Metabolic Alterations in Cardiomyocytes of Patients with Duchenne and Becker Muscular Dystrophies
Abstract
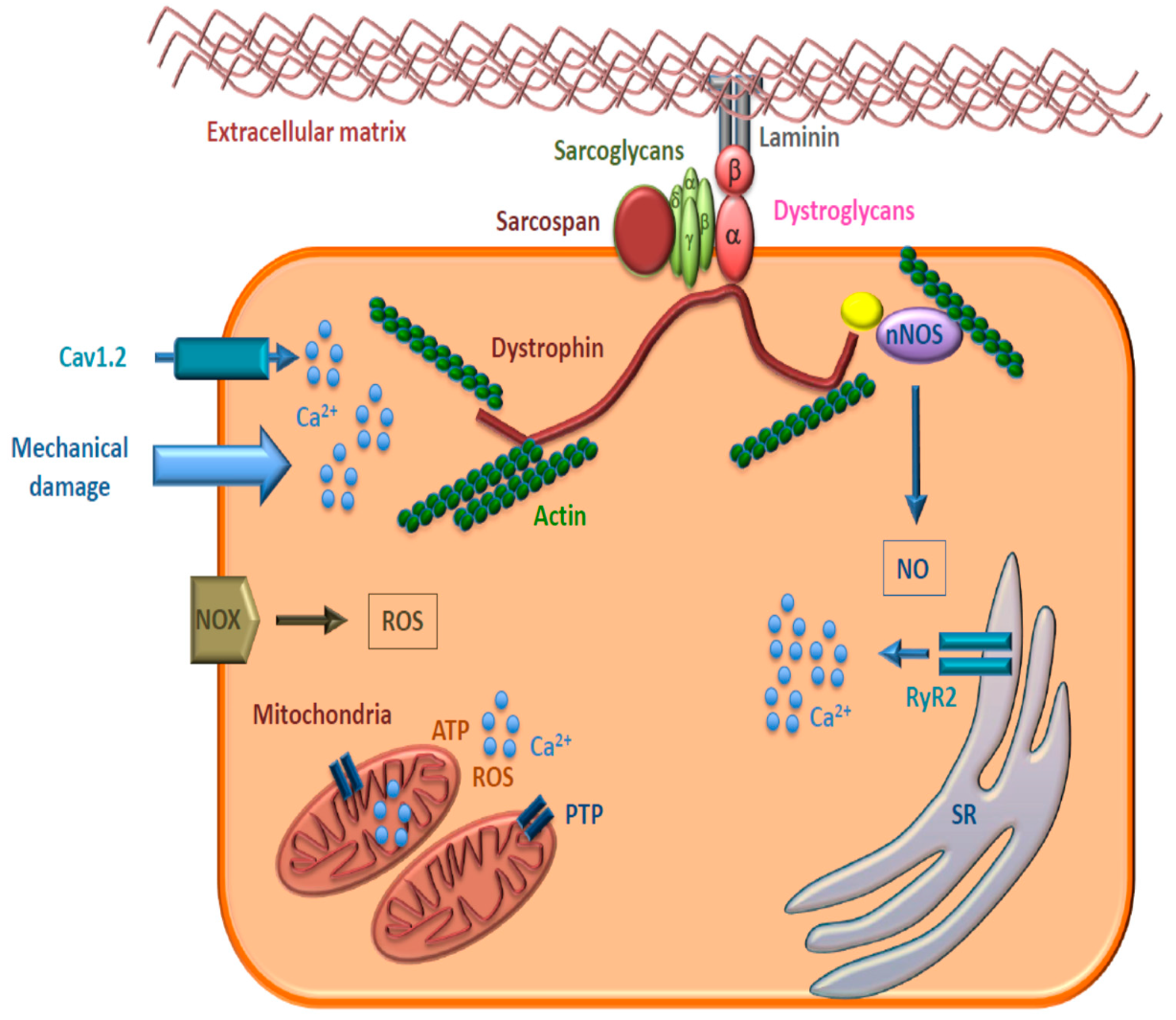
:1. Introduction
2. Materials and Methods
3. Results
3.1. Lipid Metabolism
3.2. Mitochondrial Impairment/Dysfunction
3.3. Reactive Oxygen/Nitrogen Species (ROS/RNS)
3.4. Calcium Handling
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Ervasti, J.M.; Campbell, K.P. A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J. Cell. Biol. 1993, 122, 809–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrof, B.J.; Shrager, J.B.; Stedman, H.H.; Kelly, A.M.; Sweeney, H. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc. Natl. Acad. Sci. USA 1993, 90, 3710–3714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rando, T.A. The dystrophin-glycoprotein complex, cellular signaling, and the regulation of cell survival in the muscular dystrophies. Muscle Nerve 2001, 24, 1575–1594. [Google Scholar] [CrossRef] [PubMed]
- Spence, H.J.; Dhillon, A.S.; James, M.; Winder, S.J. Dystroglycan, a scaffold for the ERK–MAP kinase cascade. EMBO Rep. 2004, 5, 484–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillis, J.M. Membrane abnormalities and Ca homeostasis in muscles of the mdx mouse, an animal model of the Duchenne muscular dystrophy: A review. Acta Physiol. Scand. 1996, 156, 397–406. [Google Scholar] [CrossRef]
- Worton, R.G.; Molnar, M.J.; Brais, B.; Karpati, G. The muscular dystrophies. In The Metabolic and Molecular Bases of Inherited Disease, 8th ed.; Scriver, C.R., Beaudet, A.L., Valle, D., Sly, W.S., Eds.; McGraw Hill: New York, NY, USA, 2001; pp. 5493–5523. [Google Scholar]
- Vita, G.; Vita, G.L.; Musumeci, O.; Rodolico, C.; Messina, S. Genetic neuromuscular disorders: Living the era of a therapeutic revolution. Part 2: Diseases of motor neuron and skeletal muscle. Neurol. Sci. 2019, 40, 671–681. [Google Scholar] [CrossRef]
- Esposito, G.; Ruggiero, R.; Savarese, M.; Savarese, G.; Tremolaterra, M.R.; Salvatore, F.; Carsana, A. Prenatal molecular diagnoses of inherited neuromuscular diseases: Duchenne/Becker muscular dystrophy, myotonic dystrophy type 1 and spinal muscular atrophy. Clin. Chem. Lab. Med. 2013, 51, 2239–2245. [Google Scholar] [CrossRef]
- Esposito, G.; Tremolaterra, M.R.; Marsocci, E.; Tandurella, I.C.M.; Fioretti, T.; Savarese, M.; Carsana, A. Precise mapping of 17 deletion breakpoints within the central hotspot deletion region (introns 50 and 51) of the DMD gene. J. Hum. Genet. 2017, 62, 1057–1063. [Google Scholar] [CrossRef] [Green Version]
- Carsana, A.; Frisso, G.; Tremolaterra, M.R.; Ricci, E.; De Rasmo, D.; Salvatore, F. A larger spectrum of intragenic STRs improves linkage analysis and localization of intragenic recombination detection in the dystrophin gene: An analysis of 93 families from Southern Italy. J. Mol. Diagn. 2007, 9, 64–69. [Google Scholar] [CrossRef] [Green Version]
- Townsend, D.; Yasuda, S.; Metzger, J. Cardiomyopathy of Duchenne muscular dystrophy: Pathogenesis and prospect of membrane sealants as a new therapeutic approach. Expert. Rev. Cardiovasc. Ther. 2007, 5, 99–109. [Google Scholar] [CrossRef]
- De Kermadec, J.M.; Bécane, H.M.; Chénard, A.; Tertrain, F.; Weiss, Y. Prevalence of left ventricular systolic dysfunction in Duchenne muscular dystrophy: An echocardiographic study. Am. Heart J. 1994, 127, 618–623. [Google Scholar] [CrossRef]
- Danialou, G.; Comtois, A.S.; Dudley, R.; Karpati, G.; Vincent, G.; Des Rosiers, C.; Petrof, B.J. Dystrophin-deficient cardiomyocytes are abnormally vulnerable to mechanical stress-induced contractile failure and injury. Faseb J. 2001, 15, 1655–1657. [Google Scholar] [CrossRef] [PubMed]
- Verhaert, D.; Richards, K.; Rafael-Fortney, J.A.; Raman, S.V. Cardiac involvement in patients with muscular dystrophies: Magnetic resonance imaging phenotype and genotypic considerations. Circ. Cardiovasc. Imaging 2011, 4, 67–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carsana, A.; Frisso, G.; Intrieri, M.; Tremolaterra, M.R.; Savarese, G.; Scapagnini, G.; Esposito, G.; Santoro, L.; Salvatore, F. A 15-year molecular analysis of Duchenne/Becker muscular dystrophy: Genetic features in a large cohort. Front. Biosci. 2010, 2E, 2547–2558. [Google Scholar]
- Coley, W.D.; Bogdanik, L.; Vila, M.C.; Yu, Q.; Van Der Meulen, J.H.; Rayavarapu, S.; Novak, J.S.; Nearing, M.; Quinn, J.L.; Saunders, A.; et al. Effect of genetic background on the dystrophic phenotype in mdx mice. Hum. Mol. Genet. 2016, 25, 130–145. [Google Scholar] [CrossRef] [Green Version]
- Roberts, N.W.; Holley-Cuthrell, J.; Gonzalez-Vega, M.; Mull, A.J.; Heydemann, A. Biochemical and Functional Comparisons of mdx and Sgcg(-/-) Muscular Dystrophy Mouse Models. BioMed Res. Int. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Timpani, C.A.; Hayes, A.; Rybalka, E. Revisiting the dystrophin-ATP connection: How half a century of research still implicates mitochondrial dysfunction in Duchenne Muscular Dystrophy aetiology. Med. Hypotheses 2015, 85, 1021–1033. [Google Scholar] [CrossRef]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, N.K.; Yadav, R.; Mukherjee, S.; Pal, L.; Sinha, N. Abnormal lipid metabolism in skeletal muscle tissue of patients with muscular dystrophy: In vitro, high-resolution NMR spectroscopy based observation in early phase of the disease. Magn. Reson. Imaging 2017, 38, 163–173. [Google Scholar] [CrossRef]
- Milad, N.; White, Z.; Tehrani, A.Y.; Sellers, S.; Rossi, F.M.V.; Bernatchez, P. Increased plasma lipid levels exacerbate muscle pathology in the mdx mouse model of Duchenne muscular dystrophy. Skelet. Muscle 2017, 7, 19. [Google Scholar] [CrossRef] [Green Version]
- Whitehead, N.P. Enhanced autophagy as a potential mechanism for the improved physiological function by simvastatin in muscular dystrophy. Autophagy 2016, 12, 705–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Podkalicka, P.; Mucha, O.; Dulak, J.; Loboda, A. Targeting angiogenesis in Duchenne muscular dystrophy. Cell Mol. Life Sci. 2019, 76, 1507–1528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buyse, G.M.; Goemans, N.; van den Hauwe, M.; Thijs, D.; de Groot, I.J.; Schara, U.; Ceulemans, B.; Meier, T.; Mertens, L. Idebenone as a novel, therapeutic approach for Duchenne muscular dystrophy: Results from a 12 month, double-blind, randomized placebo-controlled trial. Neuromuscul. Disord. 2011, 21, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Buyse, G.M.; Voit, T.; Schara, U.; Straathof, C.S.; D’Angelo, M.G.; Bernert, G.; Cuisset, J.M.; Finkel, R.S.; Goemans, N.; McDonald, C.M.; et al. Efficacy of idebenone on respiratory function in patients with Duchenne muscular dystrophy not using glucocorticoids (DELOS): A double-blind randomized placebo-controlled phase 3 trial. Lancet 2015, 385, 1748–1757. [Google Scholar] [CrossRef] [Green Version]
- Buyse, G.M.; Voit, T.; Schara, U.; Straathof, C.S.; D’Angelo, M.G.; Bernert, G.; Cuisset, J.M.; Finkel, R.S.; Goemans, N.; Rummey, C.; et al. Treatment effect of idebenone on inspiratory function in patients with Duchenne muscular dystrophy. Pediatr. Pulmonol. 2017, 52, 508–515. [Google Scholar] [CrossRef] [Green Version]
- Timpani, C.A.; Hayes, A.; Rybalka, E. Therapeutic strategies to address neuronal nitric oxide synthase deficiency and the loss of nitric oxide bioavailability in Duchenne Muscular Dystrophy. Orphanet J. Rare Dis. 2017, 12, 100. [Google Scholar] [CrossRef] [Green Version]
- Homburger, F.; Nixon, C.W.; Eppenberger, M.; Baker, J.R. Hereditary myopathy in the Syrian hamster: Studies on pathogenesis. Ann. N. Y. Acad. Sci. 1966, 138, 14–27. [Google Scholar] [CrossRef]
- Homburger, F. Disease models in Syrian hamsters. Prog. Exp. Tumor Res. 1972, 16, 69–86. [Google Scholar]
- Borowski, I.F.; Harrow, J.A.; Pritchard, E.T.; Dhalla, N.S. Changes in electrolyte and lipid contents of the myopathic hamster (UM-X7.1) skeletal and cardiac muscles. Res. Commun. Chem. Pathol. Pharmacol. 1974, 7, 443–451. [Google Scholar]
- Owens, K.; Weglicki, W.B.; Sonnenblick, E.H.; Gerz, E.W. Phospholipid and cholesterol content of ventricular tissue from the cardiomyopathic Syrian hamster. J. Mol. Cell. Cardiol. 1972, 4, 229–236. [Google Scholar] [CrossRef]
- Vecchini, A.; Binaglia, L.; Bibeau, M.; Minieri, M.; Carotenuto, F.; Di Nardo, P. Insulin deficiency and reduced expression of lipogenic enzymes in cardiomyopathic hamster. J. Lipid Res. 2001, 42, 96–105. [Google Scholar] [PubMed]
- Srivastava, N.K.; Pradhan, S.; Mittal, B.; Gowda, G.A. High resolution NMR based analysis of serum lipids in Duchenne muscular dystrophy patients and its possible diagnostic significance. NMR Biomed. 2010, 23, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, E.; Sestili, P. Reactive oxygen species in skeletal muscle signaling. J. Signal. Transduct. 2012, 982794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, B.; Tian, R. Mitochondrial dysfunction in pathophysiology of heart failure. J. Clin. Investig. 2018, 128, 3716–3726. [Google Scholar] [CrossRef] [Green Version]
- Allen, D.G.; Whitehead, N.P.; Froehner, S.C. Absence of Dystrophin Disrupts Skeletal Muscle Signaling: Roles of Ca2+, Reactive Oxygen Species, and Nitric Oxide in the Development of Muscular Dystrophy. Physiol. Rev. 2016, 96, 253–305. [Google Scholar] [CrossRef] [Green Version]
- Burelle, Y.; Khairallah, M.; Ascah, A.; Allen, B.G.; Deschepper, C.F.; Petrof, B.J.; Des Rosiers, C. Alterations in mitochondrial function as a harbinger of cardiomyopathy: Lessons from the dystrophic heart. J. Mol. Cell. Cardiol. 2010, 48, 310–321. [Google Scholar] [CrossRef] [Green Version]
- Šileikytė, J.; Forte, M. The Mitochondrial Permeability Transition in Mitochondrial Disorders. Oxid. Med. Cell. Longev. 2019, 2019, 3403075. [Google Scholar] [CrossRef] [Green Version]
- Matecki, S.; Fauconnier, J.; Lacampagne, A. Reactive Oxygen Species and Muscular Dystrophy. In Systems Biology of Free Radicals and Antioxidants; Laher, I., Ed.; Springer: Heidelberg/Berlin, Germany, 2014; pp. 3055–3079. [Google Scholar] [CrossRef]
- Cattaneo, F.; Castaldo, M.; Parisi, M.; Faraonio, R.; Esposito, G.; Ammendola, R. Formyl peptide receptor 1 modulates endothelial cell functions by NADPH oxidase-dependent VEGFR2 transactivation. Oxid. Med. Cell Longev. 2018, 2018, 2609847. [Google Scholar] [CrossRef] [Green Version]
- Ascah, A.; Khairallah, M.; Daussin, F.; Bourcier-Lucas, C.; Godin, R.; Allen, B.G.; Petrof, B.J.; Des Rosiers, C.; Burelle, Y. Stress-induced opening of the permeability transition pore in the dystrophin-deficient heart is attenuated by acute treatment with sildenafil. Am. J. Physiol. Heart. Circ. Physiol. 2010, 300, H144–H153. [Google Scholar] [CrossRef]
- Hughes, M.C.; Ramos, S.V.; Turnbull, P.C.; Edgett, B.A.; Huber, J.S.; Polidovitch, N.; Schlattner, U.; Backx, P.H.; Simpson, J.A.; Perry, C.G.R. Impairments in left ventricular mitochondrial bioenergetics precede overt cardiac dysfunction and remodelling in Duchenne muscular dystrophy. J. Physiol. 2019. [Google Scholar] [CrossRef]
- Gonzalez, D.R.; Treuer, A.V.; Lamirault, G.; Mayo, V.; Cao, Y.; Dulce, R.A.; Hare, J.M. NADPH oxidase-2 inhibition restores contractility and intracellular calcium handling and reduces arrhythmogenicity in dystrophic cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H710–H721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, I.A.; Allen, D.G. The Role of Reactive Oxygen Species in the Hearts of Dystrophin-Deficient Mdx Mice. Am. J. Physiol. Circ. Physiol. 2007, 293, H1969–H1977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brenman, J.E.; Chao, D.S.; Xia, H.; Aldape, K.; Bredt, D.S. Nitric oxide synthase complexed with dystrophin and absent from skeletal muscle sarcolemma in Duchenne muscular dystrophy. Cell 1995, 82, 743–752. [Google Scholar] [CrossRef] [Green Version]
- Danson, E.J.; Choate, J.K.; Paterson, D.J. Cardiac nitric oxide: Emerging role for nNOS in regulating physiological function. Pharmacol. Ther. 2005, 106, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, J.; Schneider, J.S.; Crassous, P.-A.; Zheng, R.; Gonzalez, J.P.; Xie, L.-H.; Beuve, A.; Fraidenraich, D.; Peluffo, R.D. Nitric Oxide Signalling Pathway in Duchenne Muscular Dystrophy Mice: Up-Regulation of L-Arginine Transporters. Biochem. J. 2013, 449, 133–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez, J.P.; Crassous, P.A.; Schneider, J.S.; Beuve, A.; Fraidenraich, D. Neuronal nitric oxide synthase localizes to utrophin expressing intercalated discs and stabilizes their structural integrity. Neuromuscul. Disord. 2015, 25, 964–976. [Google Scholar] [CrossRef]
- Wehling-Henricks, M.; Jordan, M.C.; Roos, K.P.; Deng, B.; Tidball, J.G. Cardiomyopathy in dystrophin-deficient hearts is prevented by expression of a neuronal nitric oxide synthase transgene in the myocardium. Hum. Mol. Genet. 2005, 14, 1921–1933. [Google Scholar] [CrossRef]
- Sarma, S.; Li, N.; van Oort, R.J.; Reynolds, C.; Skapura, D.G.; Wehrens, X.H. Genetic inhibition of PKA phosphorylation of RyR2 prevents dystrophic cardiomyopathy. Proc. Natl. Acad. Sci. USA 2010, 107, 13165–13170. [Google Scholar] [CrossRef] [Green Version]
- Ather, S.; Wang, W.; Wang, Q.; Li, N.; Anderson, M.E.; Wehrens, X.H. Inhibition of CaMKII phosphorylation of RyR2 prevents inducible ventricular arrhythmias in mice with Duchenne muscular dystrophy. Heart Rhythm 2013, 10, 592–599. [Google Scholar] [CrossRef]
- Fauconnier, J.; Thireau, J.; Reiken, S.; Cassan, C.; Richard, S.; Matecki, S.; Marks, A.R.; Lacampagne, A. Leaky RyR2 trigger ventricular arrhythmias in Duchenne muscular dystrophy. Proc. Natl. Acad. Sci. USA 2010, 107, 1559–1564. [Google Scholar] [CrossRef] [Green Version]
- Williams, I.A.; Allen, D.G. Intracellular calcium handling in ventricular myocytes from mdx mice. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H846–H855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Wang, W.; Wang, G.; Rodney, G.G.; Wehrens, X.H. Crosstalk between RyR2 oxidation and phosphorylation contributes to cardiac dysfunction in mice with Duchenne muscular dystrophy. J. Mol. Cell Cardiol. 2015, 89, 177–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zullo, A.; Frisso, G.; Carsana, A. Influence of physical activity on structure and function of the RyR1 calcium channel: A systematic review. Gazz. Med. Ital. Arch. Sci. Med. 2019, in press. [Google Scholar]
- Sadeghi, A.; Doyle, A.D.; Johnson, B.D. Regulation of the cardiac L-type Ca2+ channel by the actin-binding proteins alpha-actinin and dystrophin. Am. J. Physiol. Cell Physiol. 2002, 282, C1502–C1511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hohaus, A.; Person, V.; Behlke, J.; Schaper, J.; Morano, I.; Haase, H. The carboxyl-terminal region of ahnak provides a link between cardiac L-type Ca2+ channels and the actin-based cytoskeleton. FASEB J. 2002, 16, 1205–1216. [Google Scholar] [CrossRef]
- Rueckschloss, U.; Isenberg, G. Cytochalasin D reduces Ca2+ currents via cofilin-activated depolymerization of F-actin in guinea-pig cardiomyocytes. J. Physiol. 2001, 537, 363–370. [Google Scholar] [CrossRef]
- Koenig, X.; Rubi, L.; Obermair, G.J.; Cervenka, R.; Dang, X.B.; Lukacs, P.; Kummer, S.; Bittner, R.E.; Kubista, H.; Todt, H.; et al. Enhanced Currents through L-Type Calcium Channels in Cardiomyocytes Disturb the Electrophysiology of the Dystrophic Heart. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H564–H573. [Google Scholar] [CrossRef] [Green Version]
- Koenig, X.; Dysek, S.; Kimbacher, S.; Mike, A.K.; Cervenka, R.; Lukacs, P.; Nagl, K.; Dang, X.B.; Todt, H.; Bittner, R.E.; et al. Voltage-gated ion channel dysfunction precedes cardiomyopathy development in the dystrophic heart. PLoS ONE 2011, 6, e20300. [Google Scholar] [CrossRef] [Green Version]
- Rubi, L.; Todt, H.; Kubista, H.; Koenig, X.; Hilber, K. Calcium Current Properties in Dystrophin-deficient Ventricular Cardiomyocytes from Aged Mdx Mice. Physiol. Rep. 2018, 6, e13567. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhang, S.; Zhang, X.; Li, J.; Ai, X.; Zhang, L.; Yu, D.; Ge, S.; Peng, Y.; Chen, X. Blunted Cardiac Beta-Adrenergic Response as an Early Indication of Cardiac Dysfunction in Duchenne Muscular Dystrophy. Cardiovasc. Res. 2014, 103, 60–71. [Google Scholar] [CrossRef] [Green Version]
- Cserne Szappanos, H.; Muralidharan, P.; Ingley, E.; Petereit, J.; Millar, A.H.; Hool, L.C. Identification of a novel cAMP dependent protein kinase A phosphorylation site on the human cardiac calcium channel. Sci. Rep. 2017, 7, 15118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muralidharan, P.; Cserne Szappanos, H.; Ingley, E.; Hool, L.C. The Cardiac L-Type Calcium Channel AlphaSubunit Is a Target for Direct Redox Modification during Oxidative Stress-the Role of Cysteine Residues in the Alpha Interacting Domain. Clin. Exp. Pharmacol. Physiol. 2017, 44, 46–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koenig, X.; Ebner, J.; Hilber, K. Voltage-Dependent Sarcolemmal Ion Channel Abnormalities in the Dystrophin-Deficient Heart. Int. J. Mol. Sci. 2018, 19, 3296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bkaily, G.; Jacques, J. Na+-H+ exchanger and proton channel in heart failure associated with Becker and Duchenne muscular dystrophies. Can. J. Physiol. Pharmacol. 2017, 95, 1213–1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonsett, C.; Rudman, A. The dystrophin connection—ATP? Med. Hypotheses 1992, 38, 139–154. [Google Scholar] [CrossRef]


| Dysfunctional Metabolism | Molecular Alteration | Therapeutic Target | Available Drugs | Potential Therapeutic Strategy |
|---|---|---|---|---|
| Lipids | Increased cholesterol-to-phospholipid ratio | Cholesterol synthesis | Statin | |
| Mitochondria | Increased O2•− production | |||
| Impaired Ca2+ handling | ||||
| Impaired oxidative phosphorylation | Respiratory complex I function | Idebenone [24,25,26] | ||
| ROS | Increased expression of NOX2 Increased O2•− production | NOX2 | Statin [22] | NOX2 inhibition |
| RNS | Lower NO levels Impaired NOS1 activity | NO delivery NO synthesis | NO donors [27] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esposito, G.; Carsana, A. Metabolic Alterations in Cardiomyocytes of Patients with Duchenne and Becker Muscular Dystrophies. J. Clin. Med. 2019, 8, 2151. https://doi.org/10.3390/jcm8122151
Esposito G, Carsana A. Metabolic Alterations in Cardiomyocytes of Patients with Duchenne and Becker Muscular Dystrophies. Journal of Clinical Medicine. 2019; 8(12):2151. https://doi.org/10.3390/jcm8122151
Chicago/Turabian StyleEsposito, Gabriella, and Antonella Carsana. 2019. "Metabolic Alterations in Cardiomyocytes of Patients with Duchenne and Becker Muscular Dystrophies" Journal of Clinical Medicine 8, no. 12: 2151. https://doi.org/10.3390/jcm8122151





