Trastuzumab Induced Chemobrain, Atorvastatin Rescued Chemobrain with Enhanced Anticancer Effect and without Hair Loss-Side Effect
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
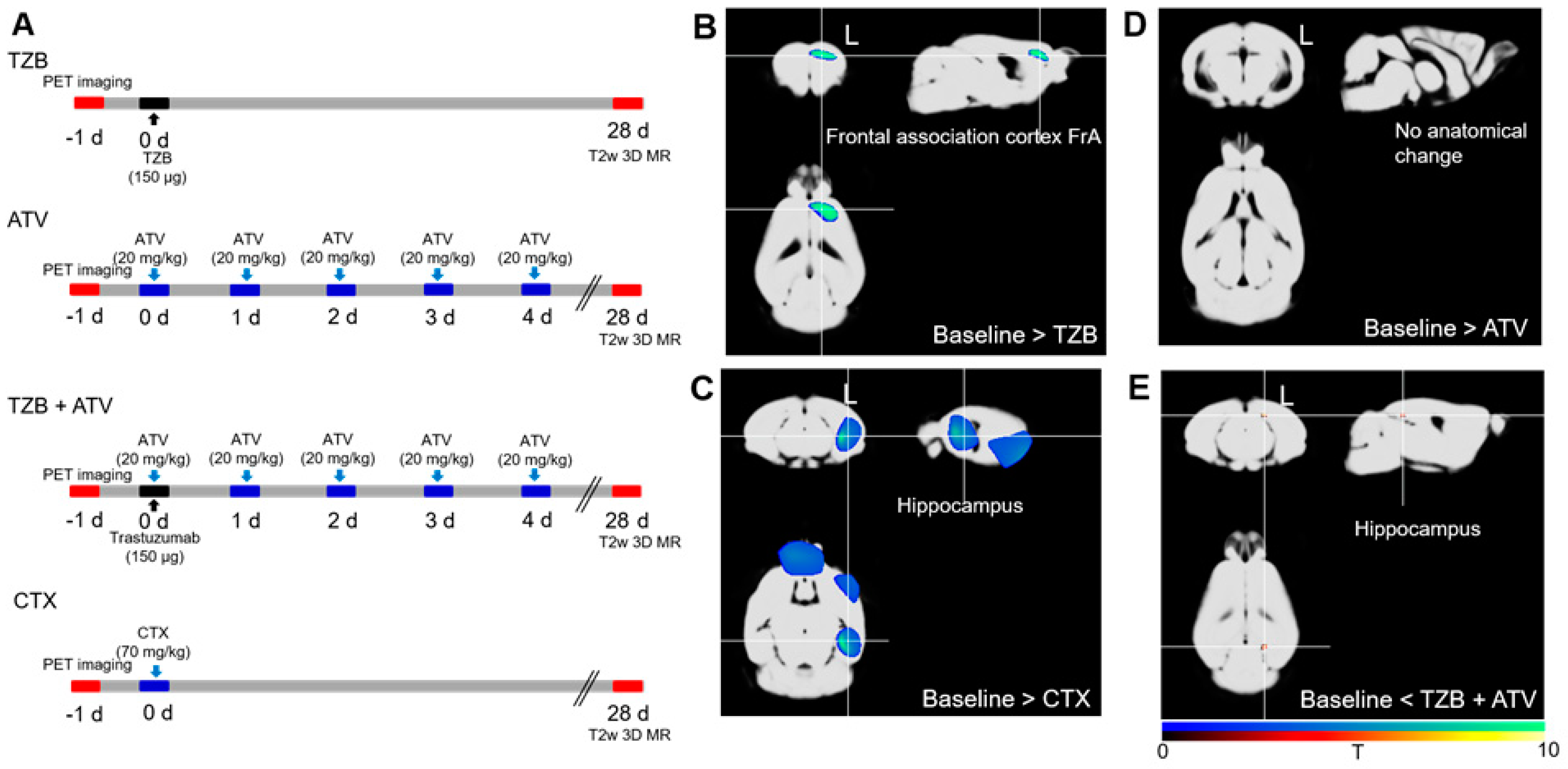
2.2. Assessment of Chemobrain Using PET SPM and MR VBM
2.2.1. Cognitive Deficit Model
2.2.2. PET Data Acquisition
2.2.3. Voxel-Based Statistical Analysis of PET Data
2.2.4. MR Data Acquisition
2.2.5. VBM Analysis of MR Data
2.3. Behavioral Analysis and Biomarker of Cognitive Deficit Model
2.3.1. Passive Avoidance Test
2.3.2. Quantitative Real-Time Reverse Transcription PCR (qPCR)
2.3.3. Assessment of Abnormal Hair Regrowth Using the Alopecia Model
2.4. Enhanced Anti-Cancer Effect of Trastuzumab after Administration of Atorvastatin
2.4.1. Cell Culture and Tumor Xenograft in Mice
2.4.2. Labelling and Purification of Alexa-488-TZB
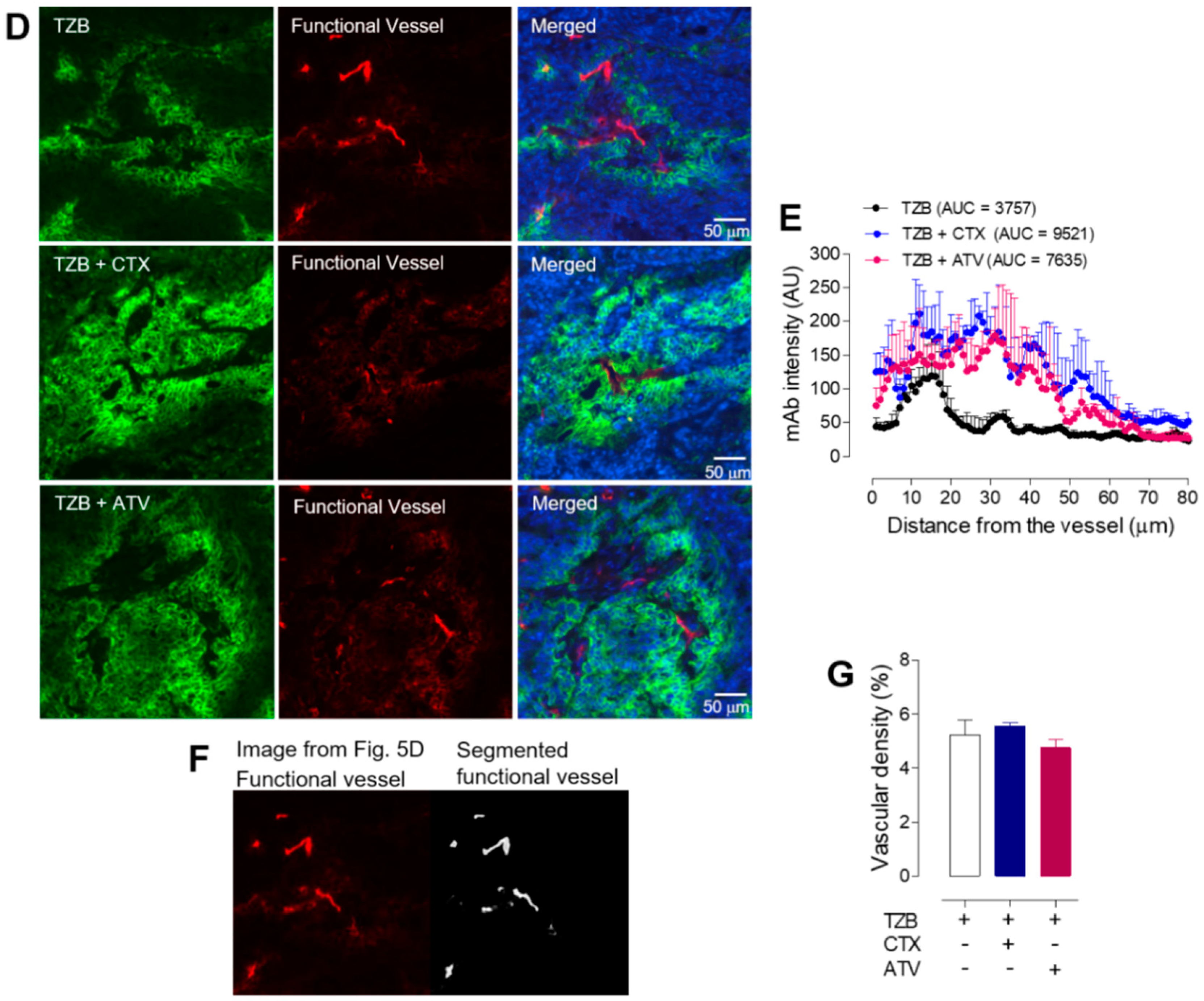
2.4.3. Immunofluorescence Staining of Tumor Tissues
2.4.4. Acquisition and Analysis of Fluorescent Images
2.4.5. Measurement of Alexa-488-TZB Accumulation in Tumor Tissues
2.4.6. Microvascular Analysis in Tumor Tissues
2.4.7. Radiomics Analysis of the Cell Nuclei in Tumor Tissues
2.5. Measurement of Tumor Growth after Co-Administration of ATV
Cell Viability Assay, Cell Lysate Analysis, and Tumor Growth Delay
2.6. Statistics
2.7. Study Approval
3. Results
3.1. Evidence of Statistical Parametric Mapping (SPM) of PET and Optimized Voxel Based Morphometry (VBM) of MR
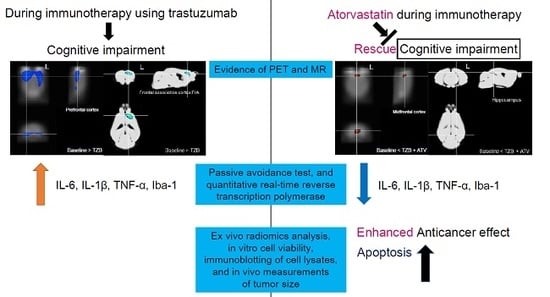
3.1.1. Trastuzumab Therapy Induced Chemo-Brain
3.1.2. Atorvastatin Has No Chemo-Brain Effect
3.1.3. Atorvastatin Rescued Cognitive Impairment during Trastuzumab Therapy
3.2. Evidence of Passive Avoidance Memory Test
3.2.1. Atorvastatin Rescued Cognitive Impairment during Trastuzumab Therapy
3.2.2. Evidence of IL-6, IL-1β, and TNF-α Levels
3.2.3. Atorvastatin Has No Abnormal Hair Regrowth Side Effect
3.2.4. Atorvastatin Enhanced Anti-Cancer Effect during Trastuzumab Therapy
4. Discussion
4.1. Atorvastatin Rescued Cognitive Impairment during Trastuzumab Therapy without Chemo-Brain and Hair-Loss Side Effect
4.2. Why Atorvastatin Therapy Could Rescue the Chemo-Brain Effect during Trastuzumab Therapy
4.3. Advantage As an Anticancer Drug of Atorvastatint during Trastuzumab Therapy
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boku, N. HER2-positive gastric cancer. Gastric Cancer 2014, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gunturu, K.S.; Woo, Y.; Beaubier, N.; Remotti, H.E.; Saif, M.W. Gastric cancer and trastuzumab: first biologic therapy in gastric cancer. Ther. Adv. Med. Oncol. 2013, 5, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Albanell, J. Mechanism of action of anti-HER2 monoclonal antibodies. Ann. Oncol. 2001, 12, S35–S41. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.J.; Hurvitz, S.A. Recent advances in the development of anti-HER2 antibodies and antibody-drug conjugates. Ann. Transl. Med. 2014, 2, 122. [Google Scholar] [CrossRef] [PubMed]
- Dokmanovic, M.; King, K.E.; Mohan, N.; Endo, Y.; Wu, W.J. Cardiotoxicity of ErbB2-targeted therapies and its impact on drug development, a spotlight on trastuzumab. Expert Opin. Drug Metab. Toxicol. 2017, 13, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994, 344, 1383–1389. [Google Scholar] [CrossRef]
- Shepherd, J.; Cobbe, S.M.; Ford, I.; Isles, C.G.; Lorimer, A.R.; MacFarlane, P.W.; McKillop, J.H.; Packard, C.J.; West of Scotland Coronary Prevention Study Group. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N. Engl. J. Med. 1995, 333, 1301–1307. [Google Scholar] [CrossRef]
- Sassano, A.; Platanias, L.C. Statins in tumor suppression. Cancer Lett. 2008, 260, 11–19. [Google Scholar] [CrossRef]
- Chan, K.K.; Oza, A.M.; Siu, L.L. The statins as anticancer agents. Clin. Cancer Res. 2003, 9, 10–19. [Google Scholar]
- Osmak, M. Statins and cancer: current and future prospects. Cancer Lett. 2012, 324, 1–12. [Google Scholar] [CrossRef]
- He, Z.; Mangala, L.S.; Theriot, C.A.; Rohde, L.H.; Wu, H.; Zhang, Y. Cell killing and radiosensitizing effects of atorvastatin in PC3 prostate cancer cells. J. Radiat. Res. 2012, 53, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Pan, Y.; Li, X.; Wang, X. Atorvastatin attenuates cognitive deficits through Akt1/caspase-3 signaling pathway in ischemic stroke. Brain Res. 2015, 10, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Martins, W.C.; dos Santos, V.V.; dos Santos, A.A.; Vandresen-Filho, S.; Dal-Cim, T.A.; de Oliveira, K.A.; Mendes-de-Aguiar, C.B.; Farina, M.; Prediger, R.D.; Viola, G.G.; et al. Atorvastatin prevents cognitive deficits induced by intracerebroventricular amyloid-β1-40 administration in mice: involvement of glutamatergic and antioxidant systems. Neurotox Res. 2015, 28, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.A.; Dager, A.D.; Panza, G.A.; Zaleski, A.L.; Meda, S.; Book, G.; Stevens, M.C.; Tartar, S.; White, C.M.; Polk, D.M.; et al. The effect of high-dose atorvastatin on neural activity and cognitive function. Am. Heart J. 2018, 197, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Ahles, T.A.; Root, J.C.; Ryan, E.L. Cancer- and cancer treatment-associated cognitive change: An update on the state of the science. J. Clin. Oncol. 2012, 30, 3675–3686. [Google Scholar] [CrossRef] [PubMed]
- Janelsins, M.C.; Kohli, S.; Mohile, S.G.; Usuki, K.; Ahles, T.A.; Morrow, G.R. An update on cancer- and chemotherapy-related cognitive dysfunction: current status. Semin. Oncol. 2011, 38, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Seretny, M.; Currie, G.L.; Sena, E.S.; Ramnarine, S.; Grant, R.; MacLeod, M.R.; Colvin, L.A.; Fallon, M. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain 2014, 155, 2461–2470. [Google Scholar] [CrossRef]
- Vichaya, E.G.; Chiu, G.S.; Krukowski, K.; Lacourt, T.E.; Kavelaars, A.; Dantzer, R.; Heijnen, C.J.; Walker, A.K. Mechanisms of chemotherapy-induced behavioral toxicities. Front. Neurosci. 2015, 9, 131. [Google Scholar] [CrossRef]
- Wang, X.M.; Walitt, B.; Saligan, L.; Tiwari, A.F.; Cheung, C.W.; Zhang, Z.J. Chemobrain: A critical review and causal hypothesis of link between cytokines and epigenetic reprogramming associated with chemotherapy. Cytokine 2015, 72, 86–96. [Google Scholar] [CrossRef]
- Lim, I.H.; Joung, H.Y.; Yu, A.R.; Shim, I.S.; Kim, J.S. PET evidence of the effect of donepezil on cognitive performance in an animal model of chemobrain. Biomed. Res. Int. 2016. [Google Scholar] [CrossRef]
- Bao, Q.; Newport, D.; Chen, M.; Stout, D.B.; Chatziioannou, A.F. Performance evaluation of the inveon dedicated PET preclinical tomograph based on the NEMA NU-4 standards. J. Nucl. Med. 2009, 50, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Lee, J.S.; Park, M.H.; Kang, H.; Lee, J.J.; Lee, H.J.; Im, K.C.; Moon, D.H.; Lim, S.M.; Oh, S.H.; et al. Assessment of cerebral glucose metabolism in cat deafness model: strategies for improving the voxel-based statistical analysis for animal PET studies. Mol. Imaging Biol. 2008, 10, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Good, C.D.; Johnsrude, I.S.; Ashburner, J.; Henson, R.N.; Friston, K.J.; Frackowiak, R.S. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage 2001, 14, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Shattuck, D.W.; Leahy, R.M. BrainSuite: an automated cortical surface identification tool. Med. Image Anal. 2002, 6, 129–142. [Google Scholar] [CrossRef]
- Delora, A.; Gonzales, A.; Medina, C.S.; Mitchell, A.; Mohed, A.F.; Jacobs, R.E.; Bearer, E.L. A simple rapid process for semi-automated brain extraction from magnetic resonance images of the whole mouse head. J. Neurosci. Methods 2016, 257, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Solmaz, V.; Delibas, D.; Inanir, S.; Erbas, O. Antipsychotic like effects of atorvastatin and melatonin in a psychosis model in rats. J. Mood 2015, 5, 120–125. [Google Scholar] [CrossRef]
- Hendrix, S.; Handjiski, B.; Peters, E.M.; Paus, R. A guide to assessing damage response pathways of the hair follicle: lessons from cyclophosphamide-induced alopecia in mice. J. Investig. Dermatol. 2005, 125, 42–51. [Google Scholar] [CrossRef]
- Huang, Y.A.; Kao, C.W.; Lie, K.K.; Huang, H.S.; Chiang, M.H.; Soo, C.R.; Chang, H.C.; Chiu, T.W.; Chao, J.I.; Eric Hwang, E. The effect of fluorescent nanodiamonds on neuronal survival and morphogenesis. Sci. Rep. 2014, 4, 6919. [Google Scholar] [CrossRef]
- Kabel, A.M.; Elkhoely, A.A. Targeting proinflammatory cytokines, oxidative stress, TGF-beta1 and STAT-3 by rosuvastatin and ubiquinone to ameliorate trastuzumab cardiotoxicity. Biomed. Pharmacother. 2017, 93, 17–26. [Google Scholar] [CrossRef]
- Coello, Y.; Richaud, S.; Magne, P.; Rossetti, Y. Vision for spatial perception and vision for action: a dissociation between the left-right and near-far dimensions. Neuropsychologia 2003, 41, 622–633. [Google Scholar] [CrossRef]
- Choi, E.K.; Kin, I.R.; Chang, O.; Kang, D.; Nam, S.J.; Lee, J.E.; Lee, S.K.; Im, Y.H.; Park, Y.H.; Yang, J.H.; et al. Impact of chemotherapy-induced alopecia distress on body image, psychosocial well-being, and depression in breast cancer patients. Psychooncology 2014, 23, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Belizário, J.E. Immunodeficient Mouse Models: An Overview. Open Immunol. J. 2009, 2, 79–85. [Google Scholar]
- Yang, Z.; Guo, L.; Liu, D.; Sun, L.; Chen, H.; Deng, Q.; Liu, Y.; Yu, M.; Ma, Y.; Guo, N.; et al. Acquisition of resistance to trastuzumab in gastric cancer cells is associated with activation of IL-6/STAT3/Jagged-1/Notch positive feedback loop. Oncotarget 2015, 6, 5072–5087. [Google Scholar] [CrossRef] [PubMed]
- Zang, C.; Lie, X.; Li, B.; He, Y.; Jing, S.; He, Y.; Wu, W.; Zhang, B.; Ma, S.; Dai, W.; et al. IL-6/STAT3/TWIST inhibition reverses ionizing radiation-induced EMT and radioresistance in esophageal squamous carcinoma. Oncotarget 2017, 8, 11228–11238. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S. Combination radioimmunotherapy approaches and quantification of immuno-PET. Nucl. Med. Mol. Imaging 2016, 50, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Trapero, I.; Cauli, O. Interleukin 6 and cognitive dysfunction. Metab. Brain Dis. 2014, 29, 593–608. [Google Scholar] [CrossRef] [PubMed]
- Aghevlian, S.; Boyle, A.J.; Reilly, R.M. Radioimmunotherapy of cancer with high linear energy transfer (LET) radiation delivered by radionuclides emitting alpha-particles or Auger electrons. Adv. Drug Deliv. Rev. 2017, 109, 102–118. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Lee, H.-J.; Kang, H.; Kim, E.-H.; Lim, Y.-C.; Park, H.; Lim, S.M.; Lee, Y.J.; Kim, J.M.; Kim, J.S. Trastuzumab Induced Chemobrain, Atorvastatin Rescued Chemobrain with Enhanced Anticancer Effect and without Hair Loss-Side Effect. J. Clin. Med. 2019, 8, 234. https://doi.org/10.3390/jcm8020234
Lee S, Lee H-J, Kang H, Kim E-H, Lim Y-C, Park H, Lim SM, Lee YJ, Kim JM, Kim JS. Trastuzumab Induced Chemobrain, Atorvastatin Rescued Chemobrain with Enhanced Anticancer Effect and without Hair Loss-Side Effect. Journal of Clinical Medicine. 2019; 8(2):234. https://doi.org/10.3390/jcm8020234
Chicago/Turabian StyleLee, Seonhwa, Hae-June Lee, Hyunji Kang, Eun-Ho Kim, Young-Cheol Lim, Hyejin Park, Sang Moo Lim, Yong Jin Lee, Jung Min Kim, and Jin Su Kim. 2019. "Trastuzumab Induced Chemobrain, Atorvastatin Rescued Chemobrain with Enhanced Anticancer Effect and without Hair Loss-Side Effect" Journal of Clinical Medicine 8, no. 2: 234. https://doi.org/10.3390/jcm8020234
APA StyleLee, S., Lee, H.-J., Kang, H., Kim, E.-H., Lim, Y.-C., Park, H., Lim, S. M., Lee, Y. J., Kim, J. M., & Kim, J. S. (2019). Trastuzumab Induced Chemobrain, Atorvastatin Rescued Chemobrain with Enhanced Anticancer Effect and without Hair Loss-Side Effect. Journal of Clinical Medicine, 8(2), 234. https://doi.org/10.3390/jcm8020234