Heterogeneity of Vascular Endothelial Cells, De Novo Arteriogenesis and Therapeutic Implications in Pancreatic Neuroendocrine Tumors
Abstract
1. Introduction
2. Heterogeneity of Vascular Endothelial Cells
3. De Novo Arteriogenesis, an Emerging Concept of Formation of New Vascular Networks
4. Tumor Arteriogenesis: Potential Target in pNETs
5. Antiangiogenic Therapy in pNETs: Challenges and Prospective
5.1. Notch Pathway: The Potential Target in pNETs
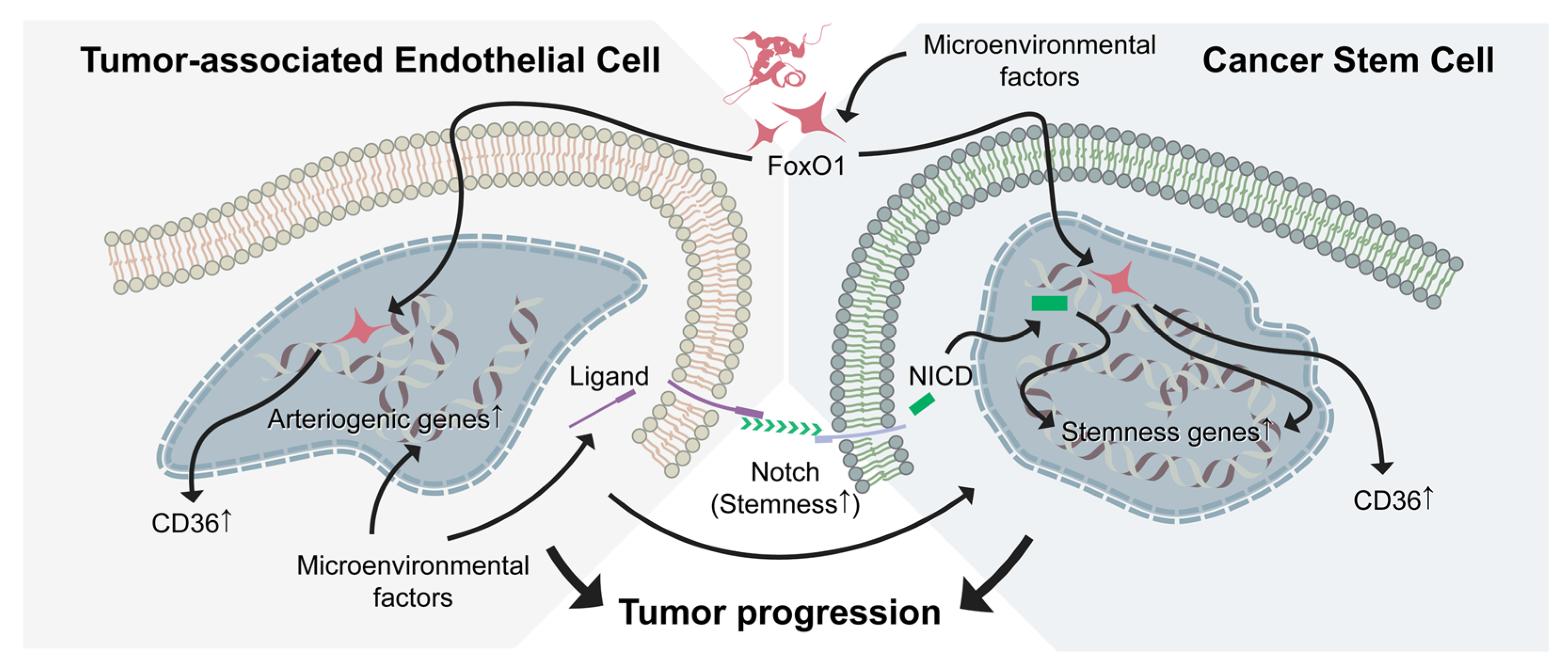
5.2. Control of pNET Progression by Targeting Vascular Niche and CSC Plasticity
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Halfdanarson, T.R.; Rabe, K.G.; Rubin, J.; Petersen, G.M. Pancreatic neuroendocrine tumors (PNETs): Incidence, prognosis and recent trend toward improved survival. Ann. Oncol. 2008, 19, 1727–1733. [Google Scholar] [CrossRef] [PubMed]
- Terris, B.; Scoazec, J.Y.; Rubbia, L.; Bregeaud, L.; Pepper, M.S.; Ruszniewski, P.; Belghiti, J.; Flejou, J.; Degott, C. Expression of vascular endothelial growth factor in digestive neuroendocrine tumours. Histopathology 1998, 32, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Casanovas, O.; Hicklin, D.J.; Bergers, G.; Hanahan, D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell 2005, 8, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.; Malik, H.Z.; Al-Mukthar, A.; Menon, K.V.; Toogood, G.J.; Lodge, J.P.; Prasad, K.R. Hepatic resection for metastatic gastrointestinal and pancreatic neuroendocrine tumours: Outcome and prognostic predictors. HPB (Oxford) 2007, 9, 345–351. [Google Scholar] [CrossRef]
- De Dosso, S.; Grande, E.; Barriuso, J.; Castellano, D.; Tabernero, J.; Capdevila, J. The targeted therapy revolution in neuroendocrine tumors: In search of biomarkers for patient selection and response evaluation. Cancer Metastasis Rev. 2013, 32, 465–477. [Google Scholar] [CrossRef]
- Sennino, B.; Ishiguro-Oonuma, T.; Wei, Y.; Naylor, R.M.; Williamson, C.W.; Bhagwandin, V.; Tabruyn, S.P.; You, W.K.; Chapman, H.A.; Christensen, J.G.; et al. Suppression of tumor invasion and metastasis by concurrent inhibition of c-Met and VEGF signaling in pancreatic neuroendocrine tumors. Cancer Discov. 2012, 2, 270–287. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Lenzi, P.; Bocci, G.; Natale, G. John Hunter and the origin of the term “angiogenesis”. Angiogenesis 2016, 19, 255–256. [Google Scholar] [CrossRef]
- Folkman, J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1995, 1, 27–31. [Google Scholar] [CrossRef]
- Carmeliet, P. Mechanisms of angiogenesis and arteriogenesis. Nat. Med. 2000, 6, 389–395. [Google Scholar] [CrossRef]
- Shalaby, F.; Rossant, J.; Yamaguchi, T.P.; Gertsenstein, M.; Wu, X.F.; Breitman, M.L.; Schuh, A.C. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature 1995, 376, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, H.F.; Brown, L.F.; Detmar, M.; Dvorak, A.M. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am. J. Pathol. 1995, 146, 1029–1039. [Google Scholar] [PubMed]
- Bernardi, R.; Guernah, I.; Jin, D.; Grisendi, S.; Alimonti, A.; Teruya-Feldstein, J.; Cordon-Cardo, C.; Simon, M.C.; Rafii, S.; Pandolfi, P.P. PML inhibits HIF-1alpha translation and neoangiogenesis through repression of mTOR. Nature 2006, 442, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Potente, M.; Gerhardt, H.; Carmeliet, P. Basic and therapeutic aspects of angiogenesis. Cell 2011, 146, 873–887. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef]
- Simons, M.; Ware, J.A. Therapeutic angiogenesis in cardiovascular disease. Nat. Rev. Drug Discov. 2003, 2, 863–871. [Google Scholar] [CrossRef]
- Mac Gabhann, F.; Peirce, S.M. Collateral capillary arterialization following arteriolar ligation in murine skeletal muscle. Microcirculation 2010, 17, 333–347. [Google Scholar] [CrossRef][Green Version]
- Ren, B.; Best, B.; Weihrauch, D.; Jones, D.W.; Dong, L.; Opansky, C.; Yuan, R.; Pritchard, K.A.; Silverstein, R. Abstract 15673: LPA/PKD-1-FoxO1-CD36 Signaling Axis Regulates Capillary Arterialization in Ischemic Conditions. Circulation 2016, 134, A15673. [Google Scholar] [CrossRef]
- Ren, B.; Deng, Y.; Mukhopadhyay, A.; Lanahan, A.A.; Zhuang, Z.W.; Moodie, K.L.; Mulligan-Kehoe, M.J.; Byzova, T.V.; Peterson, R.T.; Simons, M. ERK1/2-Akt1 crosstalk regulates arteriogenesis in mice and zebrafish. J. Clin. Invest. 2010, 120, 1217–1228. [Google Scholar] [CrossRef]
- Dong, L.; Yuan, Y.; Opansky, C.; Chen, Y.; Aguilera-Barrantes, I.; Wu, S.; Yuan, R.; Cao, Q.; Cheng, Y.C.; Sahoo, D.; et al. Diet-induced obesity links to ER positive breast cancer progression via LPA/PKD-1-CD36 signaling-mediated microvascular remodeling. Oncotarget 2017, 8, 22550–22562. [Google Scholar] [CrossRef]
- Ren, B.; Best, B.; Ramakrishnan, D.P.; Walcott, B.P.; Storz, P.; Silverstein, R.L. LPA/PKD-1-FoxO1 Signaling Axis Mediates Endothelial Cell CD36 Transcriptional Repression and Proangiogenic and Proarteriogenic Reprogramming. Arter. Thromb. Vasc. Biol. 2016, 36, 1197–1208. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Hale, J.; Srikanthan, S.; Silverstein, R.L. Lysophosphatidic acid suppresses endothelial cell CD36 expression and promotes angiogenesis via a PKD-1-dependent signaling pathway. Blood 2011, 117, 6036–6045. [Google Scholar] [CrossRef] [PubMed]
- Aitman, T.J.; Glazier, A.M.; Wallace, C.A.; Cooper, L.D.; Norsworthy, P.J.; Wahid, F.N.; Al-Majali, K.M.; Trembling, P.M.; Mann, C.J.; Shoulders, C.C.; et al. Identification of Cd36 (Fat) as an insulin-resistance gene causing defective fatty acid and glucose metabolism in hypertensive rats. Nat. Genet. 1999, 21, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Kohlenberg, J.D.; Chen, Y.; Komas, S.; Xin, G.; Yuan, G.; Cui, W.; Wu, S.; Ren, B. Abstract A09: Diet-induced obesity promotes breast cancer progression by LPA-signaling-mediated functional changes of mitochondria and angiogenesis. Cancer Res. 2015, 75, A09. [Google Scholar] [CrossRef]
- Pascual, G.; Avgustinova, A.; Mejetta, S.; Martin, M.; Castellanos, A.; Attolini, C.S.; Berenguer, A.; Prats, N.; Toll, A.; Hueto, J.A.; et al. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature 2017, 541, 41–45. [Google Scholar] [CrossRef]
- Hale, J.S.; Otvos, B.; Sinyuk, M.; Alvarado, A.G.; Hitomi, M.; Stoltz, K.; Wu, Q.; Flavahan, W.; Levison, B.; Johansen, M.L.; et al. Cancer stem cell-specific scavenger receptor CD36 drives glioblastoma progression. Stem Cells 2014, 32, 1746–1758. [Google Scholar] [CrossRef]
- Nagy, J.A.; Dvorak, H.F. Heterogeneity of the tumor vasculature: The need for new tumor blood vessel type-specific targets. Clin. Exp. Metastasis 2012, 29, 657–662. [Google Scholar] [CrossRef]
- Sitohy, B.; Nagy, J.A.; Jaminet, S.C.; Dvorak, H.F. Tumor-surrogate blood vessel subtypes exhibit differential susceptibility to anti-VEGF therapy. Cancer Res. 2011, 71, 7021–7028. [Google Scholar] [CrossRef]
- Ribatti, D. The involvement of endothelial progenitor cells in tumor angiogenesis. J. Cell Mol. Med. 2004, 8, 294–300. [Google Scholar] [CrossRef]
- Wang, R.; Chadalavada, K.; Wilshire, J.; Kowalik, U.; Hovinga, K.E.; Geber, A.; Fligelman, B.; Leversha, M.; Brennan, C.; Tabar, V. Glioblastoma stem-like cells give rise to tumour endothelium. Nature 2010, 468, 829–833. [Google Scholar] [CrossRef]
- Yuan, L.; Chan, G.C.; Beeler, D.; Janes, L.; Spokes, K.C.; Dharaneeswaran, H.; Mojiri, A.; Adams, W.J.; Sciuto, T.; Garcia-Cardena, G.; et al. A role of stochastic phenotype switching in generating mosaic endothelial cell heterogeneity. Nat. Commun. 2016, 7, 10160. [Google Scholar] [CrossRef] [PubMed]
- Regan, E.R.; Aird, W.C. Dynamical systems approach to endothelial heterogeneity. Circ. Res. 2012, 111, 110–130. [Google Scholar] [CrossRef] [PubMed]
- Ren, B. Endothelial Cells: A Key Player in Angiogenesis and Lymphangiogenesis. MOJ Cell Sci. Rep. 2015, 2. [Google Scholar] [CrossRef][Green Version]
- Chi, J.T.; Chang, H.Y.; Haraldsen, G.; Jahnsen, F.L.; Troyanskaya, O.G.; Chang, D.S.; Wang, Z.; Rockson, S.G.; van de Rijn, M.; Botstein, D.; et al. Endothelial cell diversity revealed by global expression profiling. Proc. Natl. Acad. Sci. USA 2003, 100, 10623–10628. [Google Scholar] [CrossRef] [PubMed]
- Best, B.; Moran, P.; Ren, B. VEGF/PKD-1 signaling mediates arteriogenic gene expression and angiogenic responses in reversible human microvascular endothelial cells with extended lifespan. Mol. Cell Biochem. 2018, 446, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Nolan, D.J.; Ginsberg, M.; Israely, E.; Palikuqi, B.; Poulos, M.G.; James, D.; Ding, B.S.; Schachterle, W.; Liu, Y.; Rosenwaks, Z.; et al. Molecular signatures of tissue-specific microvascular endothelial cell heterogeneity in organ maintenance and regeneration. Dev. Cell 2013, 26, 204–219. [Google Scholar] [CrossRef] [PubMed]
- Marcu, R.; Choi, Y.J.; Xue, J.; Fortin, C.L.; Wang, Y.; Nagao, R.J.; Xu, J.; MacDonald, J.W.; Bammler, T.K.; Murry, C.E.; et al. Human Organ-Specific Endothelial Cell Heterogeneity. iScience 2018, 4, 20–35. [Google Scholar] [CrossRef]
- Bentley, K.; Franco, C.A.; Philippides, A.; Blanco, R.; Dierkes, M.; Gebala, V.; Stanchi, F.; Jones, M.; Aspalter, I.M.; Cagna, G.; et al. The role of differential VE-cadherin dynamics in cell rearrangement during angiogenesis. Nat. Cell Biol. 2014, 16, 309–321. [Google Scholar] [CrossRef]
- Ren, B. FoxO1 transcriptional activities in VEGF expression and beyond: A key regulator in functional angiogenesis? J. Pathol. 2018, 245, 255–257. [Google Scholar] [CrossRef]
- Ren, B. Protein Kinase D1 Signaling in Angiogenic Gene Expression and VEGF-Mediated Angiogenesis. Front. Cell Dev. Biol. 2016, 4, 37. [Google Scholar] [CrossRef]
- Baek, K.I.; Packard, R.R.S.; Hsu, J.J.; Saffari, A.; Ma, Z.; Luu, A.P.; Pietersen, A.; Yen, H.; Ren, B.; Ding, Y.; et al. Ultrafine Particle Exposure Reveals the Importance of FOXO1/Notch Activation Complex for Vascular Regeneration. Antioxid. Redox Signal. 2018, 28, 1209–1223. [Google Scholar] [CrossRef]
- Denekamp, J. Endothelial cell proliferation as a novel approach to targeting tumour therapy. Br. J. Cancer 1982, 45, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, S.; Baluk, P.; Kaidoh, T.; Haskell, A.; Jain, R.K.; McDonald, D.M. Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am. J. Pathol. 2002, 160, 985–1000. [Google Scholar] [CrossRef]
- Orimo, A.; Weinberg, R.A. Stromal fibroblasts in cancer: A novel tumor-promoting cell type. Cell Cycle 2006, 5, 1597–1601. [Google Scholar] [CrossRef] [PubMed]
- Franklin, R.A.; Liao, W.; Sarkar, A.; Kim, M.V.; Bivona, M.R.; Liu, K.; Pamer, E.G.; Li, M.O. The cellular and molecular origin of tumor-associated macrophages. Science 2014, 344, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Ronca, R.; Van Ginderachter, J.A.; Turtoi, A. Paracrine interactions of cancer-associated fibroblasts, macrophages and endothelial cells: Tumor allies and foes. Curr. Opin. Oncol. 2018, 30, 45–53. [Google Scholar] [CrossRef]
- Wilson, W.R.; Hay, M.P. Targeting hypoxia in cancer therapy. Nat. Rev. Cancer 2011, 11, 393–410. [Google Scholar] [CrossRef]
- Krock, B.L.; Skuli, N.; Simon, M.C. Hypoxia-induced angiogenesis: Good and evil. Genes Cancer 2011, 2, 1117–1133. [Google Scholar] [CrossRef]
- Senger, D.R.; Galli, S.J.; Dvorak, A.M.; Perruzzi, C.A.; Harvey, V.S.; Dvorak, H.F. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 1983, 219, 983–985. [Google Scholar] [CrossRef]
- Ren, B.; Hoti, N.; Rabasseda, X.; Wang, Y.Z.; Wu, M. The antiangiogenic and therapeutic implications of endostatin. Methods Find. Exp. Clin. Pharm. 2003, 25, 215–224. [Google Scholar] [CrossRef]
- Ren, B.; Yee, K.O.; Lawler, J.; Khosravi-Far, R. Regulation of tumor angiogenesis by thrombospondin-1. Biochim. Biophys. Acta 2006, 1765, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Song, K.; Parangi, S.; Jin, T.; Ye, M.; Humphreys, R.; Duquette, M.; Zhang, X.; Benhaga, N.; Lawler, J.; et al. A double hit to kill tumor and endothelial cells by TRAIL and antiangiogenic 3TSR. Cancer Res. 2009, 69, 3856–3865. [Google Scholar] [CrossRef] [PubMed]
- Simons, M.; Gordon, E.; Claesson-Welsh, L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat. Rev. Mol. Cell Biol. 2016, 17, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Nagy, J.A.; Chang, S.H.; Shih, S.C.; Dvorak, A.M.; Dvorak, H.F. Heterogeneity of the tumor vasculature. Semin. Thromb. Hemost. 2010, 36, 321–331. [Google Scholar] [CrossRef]
- Dvorak, H.F. Tumor Stroma, Tumor Blood Vessels, and Antiangiogenesis Therapy. Cancer J. 2015, 21, 237–243. [Google Scholar] [CrossRef]
- Hida, K.; Hida, Y.; Amin, D.N.; Flint, A.F.; Panigrahy, D.; Morton, C.C.; Klagsbrun, M. Tumor-associated endothelial cells with cytogenetic abnormalities. Cancer Res. 2004, 64, 8249–8255. [Google Scholar] [CrossRef]
- Ricci-Vitiani, L.; Pallini, R.; Biffoni, M.; Todaro, M.; Invernici, G.; Cenci, T.; Maira, G.; Parati, E.A.; Stassi, G.; Larocca, L.M.; et al. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature 2010, 468, 824–828. [Google Scholar] [CrossRef]
- Samson, T.; Welch, C.; Monaghan-Benson, E.; Hahn, K.M.; Burridge, K. Endogenous RhoG is rapidly activated after epidermal growth factor stimulation through multiple guanine-nucleotide exchange factors. Mol. Biol. Cell 2010, 21, 1629–1642. [Google Scholar] [CrossRef]
- Franses, J.W.; Edelman, E.R. The evolution of endothelial regulatory paradigms in cancer biology and vascular repair. Cancer Res. 2011, 71, 7339–7344. [Google Scholar] [CrossRef]
- Franses, J.W.; Baker, A.B.; Chitalia, V.C.; Edelman, E.R. Stromal endothelial cells directly influence cancer progression. Sci. Transl. Med. 2011, 3, 66ra65. [Google Scholar] [CrossRef]
- Jain, R.K.; Carmeliet, P. SnapShot: Tumor angiogenesis. Cell 2012, 149, 1408. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K. Antiangiogenesis strategies revisited: From starving tumors to alleviating hypoxia. Cancer Cell 2014, 26, 605–622. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Pluda, J.M. Tumor-associated angiogenesis: Mechanisms, clinical implications, and therapeutic strategies. Semin. Oncol. 1997, 24, 203–218. [Google Scholar]
- Brem, H.; Folkman, J. Inhibition of tumor angiogenesis mediated by cartilage. J. Exp. Med. 1975, 141, 427–439. [Google Scholar] [CrossRef]
- Hainaud, P.; Contreres, J.O.; Villemain, A.; Liu, L.X.; Plouet, J.; Tobelem, G.; Dupuy, E. The role of the vascular endothelial growth factor-Delta-like 4 ligand/Notch4-ephrin B2 cascade in tumor vessel remodeling and endothelial cell functions. Cancer Res. 2006, 66, 8501–8510. [Google Scholar] [CrossRef]
- Heil, M.; Eitenmuller, I.; Schmitz-Rixen, T.; Schaper, W. Arteriogenesis versus angiogenesis: Similarities and differences. J. Cell Mol. Med. 2006, 10, 45–55. [Google Scholar] [CrossRef]
- Cai, W.J.; Koltai, S.; Kocsis, E.; Scholz, D.; Kostin, S.; Luo, X.; Schaper, W.; Schaper, J. Remodeling of the adventitia during coronary arteriogenesis. Am. J. Physiol.-Heart Circ. Physiol. 2003, 284, H31–H40. [Google Scholar] [CrossRef][Green Version]
- Lanahan, A.; Zhang, X.; Fantin, A.; Zhuang, Z.; Rivera-Molina, F.; Speichinger, K.; Prahst, C.; Zhang, J.; Wang, Y.; Davis, G.; et al. The neuropilin 1 cytoplasmic domain is required for VEGF-A-dependent arteriogenesis. Dev. Cell 2013, 25, 156–168. [Google Scholar] [CrossRef]
- Moraes, F.; Paye, J.; Mac Gabhann, F.; Zhuang, Z.W.; Zhang, J.; Lanahan, A.A.; Simons, M. Endothelial cell-dependent regulation of arteriogenesis. Circ. Res. 2013, 113, 1076–1086. [Google Scholar] [CrossRef]
- Moran, P.; Guo, Y.; Yuan, R.; Barnekow, N.; Palmer, J.; Beck, A.; Ren, B. Translating Ribosome Affinity Purification (TRAP) for RNA Isolation from Endothelial Cells In vivo. J. Vis. Exp. 2019, e59624. [Google Scholar] [CrossRef] [PubMed]
- Moran, P.; Opansky, C.; Weihrauch, D.; Yuan, R.; Jones, D.W.; Ramchandran, R.; Ren, B. Abstract 14944: Transcriptional Reprogramming of Endothelial Cells for Arteriolar Differentiation by Small Chemical Molecule via Protein Kinase D1 Signaling Pathway. Circulation 2017, 136, A14944. [Google Scholar] [CrossRef]
- Wang, H.U.; Chen, Z.F.; Anderson, D.J. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell 1998, 93, 741–753. [Google Scholar] [CrossRef]
- Weinstein, B.M.; Stemple, D.L.; Driever, W.; Fishman, M.C. Gridlock, a localized heritable vascular patterning defect in the zebrafish. Nat. Med. 1995, 1, 1143–1147. [Google Scholar] [CrossRef]
- Zhong, T.P.; Rosenberg, M.; Mohideen, M.A.; Weinstein, B.; Fishman, M.C. gridlock, an HLH gene required for assembly of the aorta in zebrafish. Science 2000, 287, 1820–1824. [Google Scholar] [CrossRef]
- Zhong, T.P.; Childs, S.; Leu, J.P.; Fishman, M.C. Gridlock signalling pathway fashions the first embryonic artery. Nature 2001, 414, 216–220. [Google Scholar] [CrossRef]
- Lawson, N.D.; Scheer, N.; Pham, V.N.; Kim, C.H.; Chitnis, A.B.; Campos-Ortega, J.A.; Weinstein, B.M. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development 2001, 128, 3675–3683. [Google Scholar]
- Weinstein, B.M.; Lawson, N.D. Arteries, veins, Notch, and VEGF. Cold Spring Harb. Symp. Quant. Biol. 2002, 67, 155–162. [Google Scholar] [CrossRef]
- Mukouyama, Y.S.; Shin, D.; Britsch, S.; Taniguchi, M.; Anderson, D.J. Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell 2002, 109, 693–705. [Google Scholar] [CrossRef]
- Visconti, R.P.; Richardson, C.D.; Sato, T.N. Orchestration of angiogenesis and arteriovenous contribution by angiopoietins and vascular endothelial growth factor (VEGF). Proc. Natl. Acad. Sci. USA 2002, 99, 8219–8224. [Google Scholar] [CrossRef]
- Hong, C.C.; Peterson, Q.P.; Hong, J.Y.; Peterson, R.T. Artery/vein specification is governed by opposing phosphatidylinositol-3 kinase and MAP kinase/ERK signaling. Curr. Biol. 2006, 16, 1366–1372. [Google Scholar] [CrossRef] [PubMed]
- Peterson, R.T.; Shaw, S.Y.; Peterson, T.A.; Milan, D.J.; Zhong, T.P.; Schreiber, S.L.; MacRae, C.A.; Fishman, M.C. Chemical suppression of a genetic mutation in a zebrafish model of aortic coarctation. Nat. Biotechnol. 2004, 22, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Kazerounian, S.; Duquette, M.; Reyes, M.A.; Lawler, J.T.; Song, K.; Perruzzi, C.; Primo, L.; Khosravi-Far, R.; Bussolino, F.; Rabinovitz, I.; et al. Priming of the vascular endothelial growth factor signaling pathway by thrombospondin-1, CD36, and spleen tyrosine kinase. Blood 2011, 117, 4658–4666. [Google Scholar] [CrossRef] [PubMed]
- Dewey, J.F. Regional tectonics. Science 1981, 214, 550–551. [Google Scholar] [CrossRef] [PubMed]
- Deindl, E.; Hoefer, I.E.; Fernandez, B.; Barancik, M.; Heil, M.; Strniskova, M.; Schaper, W. Involvement of the fibroblast growth factor system in adaptive and chemokine-induced arteriogenesis. Circ. Res. 2003, 92, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Aragones, J.; Fraisl, P.; Baes, M.; Carmeliet, P. Oxygen sensors at the crossroad of metabolism. Cell Metab. 2009, 9, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Helisch, A.; Schaper, W. Arteriogenesis: The development and growth of collateral arteries. Microcirculation 2003, 10, 83–97. [Google Scholar] [CrossRef]
- Dong, L.; Yuan, Y.; Aguilera-Barrantes, I.; Chen, Y.; Sturich, A.; Yuan, R.; Wu, S.; Silverstein, R.; Ren, B. Abstract 482: Signaling Lipid Lysophosphatidic Acid Is a Critical Link to Diet-induced Obesity, Cellular Bioenergetics and Breast Cancer Angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2015, 35, A482. [Google Scholar]
- Yu, J.L.; Rak, J.W. Host microenvironment in breast cancer development: Inflammatory and immune cells in tumour angiogenesis and arteriogenesis. Breast Cancer Res. 2003, 5, 83–88. [Google Scholar] [CrossRef]
- Skuli, N.; Majmundar, A.J.; Krock, B.L.; Mesquita, R.C.; Mathew, L.K.; Quinn, Z.L.; Runge, A.; Liu, L.; Kim, M.N.; Liang, J.; et al. Endothelial HIF-2alpha regulates murine pathological angiogenesis and revascularization processes. J. Clin. Invest. 2012, 122, 1427–1443. [Google Scholar] [CrossRef]
- Skuli, N.; Liu, L.; Runge, A.; Wang, T.; Yuan, L.; Patel, S.; Iruela-Arispe, L.; Simon, M.C.; Keith, B. Endothelial deletion of hypoxia-inducible factor-2alpha (HIF-2alpha) alters vascular function and tumor angiogenesis. Blood 2009, 114, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Eberhard, A.; Kahlert, S.; Goede, V.; Hemmerlein, B.; Plate, K.H.; Augustin, H.G. Heterogeneity of angiogenesis and blood vessel maturation in human tumors: Implications for antiangiogenic tumor therapies. Cancer Res. 2000, 60, 1388–1393. [Google Scholar] [PubMed]
- Buschmann, I.; Heil, M.; Jost, M.; Schaper, W. Influence of inflammatory cytokines on arteriogenesis. Microcirculation 2003, 10, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Rissanen, T.T.; Korpisalo, P.; Markkanen, J.E.; Liimatainen, T.; Orden, M.R.; Kholova, I.; de Goede, A.; Heikura, T.; Grohn, O.H.; Yla-Herttuala, S. Blood flow remodels growing vasculature during vascular endothelial growth factor gene therapy and determines between capillary arterialization and sprouting angiogenesis. Circulation 2005, 112, 3937–3946. [Google Scholar] [CrossRef] [PubMed]
- Schechter, J.; Goldsmith, P.; Wilson, C.; Weiner, R. Morphological evidence for the presence of arteries in human prolactinomas. J. Clin. Endocrinol. Metab. 1988, 67, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Tinsley, J.H.; Teasdale, N.R.; Yuan, S.Y. Involvement of PKCdelta and PKD in pulmonary microvascular endothelial cell hyperpermeability. Am. J. Physiol. Cell Physiol. 2004, 286, C105–C111. [Google Scholar] [CrossRef]
- Shin, D.; Garcia-Cardena, G.; Hayashi, S.; Gerety, S.; Asahara, T.; Stavrakis, G.; Isner, J.; Folkman, J.; Gimbrone, M.A., Jr.; Anderson, D.J. Expression of ephrinB2 identifies a stable genetic difference between arterial and venous vascular smooth muscle as well as endothelial cells, and marks subsets of microvessels at sites of adult neovascularization. Dev. Biol. 2001, 230, 139–150. [Google Scholar] [CrossRef]
- Elias, D.; Lasser, P.; Ducreux, M.; Duvillard, P.; Ouellet, J.F.; Dromain, C.; Schlumberger, M.; Pocard, M.; Boige, V.; Miquel, C.; et al. Liver resection (and associated extrahepatic resections) for metastatic well-differentiated endocrine tumors: A 15-year single center prospective study. Surgery 2003, 133, 375–382. [Google Scholar] [CrossRef]
- Dromain, C.; de Baere, T.; Baudin, E.; Galline, J.; Ducreux, M.; Boige, V.; Duvillard, P.; Laplanche, A.; Caillet, H.; Lasser, P.; et al. MR imaging of hepatic metastases caused by neuroendocrine tumors: Comparing four techniques. Am. J. Roentgenol. 2003, 180, 121–128. [Google Scholar] [CrossRef]
- Calderone, A. The Biological Role of Nestin((+))-Cells in Physiological and Pathological Cardiovascular Remodeling. Front. Cell Dev. Biol. 2018, 6, 15. [Google Scholar] [CrossRef]
- Yazdani, S.; Kasajima, A.; Tamaki, K.; Nakamura, Y.; Fujishima, F.; Ohtsuka, H.; Motoi, F.; Unno, M.; Watanabe, M.; Sato, Y.; et al. Angiogenesis and vascular maturation in neuroendocrine tumors. Hum. Pathol. 2014, 45, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.M.; Hislop, A.A.; Pierce, C.M.; Haworth, S.G. Prenatal origins of human intrapulmonary arteries: Formation and smooth muscle maturation. Am. J. Respir. Cell Mol. Biol. 2000, 23, 194–203. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Helmlinger, G.; Netti, P.A.; Lichtenbeld, H.C.; Melder, R.J.; Jain, R.K. Solid stress inhibits the growth of multicellular tumor spheroids. Nat. Biotechnol. 1997, 15, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Faivre, S.; Niccoli, P.; Castellano, D.; Valle, J.W.; Hammel, P.; Raoul, J.L.; Vinik, A.; Van Cutsem, E.; Bang, Y.J.; Lee, S.H.; et al. Sunitinib in pancreatic neuroendocrine tumors: Updated progression-free survival and final overall survival from a phase III randomized study. Ann. Oncol. 2017, 28, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.W.; Rizzo, P.; Pannuti, A.; Golde, T.; Osborne, B.; Miele, L. Notch signals in the endothelium and cancer “stem-like” cells: Opportunities for cancer therapy. Vasc. Cell 2012, 4, 7. [Google Scholar] [CrossRef][Green Version]
- Rafii, S.; Butler, J.M.; Ding, B.S. Angiocrine functions of organ-specific endothelial cells. Nature 2016, 529, 316–325. [Google Scholar] [CrossRef]
- Awgulewitsch, C.P.; Trinh, L.T.; Hatzopoulos, A.K. The Vascular Wall: A Plastic Hub of Activity in Cardiovascular Homeostasis and Disease. Curr. Cardiol. Rep. 2017, 19, 51. [Google Scholar] [CrossRef]
- Kohlenberg, J.D.; Chen, Y.; Best, B.; Storz, P.; Peterson, R.T.; Silverstein, R.; Ren, B. Abstract LB-338: A novel LPA-PKD1-FoxO1 pathway in endothelial cells provides an angiogenic switch via down-regulation of CD36 transcription and induction of arteriogenic responses. Cancer Res. 2013, 73. [Google Scholar] [CrossRef]
- Opansky, C.; Best, B.; Yuan, R.; Cao, Q.; Ren, B. Protein Kinase D1 Signaling is the Key to Arterial Differentiation of Vascular Endothelial Cells. Circulation 2016, 134, A14437. [Google Scholar]
- Yao, J.C.; Eisner, M.P.; Leary, C.; Dagohoy, C.; Phan, A.; Rashid, A.; Hassan, M.; Evans, D.B. Population-based study of islet cell carcinoma. Ann. Surg. Oncol. 2007, 14, 3492–3500. [Google Scholar] [CrossRef]
- Metz, D.C.; Jensen, R.T. Gastrointestinal neuroendocrine tumors: Pancreatic endocrine tumors. Gastroenterology 2008, 135, 1469–1492. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, B.; Gustafsson, B.I.; Chan, A.; Svejda, B.; Kidd, M.; Modlin, I.M. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol. Metab. Clin. N. Am. 2011, 40, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Cueto, A.; Burigana, F.; Nicolini, A.; Lugnani, F. Neuroendocrine tumors of the lung: Hystological classification, diagnosis, traditional and new therapeutic approaches. Curr. Med. Chem. 2014, 21, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Liakakos, T.; Roukos, D.H. Everolimus and sunitinib: From mouse models to treatment of pancreatic neuroendocrine tumors. Future Oncol. 2011, 7, 1025–1029. [Google Scholar] [CrossRef]
- Hori, T.; Takaori, K.; Uemoto, S. Pancreatic neuroendocrine tumor accompanied with multiple liver metastases. World J. Hepatol. 2014, 6, 596–600. [Google Scholar] [CrossRef]
- Rindi, G.; Klersy, C.; Albarello, L.; Baudin, E.; Bianchi, A.; Buchler, M.W.; Caplin, M.; Couvelard, A.; Cros, J.; de Herder, W.W.; et al. Competitive Testing of the WHO 2010 versus the WHO 2017 Grading of Pancreatic Neuroendocrine Neoplasms: Data from a Large International Cohort Study. Neuroendocrinology 2018, 107, 375–386. [Google Scholar] [CrossRef]
- Proye, C. Natural history of liver metastasis of gastroenteropancreatic neuroendocrine tumors: Place for chemoembolization. World J. Surg. 2001, 25, 685–688. [Google Scholar] [CrossRef]
- Fendrich, V.; Waldmann, J.; Bartsch, D.K.; Langer, P. Surgical management of pancreatic endocrine tumors. Nat. Rev. Clin. Oncol. 2009, 6, 419–428. [Google Scholar] [CrossRef]
- Nguyen, S.Q.; Angel, L.P.; Divino, C.M.; Schluender, S.; Warner, R.R. Surgery in malignant pancreatic neuroendocrine tumors. J. Surg. Oncol. 2007, 96, 397–403. [Google Scholar] [CrossRef]
- Villaume, K.; Blanc, M.; Gouysse, G.; Walter, T.; Couderc, C.; Nejjari, M.; Vercherat, C.; Cordier-Bussat, M.; Roche, C.; Scoazec, J.Y. VEGF secretion by neuroendocrine tumor cells is inhibited by octreotide and by inhibitors of the PI3K/AKT/mTOR pathway. Neuroendocrinology 2010, 91, 268–278. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, R.; Zhang, Y.; Jia, T.; Cao, Y.; Wahlberg, E. Differential roles of PDGFR-alpha and PDGFR-beta in angiogenesis and vessel stability. FASEB J. 2009, 23, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Raymond, E.; Dahan, L.; Raoul, J.L.; Bang, Y.J.; Borbath, I.; Lombard-Bohas, C.; Valle, J.; Metrakos, P.; Smith, D.; Vinik, A.; et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N. Engl. J. Med. 2011, 364, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.W.; Borbath, I.; Rosbrook, B.; Fernandez, K.; Raymond, E. Sunitinib in patients with pancreatic neuroendocrine tumors: Update of safety data. Future Oncol. 2019, 15. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, M.; VON Arx, C.; DE Divitiis, C.; Ottaiano, A.; Tatangelo, F.; Romano, G.M.; Tafuto, S. Antiangiogenic Therapy in Pancreatic Neuroendocrine Tumors. Anticancer Res. 2016, 36, 5025–5030. [Google Scholar] [CrossRef] [PubMed]
- Berruti, A.; Fazio, N.; Ferrero, A.; Brizzi, M.P.; Volante, M.; Nobili, E.; Tozzi, L.; Bodei, L.; Torta, M.; D’Avolio, A.; et al. Bevacizumab plus octreotide and metronomic capecitabine in patients with metastatic well-to-moderately differentiated neuroendocrine tumors: The XELBEVOCT study. BMC Cancer 2014, 14, 184. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.A.; Stuart, K.; Earle, C.C.; Clark, J.W.; Bhargava, P.; Miksad, R.; Blaszkowsky, L.; Enzinger, P.C.; Meyerhardt, J.A.; Zheng, H.; et al. Prospective study of bevacizumab plus temozolomide in patients with advanced neuroendocrine tumors. J. Clin. Oncol. 2012, 30, 2963–2968. [Google Scholar] [CrossRef] [PubMed]
- Helfrich, I.; Scheffrahn, I.; Bartling, S.; Weis, J.; von Felbert, V.; Middleton, M.; Kato, M.; Ergun, S.; Augustin, H.G.; Schadendorf, D. Resistance to antiangiogenic therapy is directed by vascular phenotype, vessel stabilization, and maturation in malignant melanoma. J. Exp. Med. 2010, 207, 491–503. [Google Scholar] [CrossRef]
- Erber, R.; Thurnher, A.; Katsen, A.D.; Groth, G.; Kerger, H.; Hammes, H.P.; Menger, M.D.; Ullrich, A.; Vajkoczy, P. Combined inhibition of VEGF and PDGF signaling enforces tumor vessel regression by interfering with pericyte-mediated endothelial cell survival mechanisms. FASEB J. 2004, 18, 338–340. [Google Scholar] [CrossRef]
- Sitohy, B.; Nagy, J.A.; Dvorak, H.F. Anti-VEGF/VEGFR therapy for cancer: Reassessing the target. Cancer Res. 2012, 72, 1909–1914. [Google Scholar] [CrossRef]
- Uri, I.; Grozinsky-Glasberg, S. Current treatment strategies for patients with advanced gastroenteropancreatic neuroendocrine tumors (GEP-NETs). Clin. Diabetes Endocrinol. 2018, 4, 16. [Google Scholar] [CrossRef]
- Raymond, E.; Kulke, M.H.; Qin, S.; Yu, X.; Schenker, M.; Cubillo, A.; Lou, W.; Tomasek, J.; Thiis-Evensen, E.; Xu, J.M.; et al. Efficacy and Safety of Sunitinib in Patients with Well-Differentiated Pancreatic Neuroendocrine Tumours. Neuroendocrinology 2018, 107, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Rinzivillo, M.; Fazio, N.; Pusceddu, S.; Spallanzani, A.; Ibrahim, T.; Campana, D.; Marconcini, R.; Partelli, S.; Badalamenti, G.; Brizzi, M.P.; et al. Sunitinib in patients with pre-treated pancreatic neuroendocrine tumors: A real-world study. Pancreatology 2018, 18, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.C.; Shah, M.H.; Ito, T.; Bohas, C.L.; Wolin, E.M.; Van Cutsem, E.; Hobday, T.J.; Okusaka, T.; Capdevila, J.; de Vries, E.G.; et al. Everolimus for advanced pancreatic neuroendocrine tumors. N. Engl. J. Med. 2011, 364, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Baldelli, R.; Barnabei, A.; Rizza, L.; Isidori, A.M.; Rota, F.; Di Giacinto, P.; Paoloni, A.; Torino, F.; Corsello, S.M.; Lenzi, A.; et al. Somatostatin analogs therapy in gastroenteropancreatic neuroendocrine tumors: Current aspects and new perspectives. Front. Endocrinol. 2014, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Caplin, M.E.; Pavel, M.; Cwikla, J.B.; Phan, A.T.; Raderer, M.; Sedlackova, E.; Cadiot, G.; Wolin, E.M.; Capdevila, J.; Wall, L.; et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N. Engl. J. Med. 2014, 371, 224–233. [Google Scholar] [CrossRef]
- Woltering, E.A.; Barrie, R.; O’Dorisio, T.M.; Arce, D.; Ure, T.; Cramer, A.; Holmes, D.; Robertson, J.; Fassler, J. Somatostatin analogues inhibit angiogenesis in the chick chorioallantoic membrane. J. Surg. Res. 1991, 50, 245–251. [Google Scholar] [CrossRef]
- Grozinsky-Glasberg, S.; Shimon, I.; Korbonits, M.; Grossman, A.B. Somatostatin analogues in the control of neuroendocrine tumours: Efficacy and mechanisms. Endocr. Relat. Cancer 2008, 15, 701–720. [Google Scholar] [CrossRef]
- Dasgupta, P. Somatostatin analogues: Multiple roles in cellular proliferation, neoplasia, and angiogenesis. Pharmacol. Ther. 2004, 102, 61–85. [Google Scholar] [CrossRef]
- Albini, A.; Florio, T.; Giunciuglio, D.; Masiello, L.; Carlone, S.; Corsaro, A.; Thellung, S.; Cai, T.; Noonan, D.M.; Schettini, G. Somatostatin controls Kaposi’s sarcoma tumor growth through inhibition of angiogenesis. FASEB J. 1999, 13, 647–655. [Google Scholar] [CrossRef]
- Mentlein, R.; Eichler, O.; Forstreuter, F.; Held-Feindt, J. Somatostatin inhibits the production of vascular endothelial growth factor in human glioma cells. Int. J. Cancer 2001, 92, 545–550. [Google Scholar] [CrossRef]
- Boehm, T.; Folkman, J.; Browder, T.; O’Reilly, M.S. Antiangiogenic therapy of experimental cancer does not induce acquired drug resistance. Nature 1997, 390, 404–407. [Google Scholar] [CrossRef] [PubMed]
- Mauceri, H.J.; Hanna, N.N.; Beckett, M.A.; Gorski, D.H.; Staba, M.J.; Stellato, K.A.; Bigelow, K.; Heimann, R.; Gately, S.; Dhanabal, M.; et al. Combined effects of angiostatin and ionizing radiation in antitumour therapy. Nature 1998, 394, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, M.; Brooks, P.C.; Shaffer, R.W.; Kincaid, C.M.; Varner, J.A.; Cheresh, D.A. Definition of two angiogenic pathways by distinct alpha v integrins. Science 1995, 270, 1500–1502. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Chen, H.; Davis-Smyth, T.; Gerber, H.P.; Nguyen, T.N.; Peers, D.; Chisholm, V.; Hillan, K.J.; Schwall, R.H. Vascular endothelial growth factor is essential for corpus luteum angiogenesis. Nat. Med. 1998, 4, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.B.; Ren, H.; Zhao, H.; Chi, Y.; Chen, K.; Zhou, B.; Liu, Y.J.; Zhang, L.; Xu, B.; Liu, B.; et al. Hypoxia-inducible factor (HIF)-1 alpha directly enhances the transcriptional activity of stem cell factor (SCF) in response to hypoxia and epidermal growth factor (EGF). Carcinogenesis 2008, 29, 1853–1861. [Google Scholar] [CrossRef] [PubMed]
- Cooke, V.G.; LeBleu, V.S.; Keskin, D.; Khan, Z.; O’Connell, J.T.; Teng, Y.; Duncan, M.B.; Xie, L.; Maeda, G.; Vong, S.; et al. Pericyte depletion results in hypoxia-associated epithelial-to-mesenchymal transition and metastasis mediated by met signaling pathway. Cancer Cell 2012, 21, 66–81. [Google Scholar] [CrossRef]
- Paez-Ribes, M.; Allen, E.; Hudock, J.; Takeda, T.; Okuyama, H.; Vinals, F.; Inoue, M.; Bergers, G.; Hanahan, D.; Casanovas, O. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell 2009, 15, 220–231. [Google Scholar] [CrossRef]
- Manegold, C.; Dingemans, A.C.; Gray, J.E.; Nakagawa, K.; Nicolson, M.; Peters, S.; Reck, M.; Wu, Y.L.; Brustugun, O.T.; Crino, L.; et al. The Potential of Combined Immunotherapy and Antiangiogenesis for the Synergistic Treatment of Advanced NSCLC. J. Thorac. Oncol. 2017, 12, 194–207. [Google Scholar] [CrossRef]
- Mack, J.J.; Iruela-Arispe, M.L. NOTCH regulation of the endothelial cell phenotype. Curr. Opin. Hematol. 2018, 25, 212–218. [Google Scholar] [CrossRef]
- Noguera-Troise, I.; Daly, C.; Papadopoulos, N.J.; Coetzee, S.; Boland, P.; Gale, N.W.; Lin, H.C.; Yancopoulos, G.D.; Thurston, G. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature 2006, 444, 1032–1037. [Google Scholar] [CrossRef]
- Ridgway, J.; Zhang, G.; Wu, Y.; Stawicki, S.; Liang, W.C.; Chanthery, Y.; Kowalski, J.; Watts, R.J.; Callahan, C.; Kasman, I.; et al. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature 2006, 444, 1083–1087. [Google Scholar] [CrossRef] [PubMed]
- Scehnet, J.S.; Jiang, W.; Kumar, S.R.; Krasnoperov, V.; Trindade, A.; Benedito, R.; Djokovic, D.; Borges, C.; Ley, E.J.; Duarte, A.; et al. Inhibition of Dll4-mediated signaling induces proliferation of immature vessels and results in poor tissue perfusion. Blood 2007, 109, 4753–4760. [Google Scholar] [CrossRef] [PubMed]
- Low, S.; Barnes, J.L.; Zammit, P.S.; Beauchamp, J.R. Delta-Like 4 Activates Notch 3 to Regulate Self-Renewal in Skeletal Muscle Stem Cells. Stem Cells 2018, 36, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Akishima-Fukasawa, Y.; Kobayashi, N.; Sano, T.; Kosuge, T.; Nimura, Y.; Kanai, Y.; Hiraoka, N. Prognostic value of tumor architecture, tumor-associated vascular characteristics, and expression of angiogenic molecules in pancreatic endocrine tumors. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2007, 13, 187–196. [Google Scholar] [CrossRef]
- Tan, G.; Cioc, A.M.; Perez-Montiel, D.; Ellison, E.C.; Frankel, W.L. Microvascular density does not correlate with histopathology and outcome in neuroendocrine tumors of the pancreas. Appl. Immunohistochem. Mol. Morphol. 2004, 12, 31–35. [Google Scholar] [CrossRef]
- Laitakari, J.; Nayha, V.; Stenback, F. Size, shape, structure, and direction of angiogenesis in laryngeal tumour development. J. Clin. Pathol. 2004, 57, 394–401. [Google Scholar] [CrossRef]
- Nayha, V.V.; Stenback, F.G. Increased angiogenesis is associated with poor prognosis of squamous cell carcinoma of the vulva. Acta Obstet. Gynecol. Scand. 2007, 86, 1392–1397. [Google Scholar] [CrossRef]
- Crabtree, J.S.; Singleton, C.S.; Miele, L. Notch Signaling in Neuroendocrine Tumors. Front. Oncol. 2016, 6, 94. [Google Scholar] [CrossRef]
- Radtke, F.; Raj, K. The role of Notch in tumorigenesis: Oncogene or tumour suppressor? Nat. Rev. Cancer 2003, 3, 756–767. [Google Scholar] [CrossRef]
- Kunnimalaiyaan, M.; Chen, H. Tumor suppressor role of Notch-1 signaling in neuroendocrine tumors. Oncologist 2007, 12, 535–542. [Google Scholar] [CrossRef]
- Kunnimalaiyaan, M.; Yan, S.; Wong, F.; Zhang, Y.W.; Chen, H. Hairy Enhancer of Split-1 (HES-1), a Notch1 effector, inhibits the growth of carcinoid tumor cells. Surgery 2005, 138, 1137–1142. [Google Scholar] [CrossRef] [PubMed]
- Kunnimalaiyaan, M.; Traeger, K.; Chen, H. Conservation of the Notch1 signaling pathway in gastrointestinal carcinoid cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 289, G636–G642. [Google Scholar] [CrossRef] [PubMed]
- Kunnimalaiyaan, M.; Vaccaro, A.M.; Ndiaye, M.A.; Chen, H. Overexpression of the NOTCH1 intracellular domain inhibits cell proliferation and alters the neuroendocrine phenotype of medullary thyroid cancer cells. J. Biol. Chem. 2006, 281, 39819–39830. [Google Scholar] [CrossRef] [PubMed]
- Nakakura, E.K.; Sriuranpong, V.R.; Kunnimalaiyaan, M.; Hsiao, E.C.; Schuebel, K.E.; Borges, M.W.; Jin, N.; Collins, B.J.; Nelkin, B.D.; Chen, H.; et al. Regulation of neuroendocrine differentiation in gastrointestinal carcinoid tumor cells by notch signaling. J. Clin. Endocrinol. Metab. 2005, 90, 4350–4356. [Google Scholar] [CrossRef]
- Krampitz, G.W.; George, B.M.; Willingham, S.B.; Volkmer, J.P.; Weiskopf, K.; Jahchan, N.; Newman, A.M.; Sahoo, D.; Zemek, A.J.; Yanovsky, R.L.; et al. Identification of tumorigenic cells and therapeutic targets in pancreatic neuroendocrine tumors. Proc. Natl. Acad. Sci. USA 2016, 113, 4464–4469. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Fernandez-Del Castillo, C.; Yilmaz, O.; Deshpande, V. Heterogeneity in signaling pathways of gastroenteropancreatic neuroendocrine tumors: A critical look at notch signaling pathway. Mod. Pathol. 2013, 26, 139–147. [Google Scholar] [CrossRef]
- Krausch, M.; Kroepil, F.; Lehwald, N.; Lachenmayer, A.; Schott, M.; Anlauf, M.; Cupisti, K.; Knoefel, W.T.; Raffel, A. Notch 1 tumor expression is lacking in highly proliferative pancreatic neuroendocrine tumors. Endocrine 2013, 44, 182–186. [Google Scholar] [CrossRef]
- Eliasz, S.; Liang, S.; Chen, Y.; De Marco, M.A.; Machek, O.; Skucha, S.; Miele, L.; Bocchetta, M. Notch-1 stimulates survival of lung adenocarcinoma cells during hypoxia by activating the IGF-1R pathway. Oncogene 2010, 29, 2488–2498. [Google Scholar] [CrossRef]
- Sriuranpong, V.; Borges, M.W.; Strock, C.L.; Nakakura, E.K.; Watkins, D.N.; Blaumueller, C.M.; Nelkin, B.D.; Ball, D.W. Notch signaling induces rapid degradation of achaete-scute homolog 1. Mol. Cell Biol. 2002, 22, 3129–3139. [Google Scholar] [CrossRef]
- Jaskula-Sztul, R.; Eide, J.; Tesfazghi, S.; Dammalapati, A.; Harrison, A.D.; Yu, X.M.; Scheinebeck, C.; Winston-McPherson, G.; Kupcho, K.R.; Robers, M.B.; et al. Tumor-suppressor role of Notch3 in medullary thyroid carcinoma revealed by genetic and pharmacological induction. Mol. Cancer Ther. 2015, 14, 499–512. [Google Scholar] [CrossRef][Green Version]
- Somnay, Y.R.; Yu, X.M.; Lloyd, R.V.; Leverson, G.; Aburjania, Z.; Jang, S.; Jaskula-Sztul, R.; Chen, H. Notch3 expression correlates with thyroid cancer differentiation, induces apoptosis, and predicts disease prognosis. Cancer 2017, 123, 769–782. [Google Scholar] [CrossRef] [PubMed]
- Lou, I.; Odorico, S.; Yu, X.M.; Harrison, A.; Jaskula-Sztul, R.; Chen, H. Notch3 as a novel therapeutic target in metastatic medullary thyroid cancer. Surgery 2018, 163, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Jin, W.Y.; Fan, Z.W.; Han, R.C. Analysis of the expression of the Notch3 receptor protein in adult lung cancer. Oncol. Lett. 2013, 5, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Udaka, N.; Yazawa, T.; Okudela, K.; Hayashi, H.; Sudo, T.; Guillemot, F.; Kageyama, R.; Kitamura, H. Basic helix-loop-helix transcription factors regulate the neuroendocrine differentiation of fetal mouse pulmonary epithelium. Development 2000, 127, 3913–3921. [Google Scholar] [PubMed]
- Marcucci, F.; Caserta, C.A.; Romeo, E.; Rumio, C. Antibody-Drug Conjugates (ADC) Against Cancer Stem-Like Cells (CSC)-Is There Still Room for Optimism? Front. Oncol. 2019, 9, 167. [Google Scholar] [CrossRef]
- Lashari, B.H.; Vallatharasu, Y.; Kolandra, L.; Hamid, M.; Uprety, D. Rovalpituzumab Tesirine: A Novel DLL3-Targeting Antibody-Drug Conjugate. Drugs R D 2018, 18, 255–258. [Google Scholar] [CrossRef]
- Saunders, L.R.; Bankovich, A.J.; Anderson, W.C.; Aujay, M.A.; Bheddah, S.; Black, K.; Desai, R.; Escarpe, P.A.; Hampl, J.; Laysang, A.; et al. A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Sci. Transl. Med. 2015, 7, 302ra136. [Google Scholar] [CrossRef]
- Kuhnert, F.; Kirshner, J.R.; Thurston, G. Dll4-Notch signaling as a therapeutic target in tumor angiogenesis. Vasc. Cell 2011, 3, 20. [Google Scholar] [CrossRef]
- Driessens, G.; Beck, B.; Caauwe, A.; Simons, B.D.; Blanpain, C. Defining the mode of tumour growth by clonal analysis. Nature 2012, 488, 527–530. [Google Scholar] [CrossRef]
- Schepers, A.G.; Snippert, H.J.; Stange, D.E.; van den Born, M.; van Es, J.H.; van de Wetering, M.; Clevers, H. Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science 2012, 337, 730–735. [Google Scholar] [CrossRef]
- Kozar, S.; Morrissey, E.; Nicholson, A.M.; van der Heijden, M.; Zecchini, H.I.; Kemp, R.; Tavare, S.; Vermeulen, L.; Winton, D.J. Continuous clonal labeling reveals small numbers of functional stem cells in intestinal crypts and adenomas. Cell Stem Cell 2013, 13, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Zomer, A.; Ellenbroek, S.I.; Ritsma, L.; Beerling, E.; Vrisekoop, N.; Van Rheenen, J. Intravital imaging of cancer stem cell plasticity in mammary tumors. Stem Cells 2013, 31, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, Y.; Yu, T.S.; McKay, R.M.; Burns, D.K.; Kernie, S.G.; Parada, L.F. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature 2012, 488, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Oshimori, N.; Oristian, D.; Fuchs, E. TGF-beta promotes heterogeneity and drug resistance in squamous cell carcinoma. Cell 2015, 160, 963–976. [Google Scholar] [CrossRef] [PubMed]
- Batlle, E.; Clevers, H. Cancer stem cells revisited. Nat. Med. 2017, 23, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Mathis, R.A.; Sokol, E.S.; Gupta, P.B. Cancer cells exhibit clonal diversity in phenotypic plasticity. Open Biol. 2017, 7. [Google Scholar] [CrossRef]
- Gupta, P.B.; Fillmore, C.M.; Jiang, G.; Shapira, S.D.; Tao, K.; Kuperwasser, C.; Lander, E.S. Stochastic state transitions give rise to phenotypic equilibrium in populations of cancer cells. Cell 2011, 146, 633–644. [Google Scholar] [CrossRef]
- Hoeck, J.D.; Biehs, B.; Kurtova, A.V.; Kljavin, N.M.; de Sousa, E.M.F.; Alicke, B.; Koeppen, H.; Modrusan, Z.; Piskol, R.; de Sauvage, F.J. Stem cell plasticity enables hair regeneration following Lgr5(+) cell loss. Nat. Cell Biol. 2017, 19, 666–676. [Google Scholar] [CrossRef]
- Lenos, K.J.; Miedema, D.M.; Lodestijn, S.C.; Nijman, L.E.; van den Bosch, T.; Romero Ros, X.; Lourenco, F.C.; Lecca, M.C.; van der Heijden, M.; van Neerven, S.M.; et al. Stem cell functionality is microenvironmentally defined during tumour expansion and therapy response in colon cancer. Nat. Cell Biol. 2018, 20, 1193–1202. [Google Scholar] [CrossRef]
- de Sousa e Melo, F.; Kurtova, A.V.; Harnoss, J.M.; Kljavin, N.; Hoeck, J.D.; Hung, J.; Anderson, J.E.; Storm, E.E.; Modrusan, Z.; Koeppen, H.; et al. A distinct role for Lgr5(+) stem cells in primary and metastatic colon cancer. Nature 2017, 543, 676–680. [Google Scholar] [CrossRef]
- Drost, J.; van Boxtel, R.; Blokzijl, F.; Mizutani, T.; Sasaki, N.; Sasselli, V.; de Ligt, J.; Behjati, S.; Grolleman, J.E.; van Wezel, T.; et al. Use of CRISPR-modified human stem cell organoids to study the origin of mutational signatures in cancer. Science 2017, 358, 234–238. [Google Scholar] [CrossRef]
- Clevers, H. STEM CELLS. What is an adult stem cell? Science 2015, 350, 1319–1320. [Google Scholar] [CrossRef]
- Ball, C.R.; Oppel, F.; Ehrenberg, K.R.; Dubash, T.D.; Dieter, S.M.; Hoffmann, C.M.; Abel, U.; Herbst, F.; Koch, M.; Werner, J.; et al. Succession of transiently active tumor-initiating cell clones in human pancreatic cancer xenografts. EMBO Mol. Med. 2017, 9, 918–932. [Google Scholar] [CrossRef]
- McGranahan, N.; Swanton, C. Clonal Heterogeneity and Tumor Evolution: Past, Present, and the Future. Cell 2017, 168, 613–628. [Google Scholar] [CrossRef]
- Jagust, P.; de Luxan-Delgado, B.; Parejo-Alonso, B.; Sancho, P. Metabolism-Based Therapeutic Strategies Targeting Cancer Stem Cells. Front. Pharmacol. 2019, 10, 203. [Google Scholar] [CrossRef]
- Ryall, J.G.; Dell’Orso, S.; Derfoul, A.; Juan, A.; Zare, H.; Feng, X.; Clermont, D.; Koulnis, M.; Gutierrez-Cruz, G.; Fulco, M.; et al. The NAD(+)-dependent SIRT1 deacetylase translates a metabolic switch into regulatory epigenetics in skeletal muscle stem cells. Cell Stem Cell 2015, 16, 171–183. [Google Scholar] [CrossRef]
- Buffie, C.G.; Bucci, V.; Stein, R.R.; McKenney, P.T.; Ling, L.; Gobourne, A.; No, D.; Liu, H.; Kinnebrew, M.; Viale, A.; et al. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature 2015, 517, 205–208. [Google Scholar] [CrossRef]
- Viale, A.; Pettazzoni, P.; Lyssiotis, C.A.; Ying, H.; Sanchez, N.; Marchesini, M.; Carugo, A.; Green, T.; Seth, S.; Giuliani, V.; et al. Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature 2014, 514, 628–632. [Google Scholar] [CrossRef]
- Sancho, P.; Burgos-Ramos, E.; Tavera, A.; Bou Kheir, T.; Jagust, P.; Schoenhals, M.; Barneda, D.; Sellers, K.; Campos-Olivas, R.; Grana, O.; et al. MYC/PGC-1alpha Balance Determines the Metabolic Phenotype and Plasticity of Pancreatic Cancer Stem Cells. Cell Metab. 2015, 22, 590–605. [Google Scholar] [CrossRef]
- Barnekow, N.; Yuan, R.; Moran, P.; Ren, B. Abstract 16740: Foxo1-Activated Cd36 Transcription Switches Arteriolar Differentiation of Endothelial Cells. Circulation 2018, 138, A16740. [Google Scholar] [CrossRef]
- Ladanyi, A.; Mukherjee, A.; Kenny, H.A.; Johnson, A.; Mitra, A.K.; Sundaresan, S.; Nieman, K.M.; Pascual, G.; Benitah, S.A.; Montag, A.; et al. Adipocyte-induced CD36 expression drives ovarian cancer progression and metastasis. Oncogene 2018, 37, 2285–2301. [Google Scholar] [CrossRef]
- Ye, H.; Adane, B.; Khan, N.; Sullivan, T.; Minhajuddin, M.; Gasparetto, M.; Stevens, B.; Pei, S.; Balys, M.; Ashton, J.M.; et al. Leukemic Stem Cells Evade Chemotherapy by Metabolic Adaptation to an Adipose Tissue Niche. Cell Stem Cell 2016, 19, 23–37. [Google Scholar] [CrossRef]
- Fumagalli, A.; Drost, J.; Suijkerbuijk, S.J.; van Boxtel, R.; de Ligt, J.; Offerhaus, G.J.; Begthel, H.; Beerling, E.; Tan, E.H.; Sansom, O.J.; et al. Genetic dissection of colorectal cancer progression by orthotopic transplantation of engineered cancer organoids. Proc. Natl. Acad. Sci. USA 2017, 114, E2357–E2364. [Google Scholar] [CrossRef]
- Fujii, M.; Shimokawa, M.; Date, S.; Takano, A.; Matano, M.; Nanki, K.; Ohta, Y.; Toshimitsu, K.; Nakazato, Y.; Kawasaki, K.; et al. A Colorectal Tumor Organoid Library Demonstrates Progressive Loss of Niche Factor Requirements during Tumorigenesis. Cell Stem Cell 2016, 18, 827–838. [Google Scholar] [CrossRef]
- Zhang, X.; Yalcin, S.; Lee, D.F.; Yeh, T.Y.; Lee, S.M.; Su, J.; Mungamuri, S.K.; Rimmele, P.; Kennedy, M.; Sellers, R.; et al. FOXO1 is an essential regulator of pluripotency in human embryonic stem cells. Nat. Cell Biol. 2011, 13, 1092–1099. [Google Scholar] [CrossRef]
- Tothova, Z.; Gilliland, D.G. FoxO transcription factors and stem cell homeostasis: Insights from the hematopoietic system. Cell Stem Cell 2007, 1, 140–152. [Google Scholar] [CrossRef]
- Sykes, S.M.; Lane, S.W.; Bullinger, L.; Kalaitzidis, D.; Yusuf, R.; Saez, B.; Ferraro, F.; Mercier, F.; Singh, H.; Brumme, K.M.; et al. AKT/FOXO signaling enforces reversible differentiation blockade in myeloid leukemias. Cell 2011, 146, 697–708. [Google Scholar] [CrossRef]
- Paik, J.H.; Kollipara, R.; Chu, G.; Ji, H.; Xiao, Y.; Ding, Z.; Miao, L.; Tothova, Z.; Horner, J.W.; Carrasco, D.R.; et al. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell 2007, 128, 309–323. [Google Scholar] [CrossRef]
- Wilhelm, K.; Happel, K.; Eelen, G.; Schoors, S.; Oellerich, M.F.; Lim, R.; Zimmermann, B.; Aspalter, I.M.; Franco, C.A.; Boettger, T.; et al. FOXO1 couples metabolic activity and growth state in the vascular endothelium. Nature 2016, 529, 216–220. [Google Scholar] [CrossRef]
- Carrasco, P.; Zuazo-Gaztelu, I.; Casanovas, O. Sprouting strategies and dead ends in anti-angiogenic targeting of NETs. J. Mol. Endocrinol. 2017, 59, R77–R91. [Google Scholar] [CrossRef]
- Chu, X.; Gao, X.; Jansson, L.; Quach, M.; Skogseid, B.; Barbu, A. Multiple microvascular alterations in pancreatic islets and neuroendocrine tumors of a Men1 mouse model. Am. J. Pathol. 2013, 182, 2355–2367. [Google Scholar] [CrossRef]
- Wagenblast, E.; Soto, M.; Gutierrez-Angel, S.; Hartl, C.A.; Gable, A.L.; Maceli, A.R.; Erard, N.; Williams, A.M.; Kim, S.Y.; Dickopf, S.; et al. A model of breast cancer heterogeneity reveals vascular mimicry as a driver of metastasis. Nature 2015, 520, 358–362. [Google Scholar] [CrossRef]
- Hendrix, M.J.; Seftor, E.A.; Seftor, R.E.; Chao, J.T.; Chien, D.S.; Chu, Y.W. Tumor cell vascular mimicry: Novel targeting opportunity in melanoma. Pharmacol. Ther. 2016, 159, 83–92. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, X.D.; Sun, M.; Zhang, X.; German, P.; Bai, S.; Ding, Z.; Tannir, N.; Wood, C.G.; Matin, S.F.; et al. Targeting MET and AXL overcomes resistance to sunitinib therapy in renal cell carcinoma. Oncogene 2016, 35, 2687–2697. [Google Scholar] [CrossRef]
- Capdevila, J.; Fazio, N.; López-López, C.; Teule, A.; Valle, J.W.; Tafuto, S.; Custodio, A.B.; Reed, N.; Raderer, M.; Grande, E.; et al. Progression-free survival (PFS) and subgroups analyses of lenvatinib in patients (pts) with G1/G2 advanced pancreatic (panNETs) and gastrointestinal (giNETs) neuroendocrine tumors (NETs): Updated results from the phase II TALENT trial (GETNE 1509). J. Clin. Oncol. 2019, 37, 332. [Google Scholar] [CrossRef]
- Capozzi, M.; De Divitiis, C.; Ottaiano, A.; von Arx, C.; Scala, S.; Tatangelo, F.; Delrio, P.; Tafuto, S. Lenvatinib, a molecule with versatile application: From preclinical evidence to future development in anti-cancer treatment. Cancer Manag. Res. 2019, 11, 3847–3860. [Google Scholar] [CrossRef]
- Grande, E.; Lopez, C.; Alonso-Gordoa, T.; Benavent, M.; Capdevila, J.; Teule, A.; Custodio, A.; Sevilla, I.; Gajate, P.; Molina-Cerrillo, J.; et al. The SUNEVO (GETNE-1408) trial to evaluate the activity and safety of thecombination of sunitinib with evofosfamide (TH-302) in patients with G1/G2 metastatic pancreatic neuroendocrine tumours (pNETs) naïve forsystemic treatment: A phase II study of the Spanish Task Force Group for Neuroendocrine and Endocrine Tumors (GETNE). J. Clin. Oncol. 2019, 37, 4105. [Google Scholar] [CrossRef]
- Ikezono, Y.; Koga, H.; Akiba, J.; Abe, M.; Yoshida, T.; Wada, F.; Nakamura, T.; Iwamoto, H.; Masuda, A.; Sakaue, T.; et al. Pancreatic Neuroendocrine Tumors and EMT Behavior Are Driven by the CSC Marker DCLK1. Mol. Cancer Res. 2017, 15, 744–752. [Google Scholar] [CrossRef]
- Rigamonti, N.; Kadioglu, E.; Keklikoglou, I.; Wyser Rmili, C.; Leow, C.C.; De Palma, M. Role of angiopoietin-2 in adaptive tumor resistance to VEGF signaling blockade. Cell Rep. 2014, 8, 696–706. [Google Scholar] [CrossRef]
- Biel, N.M.; Siemann, D.W. Targeting the Angiopoietin-2/Tie-2 axis in conjunction with VEGF signal interference. Cancer Lett. 2016, 380, 525–533. [Google Scholar] [CrossRef]
- Maione, F.; Capano, S.; Regano, D.; Zentilin, L.; Giacca, M.; Casanovas, O.; Bussolino, F.; Serini, G.; Giraudo, E. Semaphorin 3A overcomes cancer hypoxia and metastatic dissemination induced by antiangiogenic treatment in mice. J. Clin. Invest. 2012, 122, 1832–1848. [Google Scholar] [CrossRef]
- Allen, E.; Walters, I.B.; Hanahan, D. Brivanib, a dual FGF/VEGF inhibitor, is active both first and second line against mouse pancreatic neuroendocrine tumors developing adaptive/evasive resistance to VEGF inhibition. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011, 17, 5299–5310. [Google Scholar] [CrossRef]
- Hilfenhaus, G.; Gohrig, A.; Pape, U.F.; Neumann, T.; Jann, H.; Zdunek, D.; Hess, G.; Stassen, J.M.; Wiedenmann, B.; Detjen, K.; et al. Placental growth factor supports neuroendocrine tumor growth and predicts disease prognosis in patients. Endocr. Relat. Cancer 2013, 20, 305–319. [Google Scholar] [CrossRef]
- Fischer, C.; Jonckx, B.; Mazzone, M.; Zacchigna, S.; Loges, S.; Pattarini, L.; Chorianopoulos, E.; Liesenborghs, L.; Koch, M.; De Mol, M.; et al. Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell 2007, 131, 463–475. [Google Scholar] [CrossRef]
- Marconcini, R.; Faviana, P.; Campani, D.; Galli, L.; Antonuzzo, A.; Orlandini, C.; Falcone, A.; Ricci, S. Enhancer of zest homolog 2 (EZH2) expression in well and moderately differentiated pancreatic neuroendocrine tumor (pNET). Ann. Oncol. 2016, 27. [Google Scholar] [CrossRef]
- Yang, L.; Yu, X.; Yang, Y. Autotaxin upregulated by STAT3 activation contributes to invasion in pancreatic neuroendocrine neoplasms. Endocr. Connect. 2018, 7, 1299–1307. [Google Scholar] [CrossRef]
- Chen, Y.; Ramakrishnan, D.P.; Ren, B. Regulation of angiogenesis by phospholipid lysophosphatidic acid. Front. Biosci. (Landmark Ed) 2013, 18, 852–861. [Google Scholar]
- Liu, S.; Umezu-Goto, M.; Murph, M.; Lu, Y.; Liu, W.; Zhang, F.; Yu, S.; Stephens, L.C.; Cui, X.; Murrow, G.; et al. Expression of autotaxin and lysophosphatidic acid receptors increases mammary tumorigenesis, invasion, and metastases. Cancer Cell 2009, 15, 539–550. [Google Scholar] [CrossRef]
- Liu, Y.; An, S.; Ward, R.; Yang, Y.; Guo, X.X.; Li, W.; Xu, T.R. G protein-coupled receptors as promising cancer targets. Cancer Lett. 2016, 376, 226–239. [Google Scholar] [CrossRef]
- Zecchin, A.; Kalucka, J.; Dubois, C.; Carmeliet, P. How Endothelial Cells Adapt Their Metabolism to Form Vessels in Tumors. Front. Immunol. 2017, 8, 1750. [Google Scholar] [CrossRef]

| Name | Mechanisms & Functions | Reference(s) |
|---|---|---|
| CVM-1118 | Phase II clinical trial for patients with advanced NETs, including pNETs; de novo development of vascular networks via vascular mimicry, which is associated with a malignant phenotype and a poor clinical outcome. Vascular mimicry was presented in pNET animal models. | https://clinicaltrials.gov/ct2/show/NCT03600233; Chu et al., Am J Pathol 2013; Wagenblast, et al., Nature 2015; Hendrix, et al., Pharmacol Ther 2016 [211,212,213] |
| Cabozantinib | Phase III clinical trial of in patients with advanced neuroendocrine tumors after progression on everolimus (CABINET) A VEGFR, c-MET and AXL inhibitor; attenuate sunitinib therapy-mediated pro-metastasis in xenograft mouse tumor models of renal cell carcinoma. | https://clinicaltrials.gov/ct2/show/NCT03375320; Zhou et al., Oncogene, 2015 [214] |
| Lenvatinib | A multi-kinase inhibitor with a preferential antiangiogenic activity. | Capdevila, et al. J Clin Oncol, 2019; Capozzi, et al., Cancer Manag Res 2019 [215,216] |
| TH-302 (evofosfamide) | A hypoxia-activated prodrug, which is metabolized to its active form, bromo-isophosphoramide mustard (Br-IPM), under hypoxic conditions. Used in combination with sunitinib. | Grande, et al., abstract. J Clin Oncol, 2019 [217] |
| Doublecortin-like kinase 1 (DCLK1) | A potential marker for pNET CSCs; induce epithelial-mesenchymal transition (EMT). | Ikezono, et al., Mol Cancer Res, 2017 [218] |
| MEDI3617 | A monoclonal antibody targeting angiopoietin-2; in combination with VEGF-targeted therapies. | Rigamonti, et al., Cell Rep 2014; Biel, et al., Cancer Letters, 2016 [219,220] |
| Sema3A (Semaphorin 3A) | Overcome cancer hypoxia and metastatic dissemination induced by sunitinib treatment in mice. In combination with sunitinib, Sema3A synergistically enhanced RIP-Tag2 mouse survival. | Maione, F, et al., J Clin Invest, 2012 [221] |
| Brivanib | A dual FGF/VEGF inhibitor; vascular inhibition and tumor stability. | Allen, et al., Clin Cancer Res, 2011 [222] |
| PlGF signaling inhibitor? | High PIGF expression in pNET patients with poor outcome; overcome resistance to antiangiogenic factors. | Fischer et al., Cell, 2007; Hilfenhaus, et al., Endocr Relat Cancer, 2013 [223,224] |
| EZH2 inhibitor? | Sensitize tumor to sunitinib in cell lines and PDX models. | Marconcini, et al., 2016 [225] |
| Autotaxin (ATX) inhibitors? LPA receptor-specific antagonists? | High ATX expression in pNET tissues, which was associated with higher tumor grade, TNM staging and lymph node metastasis; ATX drives LPA expression, which is also linked to tumor angiogenesis and arteriolar differentiation and malignancy of tumor cells. | Liu et al., Cancer Cell, 2009; Chen, et al., Front Biosci (Landmark Ed) 2013; Ren, et al., Arterioscler Thromb Vasc Biol, 2016; Liu et al., Cancer Lett 2016; Dong et al., Oncotarget, 2017 [20]; Yang et al., Endocr. Connect, 2018 [21,226,227,228,229] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, B.; Rose, J.B.; Liu, Y.; Jaskular-Sztul, R.; Contreras, C.; Beck, A.; Chen, H. Heterogeneity of Vascular Endothelial Cells, De Novo Arteriogenesis and Therapeutic Implications in Pancreatic Neuroendocrine Tumors. J. Clin. Med. 2019, 8, 1980. https://doi.org/10.3390/jcm8111980
Ren B, Rose JB, Liu Y, Jaskular-Sztul R, Contreras C, Beck A, Chen H. Heterogeneity of Vascular Endothelial Cells, De Novo Arteriogenesis and Therapeutic Implications in Pancreatic Neuroendocrine Tumors. Journal of Clinical Medicine. 2019; 8(11):1980. https://doi.org/10.3390/jcm8111980
Chicago/Turabian StyleRen, Bin, J. Bart Rose, Yehe Liu, Renata Jaskular-Sztul, Carlo Contreras, Adam Beck, and Herbert Chen. 2019. "Heterogeneity of Vascular Endothelial Cells, De Novo Arteriogenesis and Therapeutic Implications in Pancreatic Neuroendocrine Tumors" Journal of Clinical Medicine 8, no. 11: 1980. https://doi.org/10.3390/jcm8111980
APA StyleRen, B., Rose, J. B., Liu, Y., Jaskular-Sztul, R., Contreras, C., Beck, A., & Chen, H. (2019). Heterogeneity of Vascular Endothelial Cells, De Novo Arteriogenesis and Therapeutic Implications in Pancreatic Neuroendocrine Tumors. Journal of Clinical Medicine, 8(11), 1980. https://doi.org/10.3390/jcm8111980





