Neopterin is Associated with Disease Severity and Outcome in Patients with Non-Ischaemic Heart Failure
Abstract
1. Introduction
2. Experimental Section
2.1. Study Population
2.2. Follow-Up Analysis
2.3. Measurements
2.4. Statistical Analysis
3. Results
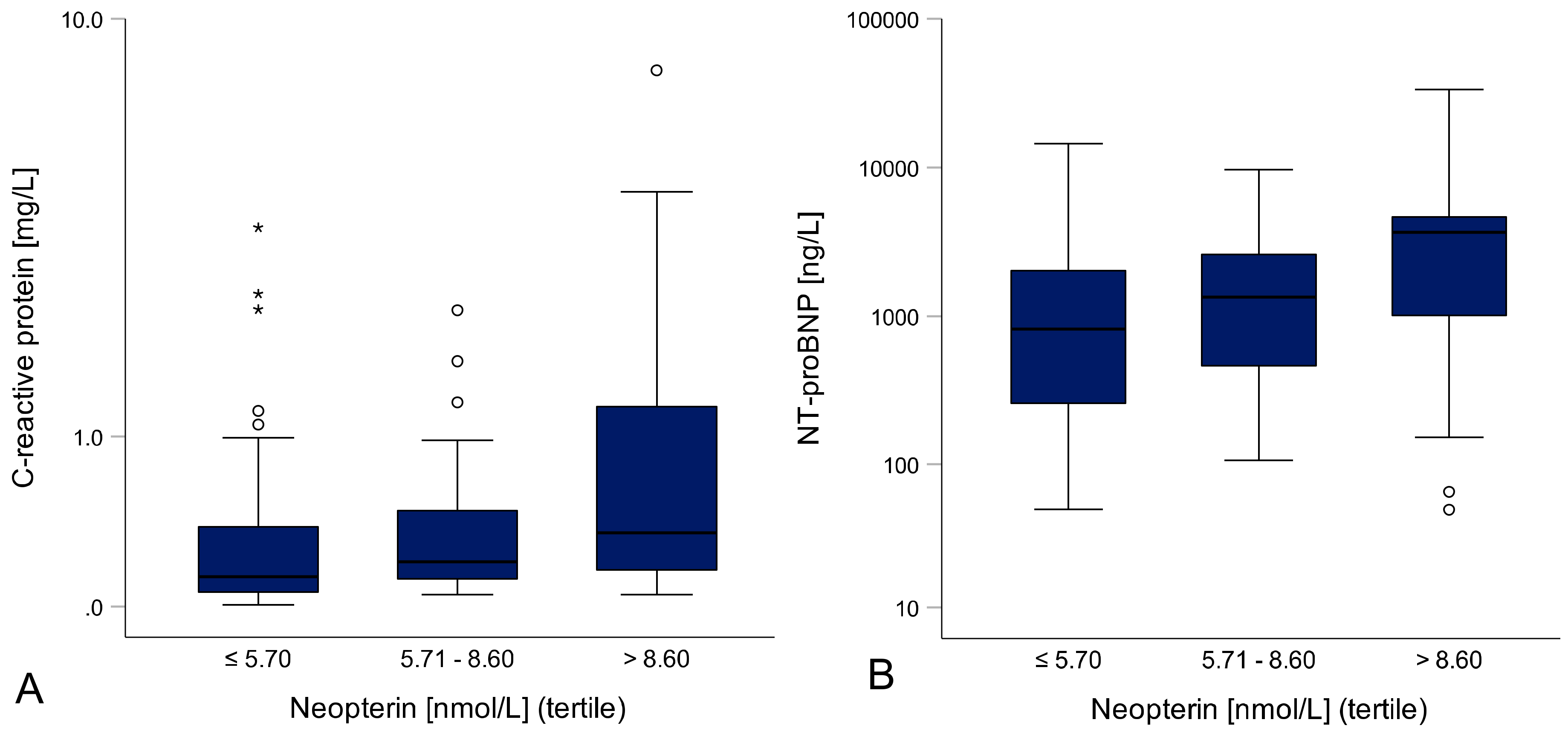
3.1. Inflammation Correlates With HF Severity and Cardiac Function
3.2. Neopterin/eGFR Ratio and HF Severity
3.3. Neopterin/eGFR Ratio and Left Ventricular Ejection Fraction
3.4. Laboratory Parameters and Event-Free Survival
3.5. Neopterin is a Predictor for an Adverse Outcome in Patients with HF
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Variable | HFrEF, LV-EF < 40% | HFmrEF, LV-EF 40–49.9% | HFpEF, LV-EF ≥ 50% | Sig. |
|---|---|---|---|---|
| n = 74 | n = 32 | n = 33 | p-Value | |
| Median (IQR) | Median (IQR) | Median (IQR) | ||
| Demographic and clinical characteristics | ||||
| Age (years) | 46.3 (36.2–55.8) | 46.3 (35.3–55.8) | 55.6 (48.2–69.2) | 0.005 |
| BMI (kg/m2) | 24.00 (21.60–27.30) | 24.76 (22.35–28.05) | 24.40 (22.00–28.90) | 0.516 |
| Heart rate (bpm) | 72 (63–85) | 69 (60–79) | 70 (60–81) | 0.385 |
| Syst. BP (mmHg) | 115 (110 – 140) | 126 (110–140) | 126 (120–150) | 0.003 |
| Laboratory measurements | ||||
| Neopterin (nmol/L) | 7.00 (5.20–9.20) | 5.35 (3.95–8.10) | 7.40 (5.90–11.50) | 0.021 |
| CRP (mg/L) | 0.24 (0.13–0.63) | 0.25 (0.07–0.63) | 0.15 (0.10–0.25) | 0.120 |
| eGFR (mL/min/1.73m2) | 76.48 (58.09–94.34) | 84.28 (71.12–101.40) | 66.15 (54.16–74.23) | 0.003 |
| Neopterin/eGFR ratio | 0.104 (0.059–0.144) | 0.061 (0.046–0.103) | 0.126 (0.082–0.190) | 0.005 |
| NT-proBNP (ng/L) | 2072 (949–3681) | 198 (96–745) | 1989 (703–4644) | <0.001 |
References
- Medzhitov, R. Inflammation 2010: New adventures of an old flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef]
- Dick, S.A.; Epelman, S. Chronic heart failure and inflammation: What do we really know? Circ. Res. 2016, 119, 159–176. [Google Scholar] [CrossRef] [PubMed]
- Torre-Amione, G.; Kapadia, S.; Lee, J.; Durand, J.B.; Bies, R.D.; Young, J.B.; Mann, D.L. Tumor necrosis factor-alpha and tumor necrosis factor receptors in the failing human heart. Circulation 1996, 93, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Vasan, R.S.; Sullivan, L.M.; Roubenoff, R.; Dinarello, C.A.; Harris, T.; Benjamin, E.J.; Sawyer, D.B.; Levy, D.; Wilson, P.W.; D’Agostino, R.B.; et al. Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: The Framingham Heart Study. Circulation 2003, 107, 1486–1491. [Google Scholar] [CrossRef]
- Mann, D.L. Innate immunity and the failing heart: The cytokine hypothesis revisited. Circ. Res. 2015, 116, 1254–1268. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, E.A.; Ponikowski, P.; Piepoli, M.F.; Banasiak, W.; Anker, S.D.; Poole-Wilson, P.A. Autonomic imbalance and immune activation in chronic heart failure—pathophysiological links. Cardiovasc. Res. 2006, 70, 434–445. [Google Scholar] [CrossRef]
- Libby, P.; Lichtman, A.H.; Hansson, G.K. Immune effector mechanisms implicated in atherosclerosis: From mice to humans. Immunity 2013, 38, 1092–1104. [Google Scholar] [CrossRef]
- Niebauer, J.; Volk, H.D.; Kemp, M.; Dominguez, M.; Schumann, R.R.; Rauchhaus, M.; Poole-Wilson, P.A.; Coats, A.J.; Anker, S.D. Endotoxin and immune activation in chronic heart failure: A prospective cohort study. Lancet 1999, 353, 1838–1842. [Google Scholar] [CrossRef]
- Hasper, D.; Hummel, M.; Kleber, F.X.; Reindl, I.; Volk, H.D. Systemic inflammation in patients with heart failure. Eur. Heart J. 1998, 19, 761–765. [Google Scholar] [CrossRef]
- Suzuki, K.; Nakaji, S.; Yamada, M.; Totsuka, M.; Sato, K.; Sugawara, K. Systemic inflammatory response to exhaustive exercise. Cytokine kinetics. Exerc. Immunol. Rev. 2002, 8, 6–48. [Google Scholar]
- Shephard, R.J. Sepsis and mechanisms of inflammatory response: Is exercise a good model? Br. J. Sports Med. 2001, 35, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Grammer, T.B.; Fuchs, D.; Boehm, B.O.; Winkelmann, B.R.; Maerz, W. Neopterin as a predictor of total and cardiovascular mortality in individuals undergoing angiography in the Ludwigshafen Risk and Cardiovascular Health study. Clin. Chem. 2009, 55, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, D.; Avanzas, P.; Arroyo-Espliguero, R.; Jenny, M.; Consuegra-Sanchez, L.; Kaski, J.C. The role of neopterin in atherogenesis and cardiovascular risk assessment. Curr. Med. Chem. 2009, 16, 4644–4653. [Google Scholar] [CrossRef] [PubMed]
- Zuo, H.; Ueland, P.M.; Ulvik, A.; Eussen, S.J.; Vollset, S.E.; Nygård, O.; Midttun, Ø.; Theofylaktopoulou, D.; Meyer, K.; Tell, G.S. Plasma biomarkers of inflammation, the kynurenine pathway, and risks of all-cause, cancer, and cardiovascular disease mortality: The Hordaland health study. Am. J. Epidemiol. 2016, 183, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; Willeit, J.; Kiechl, S.; Fuchs, D.; Jarosch, E.; Oberhollenzer, F.; Reibnegger, G.; Tilz, G.P.; Gerstenbrand, F.; Wachter, H. Increased concentrations of neopterin in carotid atherosclerosis. Atherosclerosis 1994, 106, 263–271. [Google Scholar] [CrossRef]
- Estévez-Loureiro, R.; Recio-Mayoral, A.; Sieira-Rodríguez-Moret, J.A.; Trallero-Araguás, E.; Kaski, J.C. Neopterin levels and left ventricular dysfunction in patients with chronic stable angina pectoris. Atherosclerosis 2009, 207, 514–518. [Google Scholar] [CrossRef]
- Avanzas, P.; Arroyo-Espliguero, R.; Quiles, J.; Roy, D.; Kaski, J.C. Elevated serum neopterin predicts future adverse cardiac events in patients with chronic stable angina pectoris. Eur. Heart J. 2005, 26, 457–463. [Google Scholar] [CrossRef]
- Bjørnestad, E.; Borsholm, R.A.; Svingen, G.F.T.; Pedersen, E.R.; Seifert, R.; Midttun, Ø.; Ueland, P.M.; Tell, G.S.; Bønaa, K.H.; Nygård, O. Neopterin as an effect modifier of the cardiovascular risk predicted by total homocysteine: A prospective 2-cohort study. J. Am. Heart Assoc. 2017, 6, e006500. [Google Scholar] [CrossRef]
- Ray, K.K.; Morrow, D.A.; Sabatine, M.S.; Shui, A.; Rifai, N.; Cannon, C.P.; Braunwald, E. Long-term prognostic value of neopterin: A novel marker of monocyte activation in patients with acute coronary syndrome. Circulation 2007, 115, 3071–3078. [Google Scholar] [CrossRef]
- Nathan, C.F.; Murray, H.W.; Wiebe, M.E.; Rubin, B.Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J. Exp. Med. 1983, 158, 670–689. [Google Scholar] [CrossRef]
- Eisenhut, M. Neopterin in diagnosis and monitoring of infectious diseases. J. Biomark. 2013, 2013, 196432. [Google Scholar] [CrossRef] [PubMed]
- Wirleitner, B.; Reider, D.; Ebner, S.; Böck, G.; Widner, B.; Jaeger, M.; Schennach, H.; Romani, N.; Fuchs, D. Monocyte-derived dendritic cells release neopterin. J. Leukoc. Biol. 2002, 72, 1148–1153. [Google Scholar] [PubMed]
- Gostner, J.M.; Becker, K.; Fuchs, D.; Sucher, R. Redox regulation of the immune response. Redox. Rep. 2013, 18, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.S.; Stenseth, R.; Pleym, H.; Wahba, A.; Videm, V. Neopterin predicts cardiac dysfunction following cardiac surgery. Interact. Cardiovasc. Thorac. Surg. 2015, 21, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Caruso, R.; De Chiara, B.; Campolo, J.; Verde, A.; Musca, F.; Belli, O.; Parolini, M.; Cozzi, L.; Moreo, A.; Frigerio, M.; et al. Neopterin levels are independently associated with cardiac remodeling in patients with chronic heart failure. Clin. Biochem. 2013, 46, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, E.; Hirata, Y.; Tokitsu, T.; Kusaka, H.; Tabata, N.; Tsujita, K.; Yamamuro, M.; Kaikita, K.; Watanabe, H.; Hokimoto, S.; et al. The clinical significance of plasma neopterin in heart failure with preserved left ventricular ejection fraction. ESC Heart Fail. 2016, 3, 53–59. [Google Scholar] [CrossRef]
- Karabacak, M.; Doğan, A.; Varol, E.; Uysal, B.A.; Yıldız, İ. Comparison of Inflammatory Markers in Patients with Ischemic and Non-ischemic Heart Failure. J. Am. Coll. Cardiol. 2013, 62, C137–C138. [Google Scholar] [CrossRef][Green Version]
- Sorriento, D.; Iaccarino, G. Inflammation and cardiovascular diseases: The most recent findings. Int. J. Mol. Sci. 2019, 20, 3879. [Google Scholar] [CrossRef]
- Fiordelisi, A.; Iaccarino, G.; Morisco, C.; Coscioni, E.; Sorriento, D. NFkappaB is a key player in the crosstalk between inflammation and cardiovascular diseases. Int. J. Mol. Sci. 2019, 20, 1599. [Google Scholar] [CrossRef]
- Mann, D.L. Stress-activated cytokines and the heart: From adaptation to maladaptation. Annu. Rev. Physiol. 2003, 65, 81–101. [Google Scholar] [CrossRef]
- Samsonov, M.; Fuchs, D.; Reibnegger, G.; Belenkov, J.N.; Nassonov, E.L.; Wachter, H. Patterns of serological markers for cellular immune activation in patients with dilated cardiomyopathy and chronic myocarditis. Clin. Chem. 1992, 38, 678–680. [Google Scholar] [PubMed]
- Rudzite, V.; Skards, J.I.; Fuchs, D.; Reibnegger, G.; Wachter, H. Serum kynurenine and neopterin concentrations in patients with cardiomyopathy. Immunol. Lett. 1992, 32, 125–129. [Google Scholar] [CrossRef]
- England, B.R.; Thiele, G.M.; Anderson, D.R.; Mikuls, T.R. Increased cardiovascular risk in rheumatoid arthritis: Mechanisms and implications. BMJ 2018, 361, k1036. [Google Scholar] [CrossRef] [PubMed]
- Sinicato, N.A.; da Silva Cardoso, P.A.; Appenzeller, S. Risk factors in cardiovascular disease in systemic lupus erythematosus. Curr. Cardiol. Rev. 2013, 9, 15–19. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Silverwood, R.J.; Forbes, H.J.; Abuabara, K.; Ascott, A.; Schmidt, M.; Schmidt, S.A.J.; Smeeth, L.; Langan, S.M. Severe and predominantly active atopic eczema in adulthood and long term risk of cardiovascular disease: Population based cohort study. BMJ 2018, 361, k1786. [Google Scholar] [CrossRef] [PubMed]
- Araújo, J.P.; Lourenço, P.; Azevedo, A.; Friões, F.; Rocha-Gonçalves, F.; Ferreira, A.; Bettencourt, P. Prognostic value of high-sensitivity C-reactive protein in heart failure: A systematic review. J. Card. Fail. 2009, 15, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Abrams, J. C-reactive protein, inflammation, and coronary risk: An update. Cardiol. Clin. 2003, 21, 327–331. [Google Scholar] [CrossRef]
- Avanzas, P.; Arroyo-Espliguero, R.; Kaski, J.C. Neopterin—marker of coronary artery disease activity or extension in patients with chronic stable angina? Int. J. Cardiol. 2010, 144, 74–75. [Google Scholar] [CrossRef]
- Fonseca, F.A.; Izar, M.C. High-sensitivity C-reactive protein and cardiovascular disease across countries and ethnicities. Clinics 2016, 71, 235–242. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Gambardella, J.; Santulli, G. Integrating diet and inflammation to calculate cardiovascular risk. Atherosclerosis 2016, 253, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Michels da Silva, D.; Langer, H.; Graf, T. Inflammatory and molecular pathways in heart failure-ischemia, HFpEF and transthyretin cardiac amyloidosis. Int. J. Mol. Sci. 2019, 20, 2322. [Google Scholar] [CrossRef] [PubMed]
- Riggs, K.A.; Joshi, P.H.; Khera, A.; Singh, K.; Akinmolayemi, O.; Ayers, C.R.; Rohatgi, A. Impaired HDL metabolism links GlycA, A novel inflammatory marker, with incident cardiovascular events. J. Clin. Med. 2019, 8, 2137. [Google Scholar] [CrossRef] [PubMed]


| Variable | Total | No Event * | Event * | Significance |
|---|---|---|---|---|
| n = 149 | n = 115 | n = 34 | p-Value | |
| Median (IQR) | Median | Median | ||
| Demographic and clinical characteristics | ||||
| Age (years) | 49.7 (38.5–61.7) | 48.9 | 51.4 | 0.074 |
| BMI (kg/m2) | 24.81 (22.00–27.74) | 25.25 | 23.55 | 0.025 |
| Heart rate (bpm) | 70 (60–82) | 70 | 73 | 0.220 |
| Diast. BP (mmHg) | 80 (70–85) | 80 | 77 | 0.751 |
| Syst. BP (mmHg) | 120 (110–132) | 120 | 115 | 0.193 |
| Hypertension | 45.2% | 45.5% | 44.1% | 0.884 |
| Atrial fibrillation | 9.7% | 9.7% | 9.4% | 0.952 |
| NYHA class, overall | - | - | - | 0.072 |
| NYHA class l | 22.4% | 26.3% | 9.1% | - |
| NYHA class ll | 44.2% | 43.9% | 45.5% | - |
| NYHA class lll/lV | 33.3% | 29.8% | 45.5% | - |
| Laboratory measurements | ||||
| Neopterin (nmol/L) | 6.90 (5.00–9.70) | 6.50 | 10.00 | <0.001 |
| CRP (mg/L) | 0.20 (0.10–0.63) | 0.20 | 0.20 | 0.966 |
| eGFR (mL/min/1.73m2) | 74.11 (58.39–90.47) | 78.29 | 65.57 | 0.001 |
| Neopterin/eGFR ratio | 0.097 (0.057–0.148) | 0.082 | 0.162 | <0.001 |
| NT-proBNP (ng/L) | 1340 (501–3266) | 1025 | 3835 | <0.001 |
| Hemodynamics | ||||
| LV-EF (%) | 37.0 (25.7–49.7) | 36.0 | 46.0 | 0.052 |
| Cardiac index (L/min/m2) | 1.93 (1.68–2.45) | 2.01 | 1.78 | 0.003 |
| mean PAP (mmHg) | 26.0 (19.0–33.0) | 24.0 | 32.5 | 0.001 |
| PCWP (mmHg) | 17 (11–25) | 15 | 24 | <0.001 |
| RAP (mmHg) | 9 (6–12) | 8 | 11 | 0.003 |
| Medication and Treatment | ||||
| ACE inhibitor/ARB | 77.7% | 79.8% | 70.6% | 0.256 |
| Beta-blocker | 74.8% | 78.8% | 61.8% | 0.045 |
| MRA | 34.5% | 33.3% | 38.2% | 0.598 |
| Diuretics | 57.8% | 52.6% | 75.8% | 0.018 |
| Cardiac glycosides | 2.0% | 1.8% | 2.9% | 0.666 |
| Pacemaker | 3.4% | 3.5% | 2.9% | 0.872 |
| Variable | Univariate Model | Multivariate Model | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Neopterin (nmol/L) _Ln * | 2.874 | 1.663–4.966 | <0.001 | 2.770 | 1.419–5.407 | 0.003 |
| eGFR (mL/min/1.73m2) _Ln | 0.321 | 0.174–0.593 | <0.001 | 2.723 | 0.936–7.926 | 0.066 |
| NT-proBNP (ng/L) _Ln | 1.665 | 1.253–2.214 | <0.001 | 1.368 | 0.972–1.926 | 0.072 |
| NYHA class II vs. I | 2.542 | 0.852–7.578 | 0.094 | 3.200 | 0.830–12.329 | 0.091 |
| NYHA class III/IV vs. I | 3.245 | 1.070–9.840 | 0.038 | 3.126 | 0.751–13.006 | 0.117 |
| LV-EF (%) _Ln | 2.245 | 0.989–5.096 | 0.053 | 2.884 | 1.096–7.589 | 0.032 |
| Neopterin/eGFR ratio _Ln | 1.748 | 1.420–2.152 | <0.001 | |||
| Cardiac index (L/min/m2) _Ln | 0.250 | 0.062–1.008 | 0.051 | |||
| mean PAP (mmHg) _Ln | 2.979 | 1.168–7.599 | 0.022 | |||
| PCWP (mmHg)_Ln | 2.453 | 1.203–5.002 | 0.014 | |||
| RAP (mmHg) _Ln | 3.536 | 1.584–7.894 | 0.002 | |||
| BMI (kg/m2) _Ln | 0.188 | 0.032–1.114 | 0.066 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lanser, L.; Pölzl, G.; Fuchs, D.; Weiss, G.; Kurz, K. Neopterin is Associated with Disease Severity and Outcome in Patients with Non-Ischaemic Heart Failure. J. Clin. Med. 2019, 8, 2230. https://doi.org/10.3390/jcm8122230
Lanser L, Pölzl G, Fuchs D, Weiss G, Kurz K. Neopterin is Associated with Disease Severity and Outcome in Patients with Non-Ischaemic Heart Failure. Journal of Clinical Medicine. 2019; 8(12):2230. https://doi.org/10.3390/jcm8122230
Chicago/Turabian StyleLanser, Lukas, Gerhard Pölzl, Dietmar Fuchs, Günter Weiss, and Katharina Kurz. 2019. "Neopterin is Associated with Disease Severity and Outcome in Patients with Non-Ischaemic Heart Failure" Journal of Clinical Medicine 8, no. 12: 2230. https://doi.org/10.3390/jcm8122230
APA StyleLanser, L., Pölzl, G., Fuchs, D., Weiss, G., & Kurz, K. (2019). Neopterin is Associated with Disease Severity and Outcome in Patients with Non-Ischaemic Heart Failure. Journal of Clinical Medicine, 8(12), 2230. https://doi.org/10.3390/jcm8122230





