Association of Pulse Volume Recording at Ankle with Total and Cardiovascular Mortality in Hemodialysis Patients
Abstract
:1. Introduction
2. Methods
2.1. Study Patients and Design
2.2. Hemodialysis
2.3. Measurements of Peripheral Vascular Parameters and Blood Pressures
2.4. Collection of Demographic, Medical, and Laboratory Data
2.5. Definition of Cardiovascular Mortality
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics among Study Patients
3.2. Univariate and Multivariate Correlations of %MAP
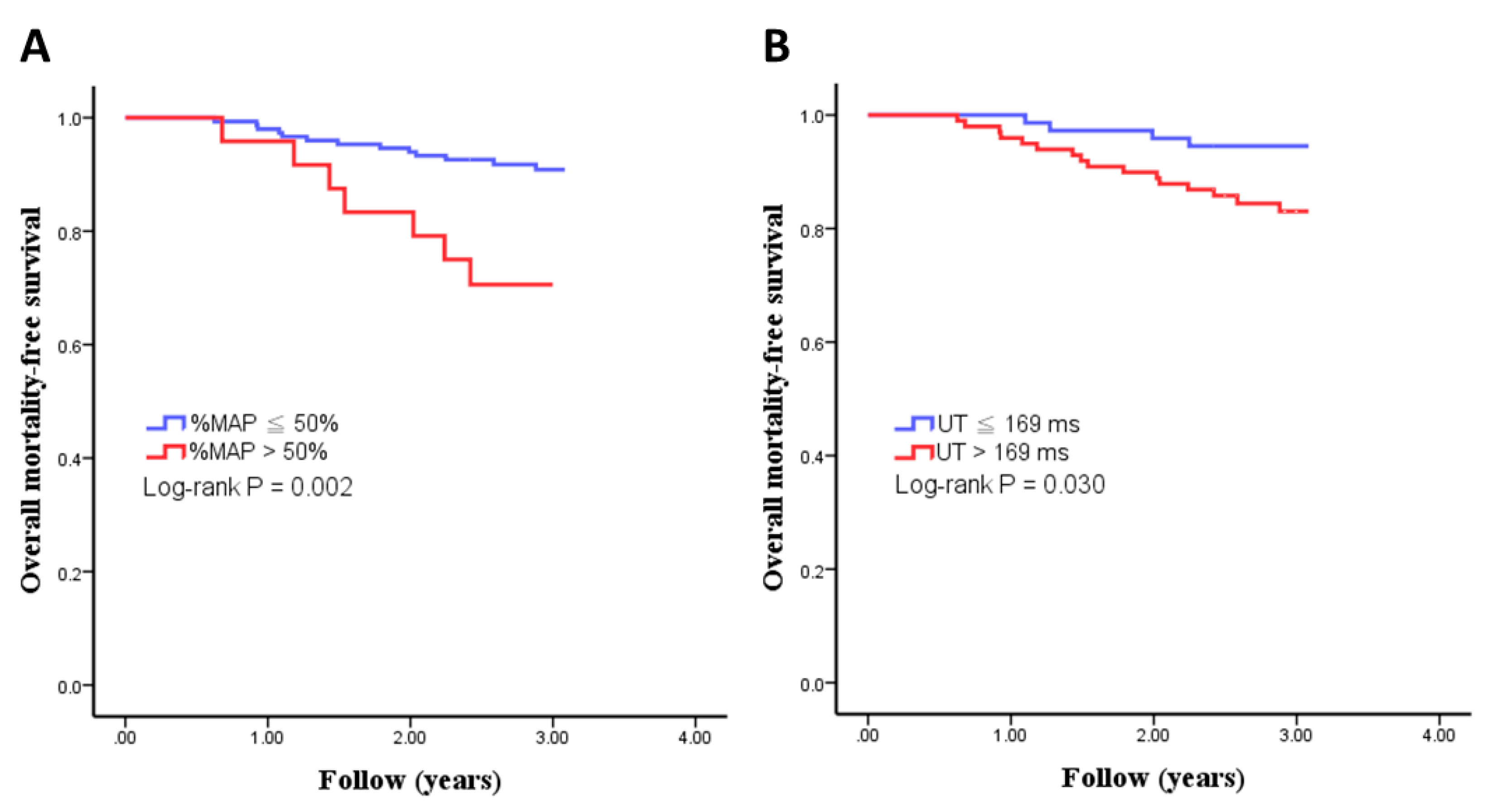
3.3. Kaplan–Meier Analyses of Overall Mortality-Free Survival in Study Patients
3.4. Major Predictors of Overall and Cardiovascular Mortality in Study Patients
4. Discussion
5. Study Limitation
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fowkes, F.G.; Aboyans, V.; Fowkes, F.J.; McDermott, M.M.; Sampson, U.K.; Criqui, M.H. Peripheral artery disease: Epidemiology and global perspectives. Nat. Rev. Cardiol. 2017, 14, 156–170. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.C.; Chiu, C.A.; Chu, C.Y.; Lee, W.H.; Su, H.M.; Lin, T.H.; Voon, W.C.; Lai, W.T.; Sheu, S.H. Chads2 score and risk of new-onset peripheral arterial occlusive disease in patients without atrial fibrillation: A nationwide cohort study in Taiwan. J. Atheroscler. Thromb. 2015, 22, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.C.; Lee, W.H.; Chiu, C.A.; Chu, C.Y.; Chen, S.C.; Su, H.M.; Lin, T.H.; Voon, W.C.; Lai, W.T.; Sheu, S.H. R2chads2 score is significantly associated with ankle-brachial index <0.9 in patients without atrial fibrillation. Atherosclerosis 2014, 236, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Su, H.M.; Chang, J.M.; Liu, W.C.; Tsai, J.C.; Tsai, Y.C.; Lin, M.Y.; Hwang, S.J.; Chen, H.C. Increasing prevalence of peripheral artery occlusive disease in hemodialysis patients: A 2-year follow-up. Am. J. Med. Sci. 2012, 343, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Asceric, R.R.; Dimkovic, N.B.; Trajkovic, G.Z.; Ristic, B.S.; Jankovic, A.N.; Duric, P.S.; Ilijevski, N.S. Prevalence, clinical characteristics, and predictors of peripheral arterial disease in hemodialysis patients: A cross-sectional study. BMC Nephrol. 2019, 20, 281. [Google Scholar] [CrossRef]
- Darling, R.C.; Raines, J.K.; Brener, B.J.; Austen, W.G. Quantitative segmental pulse volume recorder: A clinical tool. Surgery 1972, 72, 873–877. [Google Scholar]
- Kiuchi, S.; Hisatake, S.; Watanabe, I.; Toda, M.; Kabuki, T.; Oka, T.; Dobashi, S.; Ikeda, T. Pulse pressure and upstroke time are useful parameters for the diagnosis of peripheral artery disease in patients with normal ankle brachial index. Cardiol. Res. 2016, 7, 161–166. [Google Scholar] [CrossRef]
- Li, Y.H.; Lin, S.Y.; Sheu, W.H.; Lee, I.T. Relationship between percentage of mean arterial pressure at the ankle and mortality in participants with normal ankle-brachial index: An observational study. BMJ Open 2016, 6, e010540. [Google Scholar] [CrossRef]
- Yu, S.; Lu, Y.; Xiong, J.; Teliewubai, J.; Chi, C.; Ji, H.; Zhou, Y.; Fan, X.; Zhang, J.; Blacher, J.; et al. Comparison of ankle-brachial index and upstroke time in association with target organ damage: The Northern Shanghai study. J. Am. Soc. Hypertens. 2018, 12, 703–713. [Google Scholar] [CrossRef]
- Chang, L.H.; Hwu, C.M.; Chu, C.H.; Won, J.G.S.; Chen, H.S.; Lin, L.Y. Upstroke time per cardiac cycle is associated with cardiovascular prognosis in type 2 diabetes. Endocr. Pract. 2019, 25, 1109–1116. [Google Scholar] [CrossRef]
- Sheng, C.S.; Li, Y.; Huang, Q.F.; Kang, Y.Y.; Li, F.K.; Wang, J.G. Pulse waves in the lower extremities as a diagnostic tool of peripheral arterial disease and predictor of mortality in elderly chinese. Hypertension 2016, 67, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Imanishi, R.; Seto, S.; Toda, G.; Yoshida, M.; Ohtsuru, A.; Koide, Y.; Baba, T.; Yano, K. High brachial-ankle pulse wave velocity is an independent predictor of the presence of coronary artery disease in men. Hypertens. Res. 2004, 27, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Su, H.M.; Chang, J.M.; Lin, F.H.; Chen, S.C.; Voon, W.C.; Cheng, K.H.; Wang, C.S.; Lin, T.H.; Lai, W.T.; Sheu, S.H. Influence of different measurement time points on brachial-ankle pulse wave velocity and ankle-brachial index in hemodialysis patients. Hypertens. Res. 2007, 30, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.K.; Anderson, K.M.; Kannel, W.B.; Grossman, W.; Levy, D. Survival after the onset of congestive heart failure in Framingham heart study subjects. Circulation 1993, 88, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, R.B.; Lowenstein, D.H.; Klein, M.F. Combining segmental systolic pressures and plethysmography to diagnose arterial occlusive disease of the legs. Am. J. Surg. 1979, 138, 211–218. [Google Scholar] [CrossRef]
- Herraiz-Adillo, A.; Mariana-Herraiz, J.A.; Pozuelo-Carrascosa, D.P. Oscillometric and doppler ankle brachial indexes as predictors of all-cause mortality in a primary care population. Int. Angiol. 2019, 38, 256–263. [Google Scholar] [CrossRef]
- AbuRahma, A.F.; Adams, E.; AbuRahma, J.; Mata, L.A.; Dean, L.S.; Caron, C.; Sloan, J. Critical analysis and limitations of resting ankle-brachial index in the diagnosis of symptomatic peripheral arterial disease patients and the role of diabetes mellitus and chronic kidney disease. J. Vasc. Surg. 2019, in press. [Google Scholar] [CrossRef]
- Mitsutake, R.; Miura, S.; Saku, K. Association between coronary artery calcification score as assessed by multi-detector row computed tomography and upstroke time of pulse wave. Intern. Med. 2007, 46, 1833–1836. [Google Scholar] [CrossRef]
- Ma, L.; Zhao, S. Risk factors for mortality in patients undergoing hemodialysis: A systematic review and meta-analysis. Int. J. Cardiol. 2017, 238, 151–158. [Google Scholar] [CrossRef]
- Stack, A.G.; Molony, D.A.; Rahman, N.S.; Dosekun, A.; Murthy, B. Impact of dialysis modality on survival of new esrd patients with congestive heart failure in the United States. Kidney Int. 2003, 64, 1071–1079. [Google Scholar] [CrossRef]
- Tong, J.; Liu, M.; Li, H.; Luo, Z.; Zhong, X.; Huang, J.; Liu, R.; He, F.; Fu, J. Mortality and associated risk factors in dialysis patients with cardiovascular disease. Kidney Blood Press Res. 2016, 41, 479–487. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Patients with Mortality (n = 29) | Patients without Mortality (n = 168) | p Value | All Patients (n = 197) |
|---|---|---|---|---|
| Age (year) | 68 ± 12 | 60 ± 11 | <0.001 | 61 ± 12 |
| Male gender (%) | 52 | 54 | 0.854 | 53 |
| Dialysis duration (month) | 93 ± 61 | 91 ± 69 | 0.848 | 91 ± 68 |
| Diabetes mellitus (%) | 72 | 42 | 0.003 | 47 |
| Hypertension (%) | 59 | 51 | 0.424 | 52 |
| Current smoking (%) | 14 | 14 | 0.988 | 14 |
| CAD (%) | 28 | 7 | 0.001 | 10 |
| Stroke (%) | 24 | 7 | 0.004 | 10 |
| CHF (%) | 41 | 24 | 0.047 | 26 |
| Fontaine’s stages III–IV (n) | 5 | 20 | 0.425 | 25 |
| SBP (mmHg) | 162 ± 29 | 154 ± 27 | 0.234 | 155 ± 27 |
| DBP (mmHg) | 83 ± 18 | 82 ± 15 | 0.762 | 82 ± 15 |
| MAP (mmHg) | 109 ± 20 | 106 ± 18 | 0.450 | 106 ± 19 |
| BMI (kg/m2) | 22.6 ± 3.8 | 23.9 ± 3.8 | 0.103 | 23.7 ± 3.8 |
| Albumin (g/dL) | 3.6 ± 0.3 | 3.9 ± 0.3 | <0.001 | 3.9 ± 0.3 |
| Hemoglobin (g/dL) | 10.3 ± 1.7 | 10.5 ± 1.1 | 0.350 | 10.5 ± 1.2 |
| Total cholesterol (mg/dL) | 161 ± 46 | 181 ± 38 | 0.013 | 178 ± 40 |
| Triglyceride (mg/dL) | 162 ± 141 | 181 ± 38 | 0.823 | 167 ± 123 |
| Medications | ||||
| ACEI and/or ARB use (%) | 28 | 21 | 0.462 | 22 |
| β-blocker use (%) | 24 | 20 | 0.633 | 21 |
| CCB use (%) | 38 | 23 | 0.093 | 25 |
| Peripheral vascular parameters | ||||
| ABI in the lower side | 0.87 ± 0.28 | 0.97 ± 0.20 | 0.039 | 0.96 ± 0.21 |
| ABI < 0.9 in either leg (%) | 48 | 26 | 0.041 | 27 |
| ABI > 1.3 in either leg (%) | 14 | 8 | 0.389 | 9 |
| baPWV (cm/s) | 1997 ± 763 | 1927 ± 505 | 0.586 | 1936 ± 537 |
| UT (ms) | 202 ± 57 | 82 ± 36 | 0.027 | 184 ± 39 |
| UT > 169 ms (%) | 80 | 55 | 0.031 | 58 |
| %MAP | 47.7% ± 5.8% | 44.8% ± 4.5% | 0.010 | 45.1% ± 4.8% |
| %MAP > 50% (%) | 35 | 11 | 0.004 | 14 |
| Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|
| r | p | β | p | ||
| Age (year) | 0.228 | 0.003 | −0.032 | 0.609 | |
| Male gender (%) | −0.271 | <0.001 | −0.168 | 0.003 | |
| Dialysis duration (month) | −0.018 | 0.812 | |||
| Diabetes mellitus (%) | 0.327 | <0.001 | 0.021 | 0.748 | |
| Hypertension (%) | 0.006 | 0.935 | |||
| Current smoking (%) | −0.132 | 0.083 | |||
| CAD (%) | 0.078 | 0.31 | |||
| Stroke (%) | 0.185 | 0.015 | 0.076 | 0.185 | |
| CHF (%) | 0.068 | 0.373 | |||
| SBP (mmHg) | 0.249 | 0.001 | 0.121 | 0.054 | |
| DBP (mmHg) | −0.057 | 0.464 | |||
| MAP (mmHg) | 0.092 | 0.235 | |||
| BMI (kg/m2) | −0.158 | 0.038 | −0.085 | 0.144 | |
| Albumin (g/dL) | −0.229 | 0.002 | −0.095 | 0.113 | |
| Hemoglobin (g/dL) | 0.027 | 0.725 | |||
| Total cholesterol (mg/dL) | 0.118 | 0.122 | |||
| Triglyceride (mg/dL) | 0.079 | 0.302 | |||
| Medications | |||||
| ACEI and/or ARB use (%) | 0.022 | 0.776 | |||
| β-blocker use (%) | 0.113 | 0.139 | |||
| CCB use (%) | −0.02 | 0.794 | |||
| Peripheral vascular parameters | |||||
| ABI in the lower side | −0.533 | <0.001 | −0.259 | 0.01 | |
| ABI < 0.9 in either leg (%) | 0.457 | <0.001 | −0.035 | 0.699 | |
| ABI > 1.3 in either leg (%) | −0.59 | 0.443 | |||
| baPWV (cm/s) | 0.290 | <0.001 | 0.219 | 0.001 | |
| UT (ms) | 0.615 | <0.001 | 0.427 | <0.001 | |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| r | p | β | p | |
| Age (year) | 0.187 | 0.014 | −0.029 | 0.646 |
| Male gender | 0.112 | 0.145 | ||
| Dialysis duration (month) | 0.003 | 0.972 | ||
| Diabetes mellitus | 0.322 | <0.001 | 0.025 | 0.7 |
| Hypertension | 0.014 | 0.853 | ||
| Current smoking | −0.059 | 0.441 | ||
| CAD | 0.217 | 0.004 | 0.094 | 0.125 |
| Stroke | 0.096 | 0.209 | ||
| CHF | 0.184 | 0.016 | 0.07 | 0.254 |
| SBP (mmHg) | 0.058 | 0.457 | ||
| DBP (mmHg) | −0.173 | 0.024 | −0.143 | 0.017 |
| MAP (mmHg) | −0.068 | 0.382 | ||
| BMI (kg/m2) | −0.110 | 0.152 | ||
| Albumin (g/dL) | −0.129 | 0.092 | ||
| Hemoglobin (g/dL) | 0.083 | 0.277 | ||
| Total cholesterol (mg/dL) | 0.054 | 0.481 | ||
| Triglyceride (mg/dL) | 0.08 | 0.297 | ||
| Medications | ||||
| ACEI and/or ARB use | 0.2 | 0.009 | 0.094 | 0.135 |
| β-blocker use | 0.213 | 0.005 | 0.068 | 0.284 |
| CCB use (%) | 0.083 | 0.277 | ||
| Peripheral vascular parameters | ||||
| ABI in the lower side | −0.520 | <0.001 | −0.216 | 0.035 |
| ABI < 0.9 in either leg (%) | 0.497 | <0.001 | 0.090 | 0.350 |
| ABI > 1.3 in either leg (%) | −0.053 | 0.487 | ||
| baPWV (cm/s) | −0.001 | 0.992 | ||
| %MAP | 0.615 | <0.001 | 0.427 | <0.001 |
| Parameter | Total Mortality | Cardiovascular Mortality | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Age (year) | 1.070 (1.034–1.107) | <0.001 | 1.059 (1.007–1.112) | 0.025 |
| Male gender | 0.947 (0.457–1.962) | 0.883 | 1.598 (0.535–4.768) | 0.397 |
| Dialysis duration (month) | 1.000 (0.995–1.006) | 0.872 | 1.002 (0.994–1.009) | 0.644 |
| Diabetes mellitus | 3.275 (1.449–7.339) | 0.003 | 4.620 (1.287–16.585) | 0.01 |
| Hypertension | 1.378 (0.658–2.887) | 0.393 | 0.735 (0.255–2.119) | 0.567 |
| Current smoking | 1.030 (0.358–2.959) | 0.957 | 1.746 (0.487–6.260) | 0.386 |
| CAD | 4.392 (1.939–9.950) | <0.001 | 6.421 (2.136–19.300) | <0.001 |
| Stroke | 3.774 (1.609–8.853) | 0.001 | 4.758 (1.486–15.232) | 0.004 |
| CHF | 2.153 (1.028–4.509) | 0.037 | 2.285 (0.793–6.590) | 0.116 |
| Fontaine’s stages III–IV (%) | 1.427 (0.544–3.740) | 0.47 | 1.878 (0.524–6.732) | 0.333 |
| SBP (mmHg) | 1.009 (0.994–1.025) | 0.25 | 1.017 (0.995–1.039) | 0.125 |
| DBP (mmHg) | 1.004 (0.977–1.032) | 0.775 | 1.024 (0.989–1.061) | 0.182 |
| MAP (mmHg) | 1.008 (0.986–1.032) | 0.469 | 1.024 (0.993–1.056) | 0.135 |
| BMI (kg/m2) | 0.909 (0.814–1.015) | 0.094 | 0.935 (0.802–1.091) | 0.397 |
| Albumin (g/dL) | 0.197 (0.088–0.441) | <0.001 | 0.221 (0.069–0.714) | 0.013 |
| Hemoglobin (g/dL) | 0.859 (0.634–1.164) | 0.33 | 0.859 (0.634–1.164) | 0.33 |
| Total cholesterol (mg/dL) | 0.987 (0.977–0.997) | 0.012 | 0.977 (0.962–0.992) | 0.002 |
| Triglyceride (mg/dL) | 0.999 (0.996–1.003) | 0.744 | 1.001 (0.997–1.004) | 0.755 |
| Medications | ||||
| ACEI and/or ARB use | 1.482 (0.655–3.351) | 0.342 | 2.199 (0.735–6.586) | 0.148 |
| β-blocker use | 1.263 (0.539–2.958) | 0.591 | 1.605 (0.503–5.125) | 0.42 |
| CCB use | 1.895 (0.895–4.013) | 0.089 | 1.723 (0.577–5.142) | 0.324 |
| Peripheral vascular parameters | ||||
| ABI in the lower side | 0.151 (0.026–0.884) | 0.035 | 0.205 (0.015–2.851) | 0.237 |
| ABI < 0.9 in either leg | 2.375 (1.008–5.592) | 0.041 | 1.763 (0.497–6.250) | 0.373 |
| ABI > 1.3 in either leg | 1.730 (0.510–5.874) | 0.379 | 2.623 (0.557–2.365) | 0.223 |
| baPWV (cm/s) | 1.000 (0.999–1.001) | 0.606 | 1.000 (1.000–021) | 0.057 |
| UT (ms) | 1.010 (1.001–1.018) | 0.027 | 1.013 (1.021–1.025) | 0.022 |
| UT > 169 ms | 3.146 (1.051–9.411) | 0.03 | 3.172 (0.674–14.941) | 0.123 |
| %MAP | 1.121 (1.029–1.221) | 0.009 | 1.106 (0.978–1.250) | 0.108 |
| %MAP > 50% | 3.758 (1.497–9.434) | 0.002 | 4.732 (1.333–16.800) | 0.008 |
| Parameter | Total Mortality | Cardiovascular Mortality | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| UT (ms) | - | 0.15 | - | 0.103 |
| UT > 169 ms | - | 0.184 | - | 0.387 |
| %MAP | 1.098 (1.001–1.204) | 0.047 | - | 0.262 |
| %MAP > 50% | 2.900 (1.127–7.463) | 0.012 | 4.295 (1.209–15.264) | 0.024 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, W.-H.; Hsu, P.-C.; Huang, J.-C.; Chen, Y.-C.; Chen, S.-C.; Wu, P.-Y.; Lee, M.-K.; Lee, C.-S.; Yen, H.-W.; Su, H.-M. Association of Pulse Volume Recording at Ankle with Total and Cardiovascular Mortality in Hemodialysis Patients. J. Clin. Med. 2019, 8, 2045. https://doi.org/10.3390/jcm8122045
Lee W-H, Hsu P-C, Huang J-C, Chen Y-C, Chen S-C, Wu P-Y, Lee M-K, Lee C-S, Yen H-W, Su H-M. Association of Pulse Volume Recording at Ankle with Total and Cardiovascular Mortality in Hemodialysis Patients. Journal of Clinical Medicine. 2019; 8(12):2045. https://doi.org/10.3390/jcm8122045
Chicago/Turabian StyleLee, Wen-Hsien, Po-Chao Hsu, Jiun-Chi Huang, Ying-Chih Chen, Szu-Chia Chen, Pei-Yu Wu, Meng-Kuang Lee, Chee-Siong Lee, Hsueh-Wei Yen, and Ho-Ming Su. 2019. "Association of Pulse Volume Recording at Ankle with Total and Cardiovascular Mortality in Hemodialysis Patients" Journal of Clinical Medicine 8, no. 12: 2045. https://doi.org/10.3390/jcm8122045





