Volume-Controlled Versus Dual-Controlled Ventilation during Robot-Assisted Laparoscopic Prostatectomy with Steep Trendelenburg Position: A Randomized-Controlled Trial
Abstract
:1. Introduction
2. Experimental Section
2.1. Patients
2.2. Anesthetic Management
2.3. Ventilation Management
2.4. Data Collection
2.5. Statistical Analysis
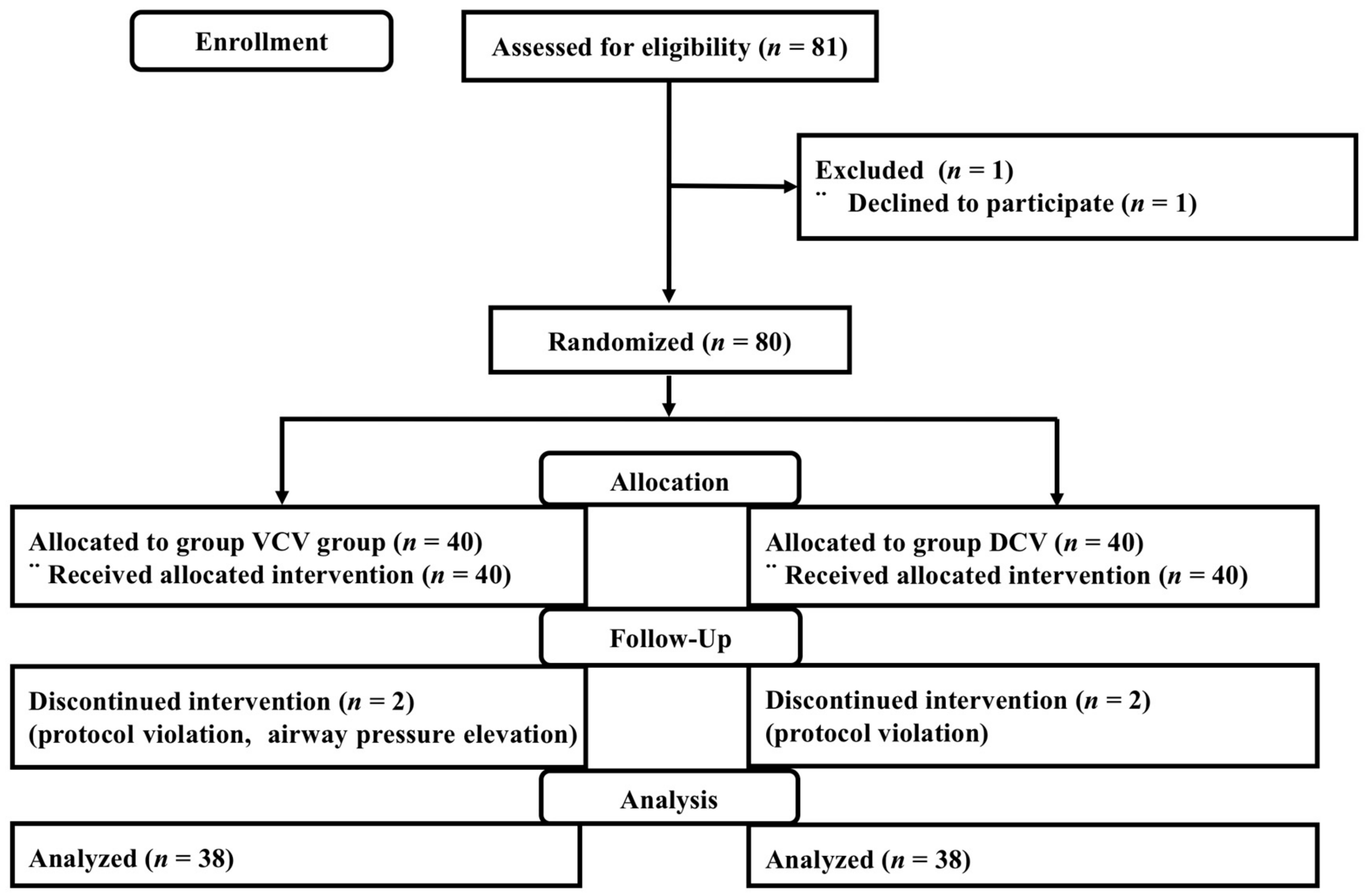
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ball, L.; Dameri, M.; Pelosi, P. Modes of mechanical ventilation for the operating room. Best Pract. Res. Clin. Anaesthesiol. 2015, 29, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Kim, H. Protective strategies for one-lung ventilation. Korean J. Anesthesiol. 2014, 67, 233–234. [Google Scholar] [CrossRef] [PubMed]
- Cadi, P.; Guenoun, T.; Journois, D.; Chevallier, J.M.; Diehl, J.L.; Safran, D. Pressure-controlled ventilation improves oxygenation during laparoscopic obesity surgery compared with volume-controlled ventilation. Br. J. Anaesth. 2008, 100, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Kalmar, A.F.; Foubert, L.; Hendrickx, J.F.; Mottrie, A.; Absalom, A.; Mortier, E.P.; Struys, M.M. Influence of steep trendelenburg position and CO2 pneumoperitoneum on cardiovascular, cerebrovascular, and respiratory homeostasis during robotic prostatectomy. Br. J. Anaesth. 2010, 104, 433–439. [Google Scholar] [CrossRef]
- Gainsburg, D.M. Anesthetic concerns for robotic-assisted laparoscopic radical prostatectomy. Minerva Anestesiol. 2012, 78, 596–604. [Google Scholar]
- Andersson, L.E.; Baath, M.; Thorne, A.; Aspelin, P.; Odeberg-Wernerman, S. Effect of carbon dioxide pneumoperitoneum on development of atelectasis during anesthesia, examined by spiral computed tomography. Anesthesiology 2005, 102, 293–299. [Google Scholar] [CrossRef]
- Sharma, K.C.; Brandstetter, R.D.; Brensilver, J.M.; Jung, L.D. Cardiopulmonary physiology and pathophysiology as a consequence of laparoscopic surgery. Chest 1996, 110, 810–815. [Google Scholar] [CrossRef]
- Oikkonen, M.; Tallgren, M. Changes in respiratory compliance at laparoscopy: Measurements using side stream spirometry. Can. J. Anaesth. 1995, 42, 495–497. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, N.Y.; Lee, K.Y.; Choi, Y.D.; Hong, J.H.; Bai, S.J. The impact of two different inspiratory to expiratory ratios (1:1 and 1:2) on respiratory mechanics and oxygenation during volume-controlled ventilation in robot-assisted laparoscopic radical prostatectomy: A randomized controlled trial. Can. J. Anaesth. 2015, 62, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, J.S.; Lee, J.H.; Shin, S.; Min, N.H.; Kim, M.S. Effect of the prolonged inspiratory to expiratory ratio on oxygenation and respiratory mechanics during surgical procedures. Medicine (Baltimore) 2016, 95, e3269. [Google Scholar] [CrossRef]
- Assad, O.M.; El Sayed, A.A.; Khalil, M.A. Comparison of volume-controlled ventilation and pressure-controlled ventilation volume guaranteed during laparoscopic surgery in trendelenburg position. J. Clin. Anesth. 2016, 34, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Merchant, R.; Chartrand, D.; Dain, S.; Dobson, G.; Kurrek, M.M.; Lagace, A.; Stacey, S.; Thiessen, B. Guidelines to the practice of anesthesia–revised edition 2015. Can. J. Anaesth. 2015, 62, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Martini, C.H.; Boon, M.; Bevers, R.F.; Aarts, L.P.; Dahan, A. Evaluation of surgical conditions during laparoscopic surgery in patients with moderate vs deep neuromuscular block. Br. J. Anaesth. 2014, 112, 498–505. [Google Scholar] [CrossRef]
- Brower, R.G.; Matthay, M.A.; Morris, A.; Schoenfeld, D.; Thompson, B.T.; Wheeler, A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1301–1308. [Google Scholar] [PubMed]
- Saravanan, S.; Kocarev, M.; Wilson, R.C.; Watkins, E.; Columb, M.O.; Lyons, G. Equivalent dose of ephedrine and phenylephrine in the prevention of post-spinal hypotension in caesarean section. Br. J. Anaesth. 2006, 96, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Cinnella, G.; Grasso, S.; Spadaro, S.; Rauseo, M.; Mirabella, L.; Salatto, P.; De Capraris, A.; Nappi, L.; Greco, P.; Dambrosio, M. Effects of recruitment maneuver and positive end-expiratory pressure on respiratory mechanics and transpulmonary pressure during laparoscopic surgery. Anesthesiology 2013, 118, 114–122. [Google Scholar] [CrossRef]
- Meininger, D.; Byhahn, C.; Mierdl, S.; Westphal, K.; Zwissler, B. Positive end-expiratory pressure improves arterial oxygenation during prolonged pneumoperitoneum. Acta Anaesthesiol. Scand. 2005, 49, 778–783. [Google Scholar] [CrossRef]
- Ahn, S.; Byun, S.H.; Chang, H.; Koo, Y.B.; Kim, J.C. Effect of recruitment maneuver on arterial oxygenation in patients undergoing robot-assisted laparoscopic prostatectomy with intraoperative 15 cmH2O positive end expiratory pressure. Korean J. Anesthesiol. 2016, 69, 592–598. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, K.S.; Jeong, J.S.; Shim, J.C.; Cho, E.S. Optimal positive end-expiratory pressure during robot-assisted laparoscopic radical prostatectomy. Korean J. Anesthesiol. 2013, 65, 244–250. [Google Scholar] [CrossRef]
- Choi, E.M.; Na, S.; Choi, S.H.; An, J.; Rha, K.H.; Oh, Y.J. Comparison of volume-controlled and pressure-controlled ventilation in steep trendelenburg position for robot-assisted laparoscopic radical prostatectomy. J. Clin. Anesth. 2011, 23, 183–188. [Google Scholar] [CrossRef]
- Tugrul, M.; Camci, E.; Karadeniz, H.; Senturk, M.; Pembeci, K.; Akpir, K. Comparison of volume controlled with pressure controlled ventilation during one-lung anaesthesia. Br. J. Anaesth. 1997, 79, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Marcy, T.W.; Marini, J.J. Inverse ratio ventilation in ards. Rationale and implementation. Chest 1991, 100, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Marini, J.J.; Ravenscraft, S.A. Mean airway pressure: Physiologic determinants and clinical importance–Part 2: Clinical implications. Crit. Care Med. 1992, 20, 1604–1616. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Kim, W.H.; Ahn, H.J.; Kim, J.A.; Yang, M.K.; Lee, C.H.; Lee, J.H.; Kim, Y.R.; Choi, J.W. The effects of prolonged inspiratory time during one-lung ventilation: A randomised controlled trial. Anaesthesia 2013, 68, 908–916. [Google Scholar] [CrossRef]
- Deiner, S.; Silverstein, J.H. Anesthesia for geriatric patients. Minerva Anestesiol. 2011, 77, 180–189. [Google Scholar]
- Diaz-Fuentes, G.; Hashmi, H.R.; Venkatram, S. Perioperative evaluation of patients with pulmonary conditions undergoing non-cardiothoracic surgery. Health Serv. Insights 2016, 9, 9–23. [Google Scholar] [CrossRef]
- Bardoczky, G.I.; d’Hollander, A.A.; Cappello, M.; Yernault, J.C. Interrupted expiratory flow on automatically constructed flow-volume curves may determine the presence of intrinsic positive end-expiratory pressure during one-lung ventilation. Anesth. Analg. 1998, 86, 880–884. [Google Scholar] [CrossRef]
- Mavros, M.N.; Velmahos, G.C.; Falagas, M.E. Atelectasis as a cause of postoperative fever: Where is the clinical evidence? Chest 2011, 140, 418–424. [Google Scholar] [CrossRef]
- Pile, J.C. Evaluating postoperative fever: A focused approach. Clevel. Clin. J. Med. 2006, 73 (Suppl. 1), S62–S66. [Google Scholar] [CrossRef]
- Bartlett, R.H.; Brennan, M.L.; Gazzaniga, A.B.; Hanson, E.L. Studies on the pathogenesis and prevention of postoperative pulmonary complications. Surg. Gynecol. Obstet. 1973, 137, 925–933. [Google Scholar] [CrossRef]
- Kokulu, S.; Günay, E.; Baki, E.D.; Ulasli, S.S.; Yilmazer, M.; Koca, B.; Arıöz, D.T.; Ela, Y.; Sivaci, R.G. Impact of a lung-protective ventilatory strategy on systemic and pulmonary inflammatory responses during laparoscopic surgery: Is it really helpful? Inflammation 2015, 38, 361–367. [Google Scholar] [CrossRef] [PubMed]

| Variables | VCV (n = 38) | DCV (n = 38) |
|---|---|---|
| Age (year) | 65.3 (6.2) | 64.5 (7.2) |
| Height (cm) | 168.8 (5.0) | 166.8 (5.2) |
| Weight (kg) | 68.9 (7.3) | 65.8 (7.3) |
| Body mass index (kg m−2) | 24.2 (2.8) | 23.7 (2.5) |
| Hypertension (n (%)) | 16 (42.1) | 15 (39.5) |
| Diabetes mellitus (n (%)) | 7 (18.4) | 3 (7.9) |
| Coronary artery disease (n (%)) | 4 (10.5) | 2 (5.3) |
| Duration of anesthesia (min) | 157.9 (27.9) | 153.4 (22.3) |
| Duration of surgery (min) | 117.7 (24.9) | 111.6 (20.9) |
| Duration of Trendelenburg position (min) | 62.4 (18.1) | 61.1 (16.3) |
| Total fluid amounts (mL) | 1505.8 (363.6) | 1413.7 (321.8) |
| Colloid amounts (mL) | 165.8 (251.8) | 155.3 (227.4) |
| Total urine output (mL) | 190.0 (155.0) | 167.9 (136.4) |
| Total blood loss (mL) | 407.9 (280.3) | 402.6 (245.8) |
| Total ephedrine amounts (mg) | 9.4 (8.7) | 7.4 (7.4) |
| Post anesthesia care unit duration (min) | 46.9 (22.5) | 45.8 (18.9) |
| Postoperative hospital stay (day) | 2.6 (0.8) | 2.5 (0.7) |
| Variables | VCV (n = 38) | DCV (n = 38) | Estimate (95% CI) | p-Value |
|---|---|---|---|---|
| pH | 0.803 * | |||
| T1 | 7.43 (0.03) | 7.43 (0.03) | −0.005 (−0.019 to 0.009) | >0.999 |
| T2 | 7.33 (0.04) | 7.34 (0.04) | −0.003 (−0.022 to 0.159) | >0.999 |
| T3 † | 7.33 (0.04) | 7.34 (0.04) | −0.012 (−0.030 to 0.005) | 0.509 |
| T4 | 7.33 (0.04) | 7.34 (0.03) | −0.006 (−0.022 to 0.010) | >0.999 |
| PaO2 (mmHg) | 0.149 * | |||
| T1 | 205.2 (38.2) | 198.1 (43.5) | 7.076 (−11.644 to 25.797) | >0.999 |
| T2 | 169.2 (34.8) | 181.8 (31.6) | −12.687 (−27.874 to 2.500) | 0.400 |
| T3 † | 168.6 (42.4) | 170.9 (36.4) | −2.268 (−20.470 to 15.934) | >0.999 |
| T4 | 177.8 (27.6) | 175.0 (38.3) | 2.768 (−12.514 to 18.051) | >0.999 |
| PaCO2 (mmHg) | 0.830 * | |||
| T1 | 32.7 (3.2) | 32.4 (3.1) | 0.305 (−1.119 to 1.730) | >0.999 |
| T2 | 44.2 (5.6) | 43.8 (6.2) | 0.437 (−2.262 to 3.136) | >0.999 |
| T3† | 44.9 (5.3) | 43.5 (6.3) | 1.403 (−1.285 to 4.092) | 0.905 |
| T4 | 44.9 (4.6) | 43.9 (4.9) | 1.039 (−1.118 to 3.197) | >0.999 |
| EtCO2 (mmHg) | 0.716 * | |||
| T1 | 35.0 (2.6) | 35.3 (2.2) | −0.368 (−1.459 to 0.723) | >0.999 |
| T2 | 43.3 (3.7) | 42.7 (4.1) | 0.658 (−1.126 to 2.442) | >0.999 |
| T3† | 42.6 (4.1) | 42.8 (3.9) | −0.125 (−1.969 to 1.719) | >0.999 |
| T4 | 43.7 (4.5) | 43.5 (3.3) | 0.211 (−1.580 to 2.001) | >0.999 |
| Variables | VCV (n = 38) | DCV (n = 38) | Estimate (95% CI) | p-Value |
|---|---|---|---|---|
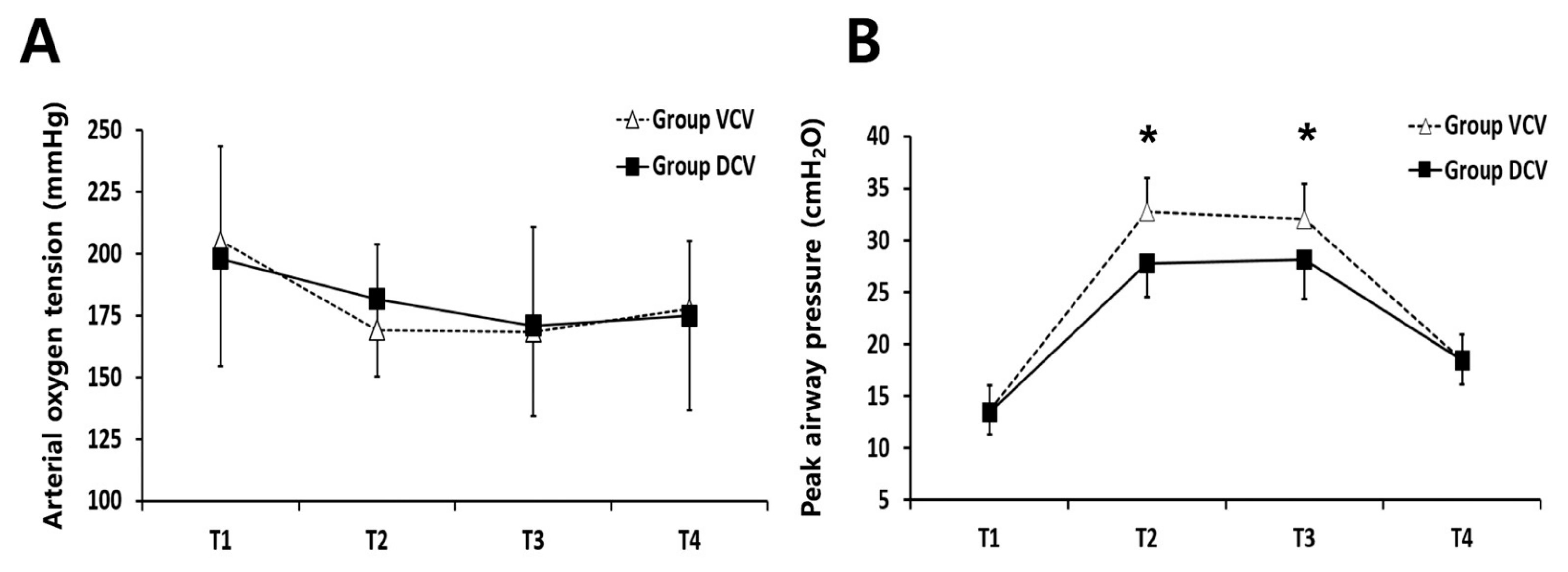
| Ppeak (cmH2O) | <0.001 * | |||
| T1 | 13.6 (2.4) | 13.4 (2.1) | 0.263 (−0.758 to 1.285) | >0.999 |
| T2 | 32.8 (3.2) | 27.8 (3.3)* | 5.026 (3.520 to 6.533) | <0.001 |
| T3 † | 32.0 (3.4) | 28.1 (3.8)* | 3.865 (2.209 to 5.520) | <0.001 |
| T4 | 18.4 (2.5) | 18.3 (2.3) | 0.131 (−0.967 to 1.230) | >0.999 |
| Pplat (cmH2O) | <0.001 * | |||
| T1 | 12.4 (2.3) | 12.4 (2.0) | 0.053 (−0.948 to 1.053) | >0.999 |
| T2 | 30.2 (4.2) | 27.5 (3.4)* | 2.711 (0.981 to 4.440) | 0.012 |
| T3 † | 29.6 (4.0) | 27.9 (4.0) | 1.713 (−0.119 to 3.546) | 0.264 |
| T4 | 15.8 (3.3) | 16.2 (2.5) | −0.368 (−1.695 to 0.958) | >0.999 |
| Pmean (cmH2O) | 0.001 * | |||
| T1 | 4.1 (0.8) | 3.8 (0.8) | 0.211 (−0.161 to 0.582) | >0.999 |
| T2 | 8.2 (0.9) | 8.6 (1.0) | −0.342 (−0.787 to 0.103) | 0.520 |
| T3 † | 8.1 (1.0) | 8.8 (1.2) | −0.625 (−1.116 to −0.134) | 0.053 |
| T4 | 5.2 (0.8) | 5.2 (0.6) | 0.026 (−0.293 to 0.345) | >0.999 |
| TV (mL) | 0.683 * | |||
| T1 | 518.9 (41.3) | 493.6 (43.4) | 25.3 (6.001 to 44.736) | 0.044 |
| T2 | 528.1 (41.3) | 500.7 (47.5) | 27.4 (6.981 to 47.650) | 0.036 |
| T3 † | 530.6 (47.8) | 511.9 (51.6) | 18.7 (−4.256 to 41.469) | 0.436 |
| T4 | 528.5 (40.6) | 506.2 (44.9) | 22.2 (2.753 to 41.879) | 0.104 |
| RR (breaths min−1) | >0.999 * | |||
| T1 | 13.4 (1.4) | 13.0 (1.4) | 0.342 (−0.295 to 0.979) | >0.999 |
| T2 | 18.6 (3.0) | 18.3 (3.5) | 0.316 (−1.184 to 1.816) | >0.999 |
| T3 † | 18.2 (3.6) | 17.7 (3.7) | 0.428 (−1.269 to 2.125) | >0.999 |
| T4 | 19.5 (3.3) | 19.2 (2.6) | 0.316 (−1.046 to 1.677) | >0.999 |
| Variables | VCV (n = 38) | DCV (n = 38) | Estimate (95% CI) | p-Value |
|---|---|---|---|---|
| MAP (mmHg) | 0.317 * | |||
| T1 | 74.9 (9.9) | 73.2 (11.3) | 1.711 (−3.135 to 6.556) | >0.999 |
| T2 | 81.3 (9.9) | 82.2 (10.1) | −0.921 (−5.498 to 3.656) | >0.999 |
| T3 † | 74.1 (8.3) | 77.4 (9.4) | −3.247 (−7.317 to 0.824) | 0.348 |
| T4 | 69.0 (11.2) | 70.4 (9.6) | −1.368 (−6.139 to 3.402) | >0.999 |
| HR (beats/min) | 0.941 * | |||
| T1 | 73.5 (11.9) | 72.4 (10.8) | 1.132 (−4.062 to 6.325) | >0.999 |
| T2 | 72.2 (11.1) | 70.6 (11.0) | 1.632 (−3.415 to 6.678) | >0.999 |
| T3 † | 70.9 (11.4) | 70.4 (10.1) | 0.436 (−4.525 to 5.397) | >0.999 |
| T4 | 71.2 (11.1) | 70.3 (9.3) | 0.816 (−3.874 to 5.505) | >0.999 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.H.; Park, I.K.; Choi, S.H.; Eum, D.; Kim, M.-S. Volume-Controlled Versus Dual-Controlled Ventilation during Robot-Assisted Laparoscopic Prostatectomy with Steep Trendelenburg Position: A Randomized-Controlled Trial. J. Clin. Med. 2019, 8, 2032. https://doi.org/10.3390/jcm8122032
Park JH, Park IK, Choi SH, Eum D, Kim M-S. Volume-Controlled Versus Dual-Controlled Ventilation during Robot-Assisted Laparoscopic Prostatectomy with Steep Trendelenburg Position: A Randomized-Controlled Trial. Journal of Clinical Medicine. 2019; 8(12):2032. https://doi.org/10.3390/jcm8122032
Chicago/Turabian StylePark, Jin Ha, In Kyeong Park, Seung Ho Choi, Darhae Eum, and Min-Soo Kim. 2019. "Volume-Controlled Versus Dual-Controlled Ventilation during Robot-Assisted Laparoscopic Prostatectomy with Steep Trendelenburg Position: A Randomized-Controlled Trial" Journal of Clinical Medicine 8, no. 12: 2032. https://doi.org/10.3390/jcm8122032





