CHADS2, CHA2DS2ASc, and New ABCD Scores Predict the Risk of Peripheral Arterial Disease in Patients with Sleep Apnea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Study Population
2.3. Study Outcome
2.4. Criteria and Definitions of Variables
2.5. Statistical Analysis
3. Results
3.1. Study Population
3.2. Similar PAD Risks in SA Patients and Control Subjects
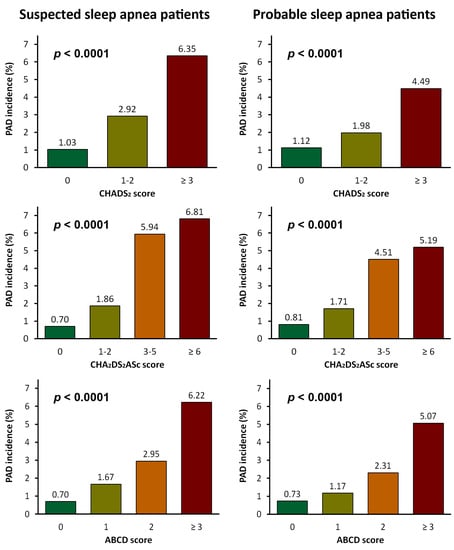
3.3. CHADS2 and CHA2DS2ASc Scores Predicts PAD Risks in SA Patients
3.4. New Scoring System Predicting PAD Risks in SA Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

| Study Arm PM-A | Study Arm PM-B | |||||
|---|---|---|---|---|---|---|
| PM-Suspected SA | Control A-PM | p-Value | PM-Probable SA | Control B-PM | p-Value | |
| n | 7985 | 31,940 | 3164 | 12,656 | ||
| Sex, n (%) | ||||||
| Female | 2837 (36%) | 11,348 (36%) | 637 (20%) | 2548 (20%) | ||
| Male | 5148 (64%) | 20,592 (64%) | 2527 (80%) | 10,108 (80%) | ||
| Age (year), mean ± SD | 44.5 ± 13.3 | 44.5 ± 13.3 | >0.99 | 44.7 ± 11.9 | 44.7 ± 11.8 | >0.99 |
| Age (year), n (%) | >0.99 | >0.99 | ||||
| ≤40 | 3258 (41%) | 13,013 (41%) | 1203 (38%) | 4804 (38%) | ||
| 40 < age ≤ 50 | 2143 (27%) | 8588 (27%) | 958 (30%) | 3842 (30%) | ||
| >50 | 2584 (32%) | 10,339 (32%) | 1003 (32%) | 4010 (32%) | ||
| Residency | 0.5303 | 0.585 | ||||
| Northern Taiwan | 3647 (46%) | 14,713 (46%) | 1345 (43%) | 5448 (43%) | ||
| Other areas | 4338 (54%) | 17,227 (54%) | 1819 (57%) | 7208 (57%) | ||
| Monthly income (NT$), n (%) | 0.9639 | 0.9427 | ||||
| ≤24,000 | 4342 (54%) | 17,359 (54%) | 1427 (45%) | 5699 (45%) | ||
| >24,000 | 3643 (46%) | 14,581 (46%) | 1737 (55%) | 6957 (55%) | ||
| CCI score, mean ± SD | 0.7 ± 0.9 | 0.7 ± 0.9 | >0.99 | 0.7 ± 0.9 | 0.7 ± 0.9 | >0.99 |
| CCI score, n (%) | >0.99 | >0.99 | ||||
| = 0 | 4199 (53%) | 16,796 (53%) | 1580 (50%) | 6320 (50%) | ||
| = 1 | 2499 (31%) | 9996 (31%) | 1037 (33%) | 4148 (33%) | ||
| ≥ 2 | 1287 (16%) | 5148 (16%) | 547 (17%) | 2188 (17%) | ||
| Underlying diseases, n (%) | ||||||
| Heart disease | 12 (0%) | 48 (0%) | >0.99 | 7 (0%) | 28 (0%) | >0.99 |
| Myocardial infarction | 3 (0%) | 24 (0%) | 0.2481 | 1 (0%) | 17 (0%) | 0.1253 |
| Congestive heart failure | 9 (0%) | 24 (0%) | 0.2961 | 6 (0%) | 11 (0%) | 0.1147 |
| Peripheral vascular disease | 0 (%) | 0 (%) | 0.2961 | 0 (%) | 0 (%) | 0.1147 |
| Major neurological disorder | 190 (2%) | 760 (2%) | >0.99 | 76 (2%) | 304 (2%) | >0.99 |
| Cerebral vascular disease | 190 (2%) | 757 (2%) | 0.9607 | 76 (2%) | 304 (2%) | >0.99 |
| Dementia | 0 (0%) | 3 (0%) | 0.3865 | 0 (%) | 0 (%) | >0.99 |
| Hemiplegia | 1 (0%) | 4 (0%) | >0.99 | 1 (0%) | 4 (0%) | >0.99 |
| Chronic pulmonary disease | 1519 (19%) | 6076 (19%) | >0.99 | 657 (21%) | 2628 (21%) | >0.99 |
| Connective tissue disease | 12 (0%) | 48 (0%) | >0.99 | 3 (0%) | 12 (0%) | >0.99 |
| Peptic ulcer disease | 1903 (24%) | 7612 (24%) | >0.99 | 770 (24%) | 3080 (24%) | >0.99 |
| Liver disease | 1189 (15%) | 4756 (15%) | >0.99 | 530 (17%) | 2120 (17%) | >0.99 |
| Diabetes mellitus | 345 (4%) | 1380 (4%) | >0.99 | 151 (5%) | 604 (5%) | >0.99 |
| Renal disease | 23 (0%) | 92 (0%) | >0.99 | 7 (0%) | 28 (0%) | >0.99 |
| Cancer | 72 (1%) | 286 (1%) | 0.9577 | 26 (1%) | 104 (1%) | >0.99 |
| Suspected SA | Probable SA | Proposed ABCD Score | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Maximal Model | Reduced Model † | Maximal Model | Reduced Model † | ||||||
| HR (95%CI) | p-Value | HR (95%CI) | p-Value | HR (95%CI) | p-Value | HR (95%CI) | p-Value | ||
| Male (vs. female) | 1.00 (0.77–1.30) | 0.9909 | 1.07 (0.62–1.86) | 0.7985 | |||||
| Age (year) (vs. ≤ 50): | |||||||||
| 50 < age ≤ 65 | 2.07 (1.45–2.96) | <0.0001 | 2.25 (1.59–3.20) | <0.0001 | 2.09 (1.16–3.77) | 0.0142 | 2.60 (1.52–4.48) | 0.0005 | 1 |
| >65 | 3.13 (2.07–4.74) | <0.0001 | 3.95 (2.70–5.79) | <0.0001 | 4.08 (1.93–8.62) | 0.0002 | 7.17 (4.07–12.63) | <0.0001 | 2 |
| Residency in Northern Taiwan (vs. other areas) | 0.80 (0.62–1.04) | 0.0950 | 0.80 (0.51–1.26) | 0.3356 | |||||
| Monthly income (NT$) > 24,000 (≤24,000) | 0.74 (0.53–1.02) | 0.0660 | 0.83 (0.49–1.40) | 0.4757 | |||||
| Underlying diseases (with vs. without): | |||||||||
| Hypertension | 1.62 (1.18–2.22) | 0.0029 | 1.70 (1.24–2.32) | 0.0009 | 1.18 (0.68–2.02) | 0.5584 | 1 | ||
| Myocardial infarction | 1.28 (0.62–2.63) | 0.5027 | 1.10 (0.26–4.63) | 0.8991 | |||||
| Congestive heart failure | 0.84 (0.52–1.36) | 0.4794 | 1.10 (0.48–2.53) | 0.8252 | |||||
| Cerebral vascular disease | 1.52 (1.10–2.11) | 0.0123 | 1.61 (1.17–2.21) | 0.0031 | 1.44 (0.79–2.65) | 0.2372 | 1 | ||
| Dementia | 1.20 (0.55–2.62) | 0.6415 | 2.27 (0.77–6.71) | 0.1382 | |||||
| Hemiplegia | 0.87 (0.35–2.17) | 0.7655 | 1.56 (0.45–5.44) | 0.4849 | |||||
| Chronic pulmonary disease | 1.39 (1.05–1.84) | 0.0214 | 1.04 (0.63–1.72) | 0.8743 | |||||
| Connective tissue disease | 1.42 (0.72–2.78) | 0.3110 | 1.04 (0.25–4.29) | 0.9612 | |||||
| Peptic ulcer disease | 1.24 (0.94–1.63) | 0.1358 | 1.22 (0.75–1.99) | 0.4271 | |||||
| Liver disease | 0.89 (0.65–1.21) | 0.4495 | 0.86 (0.49–1.48) | 0.5811 | |||||
| Diabetes mellitus | 1.74 (1.28–2.36) | 0.0003 | 1.74 (1.29–2.34) | 0.0003 | 1.69 (0.98–2.94) | 0.0608 | 1 | ||
| Renal disease | 1.35 (0.88–2.08) | 0.1742 | 1.36 (0.63-2.93) | 0.4376 | |||||
| Cancer | 1.00 (0.77–1.30) | 0.9909 | ‡ | 0.9766 | |||||


References
- Donovan, L.M.; Kapur, V.K. Prevalence and characteristics of central compared to obstructive sleep apnea: Analyses from the sleep heart health study cohort. Sleep 2016, 39, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Jonas, D.E.; Amick, H.R.; Feltner, C.; Weber, R.P.; Arvanitis, M.; Stine, A.; Lux, L.; Harris, R.P. Screening for obstructive sleep apnea in adults: Evidence report and systematic review for the us preventive services task force. JAMA 2017, 317, 415–433. [Google Scholar] [CrossRef] [PubMed]
- Kuniyoshi, F.H.; Pusalavidyasagar, S.; Singh, P.; Somers, V.K. Cardiovascular consequences of obstructive sleep apnoea. Indian J. Med. Res. 2010, 131, 196–205. [Google Scholar]
- Pande, R.L.; Perlstein, T.S.; Beckman, J.A.; Creager, M.A. Secondary prevention and mortality in peripheral artery disease: National Health and Nutrition Examination Study, 1999 to 2004. Circulation 2011, 124, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; Ballew, S.H.; Coresh, J.; Arima, H.; Arnlov, J.; Cirillo, M.; Ebert, N.; Hiramoto, J.S.; Kimm, H.; Shlipak, M.G.; et al. Measures of chronic kidney disease and risk of incident peripheral artery disease: A collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 2017, 5, 718–728. [Google Scholar] [CrossRef]
- Criqui, M.H.; Aboyans, V. Epidemiology of peripheral artery disease. Circ. Res. 2015, 116, 1509–1526. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, C.A.; Adam, L.; Weisser-Thomas, J.; Pingel, S.; Vogel, G.; Klarmann-Schulz, U.; Nickenig, G.; Pizarro, C.; Skowasch, D. High prevalence of peripheral arterial disease in patients with obstructive sleep apnoea. Clin. Res. Cardiol. 2015, 104, 719–726. [Google Scholar] [CrossRef]
- Chen, J.C.; Koo, M.; Hwang, J.H. Risks of peripheral arterial occlusive disease in patients with obstructive sleep apnoea: A population-based case-control study. Clin. Otolaryngol. 2015, 40, 437–442. [Google Scholar] [CrossRef]
- Gage, B.F.; Waterman, A.D.; Shannon, W.; Boechler, M.; Rich, M.W.; Radford, M.J. Validation of clinical classification schemes for predicting stroke: Results from the National Registry of Atrial Fibrillation. JAMA 2001, 285, 2864–2870. [Google Scholar] [CrossRef]
- Hsu, P.C.; Chiu, C.A.; Chu, C.Y.; Lee, W.H.; Su, H.M.; Lin, T.H.; Voon, W.C.; Lai, W.T.; Sheu, S.H. CHADS2 score and risk of new-onset peripheral arterial occlusive disease in patients without atrial fibrillation: A nationwide cohort study in Taiwan. J. Atheroscler. Thromb. 2015, 22, 490–498. [Google Scholar] [CrossRef]
- Hsu, P.C.; Lin, T.H.; Lee, W.H.; Chu, C.Y.; Chiu, C.A.; Lee, H.H.; Su, H.M.; Voon, W.C.; Lai, W.T.; Sheu, S.H. Association between the CHADS2 score and an ankle-brachial index of 0.9 in patients without atrial fibrillation. J. Atheroscler. Thromb. 2014, 21, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.J.; Yang, C.J.; Kung, Y.T.; Sheu, C.C.; Shen, Y.T.; Chang, P.Y.; Huang, M.S.; Chiu, H.C. Metformin decreases lung cancer risk in diabetic patients in a dose-dependent manner. Lung Cancer 2014, 86, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.M.; Tsai, M.J.; Wei, P.J.; Su, Y.C.; Yang, C.J.; Wu, M.N.; Hsu, C.Y.; Hwang, S.J.; Chong, I.W.; Huang, M.S. Erectile dysfunction in patients with sleep apnea—A nationwide population-based study. PLoS ONE 2015, 10, e0132510. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.J.; Wu, P.H.; Sheu, C.C.; Hsu, Y.L.; Chang, W.A.; Hung, J.Y.; Yang, C.J.; Yang, Y.H.; Kuo, P.L.; Huang, M.S. Cysteinyl leukotriene receptor antagonists decrease cancer risk in asthma patients. Sci. Rep. 2016, 6, 23979. [Google Scholar] [CrossRef] [PubMed]
- Shiao, T.H.; Liu, C.J.; Luo, J.C.; Su, K.C.; Chen, Y.M.; Chen, T.J.; Chou, K.T.; Shiao, G.M.; Lee, Y.C. Sleep apnea and risk of peptic ulcer bleeding: A nationwide population-based study. Am. J. Med. 2013, 126, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Chou, P.S.; Chang, W.C.; Chou, W.P.; Liu, M.E.; Lai, C.L.; Liu, C.K.; Ku, Y.C.; Tsai, S.J.; Chou, Y.H.; Chang, W.P. Increased risk of benign prostate hyperplasia in sleep apnea patients: A nationwide population-based study. PLoS One 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Su, V.Y.; Liu, C.J.; Wang, H.K.; Wu, L.A.; Chang, S.C.; Perng, D.W.; Su, W.J.; Chen, Y.M.; Lin, E.Y.; Chen, T.J.; et al. Sleep apnea and risk of pneumonia: A nationwide population-based study. CMAJ 2014, 186, 415–421. [Google Scholar] [CrossRef]
- Lee, Y.C.; Hung, S.Y.; Wang, H.K.; Lin, C.W.; Wang, H.H.; Chen, S.W.; Chang, M.Y.; Ho, L.C.; Chen, Y.T.; Liou, H.H.; et al. Sleep apnea and the risk of chronic kidney disease: A nationwide population-based cohort study. Sleep 2015, 38, 213–221. [Google Scholar] [CrossRef]
- Su, V.Y.; Chen, Y.T.; Lin, W.C.; Wu, L.A.; Chang, S.C.; Perng, D.W.; Su, W.J.; Chen, Y.M.; Chen, T.J.; Lee, Y.C.; et al. Sleep apnea and risk of panic disorder. Ann. Fam. Med. 2015, 13, 325–330. [Google Scholar] [CrossRef]
- Lin, C.S.; Chen, S.J.; Sung, C.C.; Lin, C.L.; Lin, S.H.; Cheng, S.M.; Wang, I.K.; Huang, W.S.; Kao, C.H. Hemodialysis is associated with increased peripheral artery occlusive disease risk among patients with end-stage renal disease: A nationwide population-based cohort study. Medicine 2015, 94. [Google Scholar] [CrossRef]
- Chuang, Y.W.; Yu, M.C.; Lin, C.L.; Yu, T.M.; Shu, K.H.; Huang, S.T.; Kao, C.H. Risk of peripheral arterial occlusive disease in patients with rheumatoid arthritis. A nationwide population-based cohort study. Thromb. Haemost. 2016, 115, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Deyo, R.A.; Cherkin, D.C.; Ciol, M.A. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J. Clin. Epidemiol. 1992, 45, 613–619. [Google Scholar] [CrossRef]
- Pizarro, C.; Schaefer, C.; Kimeu, I.; Pingel, S.; Horlbeck, F.; Tuleta, I.; Nickenig, G.; Skowasch, D. Underdiagnosis of obstructive sleep apnoea in peripheral arterial disease. Respiration 2015. [Google Scholar] [CrossRef]
- Schahab, N.; Sudan, S.; Schaefer, C.; Tiyerili, V.; Steinmetz, M.; Nickenig, G.; Skowasch, D.; Pizarro, C. Sleep apnoea is common in severe peripheral arterial disease. PLoS ONE 2017, 12, e0181733. [Google Scholar] [CrossRef] [PubMed]
- Nagayoshi, M.; Lutsey, P.L.; Benkeser, D.; Wassel, C.L.; Folsom, A.R.; Shahar, E.; Iso, H.; Allison, M.A.; Criqui, M.H.; Redline, S. Association of sleep apnea and sleep duration with peripheral artery disease: The Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis 2016, 251, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Somers, V.K.; White, D.P.; Amin, R.; Abraham, W.T.; Costa, F.; Culebras, A.; Daniels, S.; Floras, J.S.; Hunt, C.E.; Olson, L.J.; et al. Sleep apnea and cardiovascular disease: An American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation 2008, 118, 1080–1111. [Google Scholar] [PubMed]
- Hsu, P.C.; Lee, W.H.; Lee, H.C.; Tsai, W.C.; Chu, C.Y.; Chen, Y.C.; Lee, C.S.; Lin, T.H.; Voon, W.C.; Sheu, S.H.; et al. Association between modified CHA2DS2-VASc Score with Ankle-Brachial index <0.9. Sci. Rep. 2018, 8, 1175. [Google Scholar] [CrossRef]





| Study Arm A | Study Arm B | |||||
|---|---|---|---|---|---|---|
| Suspected SA | Control A | p-Value | Probable SA | Control B | p-Value | |
| n | 10,702 | 214,040 | 4242 | 84,840 | ||
| Sex, n (%) | ||||||
| Female | 3967 (37%) | 79,340 (37%) | 932 (22%) | 18,640 (22%) | ||
| Male | 6735 (63%) | 134,700 (63%) | 3310 (78%) | 66,200 (78%) | ||
| Age (year), mean ± SD | 47.6 ± 14.8 | 47.6 ± 14.8 | >0.99 | 47.5 ± 13.2 | 47.5 ± 13.2 | >0.99 |
| Age (year), n (%) | >0.99 | >0.99 | ||||
| ≤40 | 3668 (34%) | 73,360 (34%) | 1348 (32%) | 26,960 (32%) | ||
| 40 < age ≤ 50 | 2669 (25%) | 53,380 (25%) | 1190 (28%) | 23,800 (28%) | ||
| >50 | 4365 (41%) | 87,300 (41%) | 1704 (40%) | 34,080 (40%) | ||
| Residency | <0.0001 | <0.0001 | ||||
| Northern Taiwan | 4912 (46%) | 106,227 (50%) | 1825 (43%) | 41,908 (49%) | ||
| Other areas | 5790 (54%) | 107,813 (50%) | 2417 (57%) | 42,932 (51%) | ||
| Monthly income (NT$), n (%) | <0.0001 | <0.0001 | ||||
| ≤24,000 | 6140 (57%) | 135,967 (64%) | 2065 (49%) | 50,746 (60%) | ||
| >24,000 | 4562 (43%) | 78,073 (36%) | 2177 (51%) | 34,094 (40%) | ||
| CCI score, mean ± SD | 1.5 ± 1.9 | 0.9 ± 1.6 | <0.0001 | 1.5 ± 1.8 | 0.9 ± 1.5 | <0.0001 |
| CCI score, n (%) | <0.0001 | <0.0001 | ||||
| = 0 | 4203 (39%) | 124,704 (58%) | 1581 (37%) | 49,278 (58%) | ||
| = 1 | 2723 (25%) | 43,992 (21%) | 1121 (26%) | 17,941 (21%) | ||
| ≥2 | 3776 (35%) | 45,344 (21%) | 1540 (36%) | 17,621 (21%) | ||
| Underlying diseases, n (%) | ||||||
| Heart disease | 519 (5%) | 5293 (2%) | <0.0001 | 211 (5%) | 1819 (2%) | <0.0001 |
| Myocardial infarction | 138 (1%) | 1552 (1%) | <0.0001 | 63 (1%) | 613 (1%) | <0.0001 |
| Congestive heart failure | 416 (4%) | 4206 (2%) | <0.0001 | 165 (4%) | 1371 (2%) | <0.0001 |
| Peripheral vascular disease | 57 (1%) | 668 (0%) | <0.0001 | 21 (0%) | 279 (0%) | 0.0682 |
| Major neurological disorder | 1148 (11%) | 13,217 (6%) | <0.0001 | 496 (12%) | 4661 (5%) | <0.0001 |
| Cerebral vascular disease | 1087 (10%) | 12,379 (6%) | <0.0001 | 474 (11%) | 4404 (5%) | <0.0001 |
| Dementia | 118 (1%) | 1570 (1%) | <0.0001 | 46 (1%) | 423 (0%) | <0.0001 |
| Hemiplegia | 89 (1%) | 1590 (1%) | 0.2980 | 35 (1%) | 619 (1%) | 0.4772 |
| Chronic pulmonary disease | 2956 (28%) | 33,877 (16%) | <0.0001 | 1237 (29%) | 12,803 (15%) | <0.0001 |
| Connective tissue disease | 233 (2%) | 2656 (1%) | <0.0001 | 82 (2%) | 907 (1%) | <0.0001 |
| Peptic ulcer disease | 3387 (32%) | 41,937 (20%) | <0.0001 | 1354 (32%) | 16,613 (20%) | <0.0001 |
| Liver disease | 2302 (22%) | 26,786 (13%) | <0.0001 | 1003 (24%) | 11,462 (14%) | <0.0001 |
| Diabetes mellitus | 1387 (13%) | 20,632 (10%) | <0.0001 | 584 (14%) | 8118 (10%) | <0.0001 |
| Renal disease | 451 (4%) | 5501 (3%) | <0.0001 | 192 (5%) | 2079 (2%) | <0.0001 |
| Cancer | 578 (5%) | 7524 (4%) | <0.0001 | 191 (5%) | 2828 (3%) | <0.0001 |
| Study arm A Suspected SA | Study arm B Probable SA | |||
|---|---|---|---|---|
| HR (95%CI) | p-Value | HR (95%CI) | p-Value | |
| All patients | 0.1093 | 0.81 (0.65–1.03) | 0.90 (0.79–1.02) | 0.0838 |
| Stratified analyses | ||||
| Female | 0.96 (0.78–1.18) | 0.6916 | 0.90 (0.56–1.45) | 0.6602 |
| Male | 0.85 (0.72–1.01) | 0.0695 | 0.79 (0.61–1.04) | 0.0880 |
| Age ≤ 50 | 0.84 (0.65–1.08) | 0.1804 | 0.81 (0.54–1.23) | 0.3246 |
| Age > 50 | 0.89 (0.77–1.04) | 0.1598 | 0.81 (0.61–1.07) | 0.1337 |
| Score | Suspected SA | Probable SA | ||
|---|---|---|---|---|
| HR (95%CI) | p-Value | HR (95%CI) | p-Value | |
| CHADS2 | ||||
| 0 | 1.00 | 1.00 | ||
| 1–2 | 3.18 (2.32–4.36) | <0.0001 | 1.90 (1.12–3.23) | 0.0179 |
| ≥ 3 | 7.68 (5.47–10.77) | <0.0001 | 4.76 (2.68–8.45) | <0.0001 |
| CHA2DS2ASc | ||||
| 0 | 1.00 | 1.00 | ||
| 1-2 | 2.83 (1.83–4.37) | <0.0001 | 2.27 (1.21–4.28) | 0.0108 |
| 3-5 | 9.95 (6.45–15.35) | <0.0001 | 6.56 (3.45–12.48) | <0.0001 |
| ≥6 | 13.76 (7.60–24.92) | <0.0001 | 9.37 (3.07–28.57) | <0.0001 |
| Score | Suspected SA | Probable SA | ||
|---|---|---|---|---|
| HR (95%CI) | p-Value | HR (95%CI) | p-Value | |
| ABCD | ||||
| 0 | 1.00 | 1.00 | ||
| 1 | 1.11 (0.76–1.61) | 0.6027 | 1.69 (0.77–3.69) | 0.1927 |
| 2 | 2.07 (1.46–2.94) | <0.0001 | 3.46 (1.68–7.13) | 0.0008 |
| ≥3 | 3.93 (2.83–5.47) | <0.0001 | 8.57 (4.52–16.24) | <0.0001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, K.-L.; Kuo, C.-Y.; Tsai, Y.-C.; Hung, J.-Y.; Sheu, C.-C.; Yang, C.-J.; Hsu, C.-Y.; Wu, M.-N.; Tsai, M.-J. CHADS2, CHA2DS2ASc, and New ABCD Scores Predict the Risk of Peripheral Arterial Disease in Patients with Sleep Apnea. J. Clin. Med. 2019, 8, 188. https://doi.org/10.3390/jcm8020188
Wu K-L, Kuo C-Y, Tsai Y-C, Hung J-Y, Sheu C-C, Yang C-J, Hsu C-Y, Wu M-N, Tsai M-J. CHADS2, CHA2DS2ASc, and New ABCD Scores Predict the Risk of Peripheral Arterial Disease in Patients with Sleep Apnea. Journal of Clinical Medicine. 2019; 8(2):188. https://doi.org/10.3390/jcm8020188
Chicago/Turabian StyleWu, Kuan-Li, Chia-Yu Kuo, Yu-Chen Tsai, Jen-Yu Hung, Chau-Chyun Sheu, Chih-Jen Yang, Chung-Yao Hsu, Meng-Ni Wu, and Ming-Ju Tsai. 2019. "CHADS2, CHA2DS2ASc, and New ABCD Scores Predict the Risk of Peripheral Arterial Disease in Patients with Sleep Apnea" Journal of Clinical Medicine 8, no. 2: 188. https://doi.org/10.3390/jcm8020188
APA StyleWu, K.-L., Kuo, C.-Y., Tsai, Y.-C., Hung, J.-Y., Sheu, C.-C., Yang, C.-J., Hsu, C.-Y., Wu, M.-N., & Tsai, M.-J. (2019). CHADS2, CHA2DS2ASc, and New ABCD Scores Predict the Risk of Peripheral Arterial Disease in Patients with Sleep Apnea. Journal of Clinical Medicine, 8(2), 188. https://doi.org/10.3390/jcm8020188