlíková, Kateřina Pavelcová, Karel Pavelka, Ladislav Šenolt, and Blanka Stibůrková. 2019. "Interaction of the p.Q141K Variant of the ABCG2 Gene with Clinical Data and Cytokine Levels in Primary Hyperuricemia and Gout" Journal of Clinical Medicine 8, no. 11: 1965. https://doi.org/10.3390/jcm8111965






