Tortuosity of the Internal Carotid Artery and Its Clinical Significance in the Development of Aneurysms
Abstract
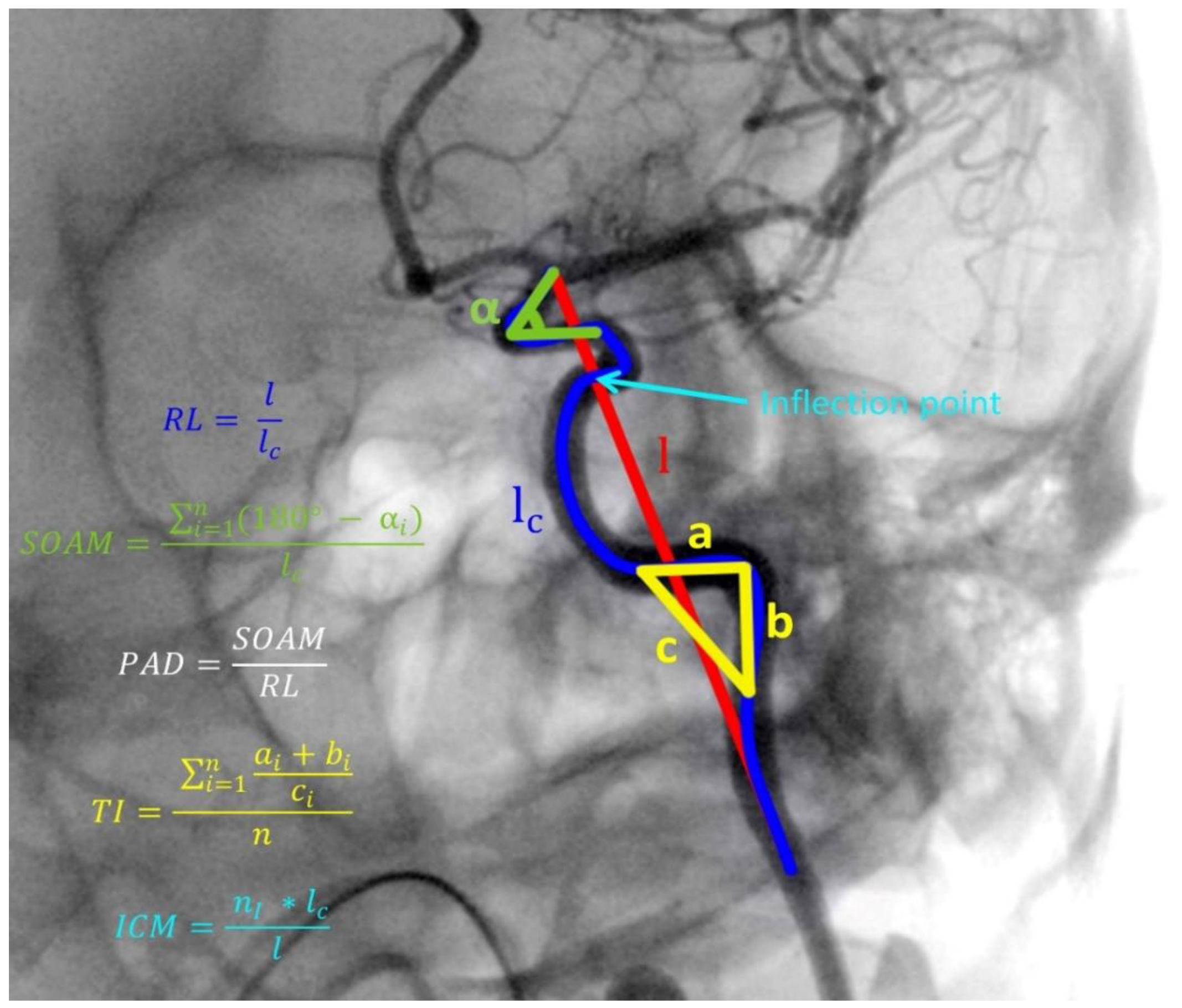
:1. Introduction
2. Experimental Section
3. Results
3.1. Study Group Characteristics
3.2. Risk Factors for Aneurysm Presence
3.3. Association of Risk Factors with Tortuosity
3.4. Additional Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Owen, C.G.; Newsom, R.S.B.; Rudnicka, A.R.; Barman, S.A.; Woodward, E.G.; Ellis, T.J. Diabetes and the tortuosity of vessels of the bulbar conjunctiva. Ophthalmology 2008, 6, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.D.; Martinez-Perez, E.; Jabbar, A.S.; Hassan, A.; Witt, N.W.; Mistry, P.D.; Chapman, N.; Stanton, A.V.; Beevers, G.; Pedrinelli, R.; et al. Quantification of topological changes in retinal vascular architecture in essential and malignant hypertension. J. Hypertens. 2006, 24, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shen, C.; Ji, Y.; Feng, Y.; Ma, G.; Liu, N. Clinical implication of coronary tortuosity in patients with coronary artery disease. PLoS One 2011, 6, 24232. [Google Scholar] [CrossRef] [PubMed]
- Sasongko, M.B.; Wong, T.Y.; Nguyen, T.T.; Cheung, C.Y.; Shaw, J.E.; Wang, J.J. Retinal vascular tortuosity in persons with diabetes and diabetic retinopathy. Diabetologia 2011, 54. [Google Scholar] [CrossRef] [PubMed]
- Amemiya, T.; Bhutto, I.A. Retinal vascular changes and systemic diseases: Corrosion cast demonstration. Ital. J. Anat. Embryol. 2001, 106, 237–244. [Google Scholar] [PubMed]
- Han, H.C. Twisted blood vessels: Symptoms, etiology and biomechanical mechanisms. J. Vasc. Res. 2012, 49, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Ruan, L.-T.; Duan, Y.-Y.; Cao, T.-S.; Zhuang, L.; Huang, L. Color and power Doppler sonography of extracranial and intracranial arteries in Moyamoya disease. J. Clin. Ultrasound 2006, 34, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Kim, S.M.; Kang, D.-W.; Kwon, S.U.; Suh, D.C.; Kim, J.S. Vascular tortuosity may be related to intracranial artery atherosclerosis. Int. J. Stroke 2015, 10, 1081–1086. [Google Scholar] [CrossRef]
- Spangler, K.M.; Challa, V.R.; Moody, D.M.; Bell, M.A. Arteriolar tortuosity of the white matter in aging and hypertension. A microradiographic study. J. Neuropathol. Exp. Neurol. 1994, 53, 22–26. [Google Scholar] [CrossRef]
- Dobrin, P.B.; Schwarcz, T.H.; Baker, W.H. Mechanisms of arterial and aneurysmal tortuosity. Surgery 1988, 104, 568–571. [Google Scholar] [PubMed]
- Lee, K.M.; Choi, S.Y.; Kim, M.U.; Lee, D.Y.; Kim, K.A.; Park, S. Effects of anatomical characteristics as factors in abdominal aortic aneurysm rupture: CT aortography analysis. Medicine (Baltimore). 2017, 96, 7236. [Google Scholar] [CrossRef] [PubMed]
- Majeski, J. Splenic artery tortuosity simulating a splenic artery aneurysm. South. Med. J. 1998, 91, 949–951. [Google Scholar] [CrossRef] [PubMed]
- Labeyrie, P.-E.; Braud, F.; Gakuba, C.; Gaberel, T.; Orset, C.; Goulay, R.; Emery, E.; Courthéoux, P.; Touzé, E. Cervical artery tortuosity is associated with intracranial aneurysm. Int. J. Stroke 2017, 12, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Lee, S.H.; Kwun, B.D.; Kang, H.G.; Hong, K.-S.; Kang, D.-W.; Kim, J.S.; Kwon, S.U. Intracranial aneurysm is associated with high intracranial artery tortuosity. World Neurosurg. 2018, 112, 876–880. [Google Scholar] [CrossRef] [PubMed]
- Kliś, K.M.; Krzyżewski, R.M.; Kwinta, B.M.; Stachura, K.; Moskała, M.; Tomaszewski, K.A. Computer-aided analysis of middle cerebral artery tortuosity: Association with aneurysm development. J. Neurosurg. 2017, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Virgilio, F.; Maurel, B.; Davis, M.; Hamilton, G.; Mastracci, T.M. Vertebral tortuosity index in patients with non-connective tissue disorder-related aneurysm disease. Eur. J. Vasc. Endovasc. Surg. 2017, 53, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Krzyżewski, R.M.; Kliś, K.M.; Kwinta, B.M.; Gackowska, M.; Stachura, K.; Starowicz-Filip, A.; Thompson, A.; Gąsowski, J. Analysis of anterior cerebral artery tortuosity: association with anterior communicating artery aneurysm rupture. World Neurosurg. 2018. [Google Scholar] [CrossRef] [PubMed]
- Rikhtegar, F.; Knight, J.A.; Olgac, U.; Saur, S.C.; Poulikakos, D.; Marshall, W.; Cattin, P.C.; Alkadhi, H.; Kurtcuoglu, V. Choosing the optimal wall shear parameter for the prediction of plaque location-A patient-specific computational study in human left coronary arteries. Atherosclerosis 2012, 221, 432–437. [Google Scholar] [CrossRef]
- Meng, H.; Tutino, V.M.; Xiang, J.; Siddiqui, A. High WSS or Low WSS? Complex interactions of hemodynamics with intracranial aneurysm initiation, growth, and rupture: toward a unifying hypothesis. Am. J. Neuroradiol. 2014, 35, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
- Hoi, Y.; Zhou, Y.-Q.; Zhang, X.; Henkelman, R.M.; Steinman, D.A. Correlation between local hemodynamics and lesion distribution in a novel aortic regurgitation murine model of atherosclerosis. Ann. Biomed. Eng. 2011, 39, 1414–1422. [Google Scholar] [CrossRef]
- Sugiyama, S.; Niizuma, K.; Nakayama, T.; Shimizu, H.; Endo, H.; Inoue, T.; Fujimura, M.; Ohta, M.; Takahashi, A.; Tominaga, T. Relative residence time prolongation in intracranial aneurysms: A possible association with atherosclerosis. Neurosurgery 2013, 73, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Q.; Han, H.-C. An in vivo rat model of artery buckling for studying wall remodeling. Ann. Biomed. Eng. 2014, 42, 1658–1667. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Lee, J.; Basu, R.; Sakamuri, S.S.V.P.; Wang, X.; Fan, D.; Kassiri, Z. Divergent roles of matrix metalloproteinase 2 in pathogenesis of thoracic aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 888–898. [Google Scholar] [CrossRef] [PubMed]
- Togay-Işikay, C.; Kim, J.; Betterman, K.; Andrews, C.; Meads, D.; Tesh, P.; Tegeler, C.; Oztuna, D. Carotid artery tortuosity, kinking, coiling: Stroke risk factor, marker, or curiosity? Acta Neurol. Belg. 2005, 105, 68–72. [Google Scholar] [PubMed]
- Chiha, J.; Mitchell, P.; Gopinath, B.; Burlutsky, G.; Kovoor, P.; Thiagalingam, A. Gender differences in the prevalence of coronary artery tortuosity and its association with coronary artery disease. IJC Hear. Vasc. 2017, 14, 23–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horikoshi, T.; Akiyama, I.; Yamagata, Z.; Sugita, M.; Nukui, H. Magnetic resonance angiographic evidence of sex-linked variations in the circle of willis and the occurrence of cerebral aneurysms. J. Neurosurg. 2002, 96, 697–703. [Google Scholar] [CrossRef]
- Lindekleiv, H.M.; Valen-Sendstad, K.; Morgan, M.K.; Mardal, K.-A.; Faulder, K.; Magnus, J.H.; Waterloo, K.; Romner, B.; Ingebrigtsen, T. Sex differences in intracranial arterial bifurcations. Gend. Med. 2010, 7, 149–155. [Google Scholar] [CrossRef]
- Hamdan, A.; Barnes, J.; Mitchell, P. Subarachnoid hemorrhage and the female sex: Analysis of risk factors, aneurysm characteristics, and outcomes. J. Neurosurg. 2014, 121, 1367–1373. [Google Scholar] [CrossRef]
- Krzyżewski, R.M.; Kliś, K.M.; Kucala, R.; Polak, J.; Kwinta, B.M.; Starowicz-Filip, A.; Stachura, K.; Piszczek, K.; Moskała, M.; Tomaszewski, K.A. Intracranial aneurysm distribution and characteristics according to gender. Br. J. Neurosurg. 2018, 32, 541–543. [Google Scholar] [CrossRef]
- Sehba, F.A.; Mostafa, G.; Knopman, J.; Friedrich, V.; Bederson, J.B. Acute alterations in microvascular basal lamina after subarachnoid hemorrhage. J. Neurosurg. 2004, 633–640. [Google Scholar] [CrossRef]
- Vikman, P.; Beg, S.; Khurana, T.; Hansen-Schwartz, J.; Edvinsson, L.; Edvinsson, L. Gene expression and molecular changes in cerebral arteries following subarachnoid hemorrhage in the rat. J. Neurosurg. 2006, 105, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Kylstra, J.A.; Wierzbicki, T.; Wolbarsht, M.L.; Landers, M.B.; Stefansson, E. The relationship between retinal vessel tortuosity, diameter, and transmural pressure. Graefes Arch. Clin. Exp. Ophthalmol. 1986, 224, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Jośko, J. Cerebral angiogenesis and expression of VEGF after subarachnoid hemorrhage (SAH) in rats. Brain Res. 2003, 981, 58–69. [Google Scholar] [CrossRef]
- Ostrowski, R.P.; Colohan, A.R.; Zhang, J.H. Molecular mechanisms of early brain injury after subarachnoid hemorrhage. Neurol. Res. 2006, 28, 399–414. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.; Ho, C.E.H.; Tan, Q.S.W.; Luu, C.D.; Moe, K.T.; Cheung, C.Y.; Wong, T.Y.; Barathi, V.A. Fluvastatin downregulates VEGF-A expression in TNF-α–induced retinal vessel tortuosity. Investig. Opthalmology Vis. Sci. 2011, 52, 7423–7431. [Google Scholar] [CrossRef]
- Lee, A.Y.; Sanyal, A.; Xiao, Y.; Shadfan, R.; Han, H.-C. Mechanical instability of normal and aneurysmal arteries. J. Biomech. 2014, 47, 3868–3875. [Google Scholar] [CrossRef] [Green Version]
- Baccin, C.E.; Krings, T.; Alvarez, H.; Ozanne, A.; Lasjaunias, P. Multiple mirror-like intracranial aneurysms. Report of a case and review of the literature. Acta Neurochir. 2006, 148, 1091–1095. [Google Scholar] [CrossRef]
- Hutchins, G.M.; Miner, M.M.; Bulkley, B.H. Tortuosity as an index of the age and diameter increase of coronary collateral vessels in patients after acute myocardial infarction. Am. J. Cardiol. 1978, 41, 210–215. [Google Scholar] [CrossRef]

| Variable | ICA Aneurysm (n = 149) | No ICA Aneurysm (n = 149) | p-Value |
|---|---|---|---|
| Female sex (%) | 83.89 (125) | 68.46 (102) | 0.002 |
| Age (years) ± SD | 57.49 ± 12 | 57.48 ± 13.32 | 0.993 |
| Risk Factors | |||
| Diabetes mellitus (%) | 9.40 (14) | 12.08 (18) | 0.454 |
| Smoking (%) | 12.75 (19) | 12.75 (19) | 0.999 |
| Hypertension (%) | 47.65 (71) | 47.65 (71) | 0.999 |
| Alcoholism (%) | 0 (0) | 4.70 (7) | 0.007 |
| Ischemic heart disease (%) | 1.34 (2) | 3.36 (5) | 0.251 |
| History of heart attack (%) | 0.67 (1) | 2.68 (4) | 0.176 |
| History of ischemic stroke (%) | 4.70 (7) | 14.09 (21) | 0.005 |
| History of subarachnoid hemorrhage (%) | 9.40 (14) | 2.68 (4) | 0.015 |
| Atrial fibrillation (%) | 1.34 (2) | 2.01 (3) | 0.652 |
| Lungs diseases (%) | 4.70 (7) | 4.03 (6) | 0.777 |
| Hyperthyroidism (%) | 2.68 (4) | 2.01 (3) | 0.702 |
| Hypothyroidism (%) | 3.36 (5) | 4.70 (7) | 0.556 |
| Hypercholesterolemia (%) | 5.37 (8) | 8.05 (12) | 0.354 |
| Current Medications | |||
| ASA (%) | 26.17 (39) | 12.08 (18) | 0.002 |
| β -blockers (%) | 14.09 (21) | 15.44 (23) | 0.744 |
| ACEI (%) | 18.12 (27) | 10.74 (16) | 0.070 |
| AT2-blockers (%) | 2.68 (4) | 0 (0) | 0.044 |
| Calcium channel blockers (%) | 6.04 (9) | 5.37 (8) | 0.803 |
| Diuretics (%) | 9.4 (14) | 8.05 (12) | 0.681 |
| Steroids (%) | 2.01 (3) | 0.67 (1) | 0.314 |
| Antidiabetic therapy (%) | 4.03 (6) | 2.68 (4) | 0.520 |
| Insulin (%) | 1.34 (2) | 2.68 (4) | 0.409 |
| Heparin (%) | 0.67 (1) | 0.67 (1) | 0.999 |
| Anticoagulants (%) | 5.37 (8) | 6.04 (9) | 0.803 |
| Nitrates (%) | 0.67 (1) | 0 (0) | 0.316 |
| Statins (%) | 8.72 (13) | 2.68 (4) | 0.025 |
| Artery Sizes | |||
| Mean ICA diameter ± SD (mm) | 4.01 ± 1.01 | 4.07 ± 1.18 | 0.617 |
| C6 segment diameter ± SD (mm) | 3.77 ± 0.88 | 3.99 ± 0.82 | 0.023 |
| C7 segment diameter ± SD (mm) | 2.88 ± 0.77 | 2.95 ± 0.67 | 0.392 |
| Tortuosity Descriptors | |||
| Relative Length ± SD | 0.46 ± 0.19 | 0.51 ± 0.17 | 0.023 |
| Sum of Angle Metrics ± SD | 0.39 ± 0.21 | 0.32 ± 0.21 | 0.003 |
| Product of Angle Distance ± SD | 0.38 ± 0.19 | 0.32 ± 0.21 | 0.011 |
| Triangular Index ± SD | 0.30 ± 0.11 | 0.27 ± 0.14 | 0.034 |
| Inflection Count Metric ± SD | 0.30 ± 0.16 | 0.22 ± 0.12 | <0.001 |
| Tortuosity Descriptor | Women (n = 227) | Men (n = 71) | p-Value |
|---|---|---|---|
| Relative Length ± SD | 0.49 ± 0.19 | 0.48 ± 0.18 | 0.812 |
| Sum of Angle Metrics ± SD | 0.37 ± 0.21 | 0.31 ± 0.21 | 0.028 |
| Product of Angle Distance ± SD | 0.37 ± 0.20 | 0.30 ± 0.20 | 0.016 |
| Triangular Index ± SD | 0.30 ± 0.13 | 0.25 ± 0.10 | 0.006 |
| Inflection Count Metric ± SD | 0.27 ± 0.15 | 0.23 ± 0.13 | 0.046 |
| Hypertension (n = 142) | No hypertension (n = 156) | ||
| Relative Length ± SD | 0.49 ± 0.18 | 0.48 ± 0.19 | 0.900 |
| Sum of Angle Metrics ± SD | 0.37 ± 0.22 | 0.35 ± 0.20 | 0.327 |
| Product of Angle Distance ± SD | 0.36 ± 0.21 | 0.35 ± 0.19 | 0.543 |
| Triangular Index ± SD | 0.30 ± 0.14 | 0.28 ± 0.11 | 0.210 |
| Inflection Count Metric ± SD | 0.27 ± 0.16 | 0.25 ± 0.13 | 0.204 |
| Diabetes Mellitus (n = 32) | No diabetes Mellitus (n = 266) | ||
| Relative Length ± SD | 0.47 ± 0.16 | 0.49 ± 0.19 | 0.573 |
| Sum of Angle Metrics ± SD | 0.35 ± 0.21 | 0.36 ± 0.21 | 0.811 |
| Product of Angle Distance ± SD | 0.35 ± 0.21 | 0.35 ± 0.20 | 0.953 |
| Triangular Index ± SD | 0.30 ± 0.17 | 0.28 ± 0.12 | 0.372 |
| Inflection Count Metric ± SD | 0.25 ± 0.13 | 0.26 ± 0.15 | 0.690 |
| Smoking (n = 38) | No Smoking (n = 260) | ||
| Relative Length ± SD | 0.51 ± 0.18 | 0.48 ± 0.19 | 0.312 |
| Sum of Angle Metrics ± SD | 0.37 ± 0.21 | 0.36 ± 0.21 | 0.758 |
| Product of Angle Distance ± SD | 0.38 ± 0.21 | 0.35 ± 0.20 | 0.379 |
| Triangular Index ± SD | 0.27 ± 0.11 | 0.29 ± 0.13 | 0.324 |
| Inflection Count Metric ± SD | 0.26 ± 0.13 | 0.26 ± 0.15 | 0.989 |
| Hypercholesterolemia (n = 20) | No Hypercholesterolemia (n = 278) | ||
| Relative Length ± SD | 0.52 ± 0.17 | 0.48 ± 0.19 | 0.403 |
| Sum of Angle Metrics ± SD | 0.31 ± 0.20 | 0.36 ± 0.21 | 0.268 |
| Product of Angle Distance ± SD | 0.31 ± 0.20 | 0.36 ± 0.20 | 0.336 |
| Triangular Index ± SD | 0.26 ± 0.11 | 0.29 ± 0.13 | 0.295 |
| Inflection Count Metric ± SD | 0.23 ± 0.11 | 0.26 ± 0.15 | 0.360 |
| History of Heart Attack (n = 5) | No History of Heart Attack (n = 293) | ||
| Relative Length ± SD | 0.48 ± 0.14 | 0.48 ± 0.19 | 0.964 |
| Sum of Angle Metrics ± SD | 0.40 ± 0.32 | 0.36 ± 0.21 | 0.658 |
| Product of Angle Distance ± SD | 0.43 ± 0.37 | 0.35 ± 0.20 | 0.379 |
| Triangular Index ± SD | 0.26 ± 0.08 | 0.29 ± 0.13 | 0.653 |
| Inflection Count Metric ± SD | 0.21 ± 0.07 | 0.26 ± 0.14 | 0.447 |
| History of Ischemic Stroke (n = 28) | No History of Ischemic Stroke (n = 270) | ||
| Relative Length ± SD | 0.48 ± 0.16 | 0.48 ± 0.19 | 0.883 |
| Sum of Angle Metrics ± SD | 0.36 ± 0.22 | 0.36 ± 0.21 | 0.999 |
| Product of Angle Distance ± SD | 0.33 ± 0.23 | 0.36 ± 0.20 | 0.545 |
| Triangular Index ± SD | 0.30 ± 0.19 | 0.29 ± 0.12 | 0.582 |
| Inflection Count Metric ± SD | 0.25 ± 0.14 | 0.26 ± 0.14 | 0.724 |
| History of Subarachnoid Hemorrhage (n = 18) | No history of Subarachnoid Hemorrhage (n = 280) | ||
| Relative Length ± SD | 0.53 ± 0.18 | 0.48 ± 0.19 | 0.284 |
| Sum of Angle Metrics ± SD | 0.42 ± 0.22 | 0.35 ± 0.21 | 0.176 |
| Product of Angle Distance ± SD | 0.46 ± 0.22 | 0.35 ± 0.20 | 0.024 |
| Triangular Index ± SD | 0.30 ± 0.08 | 0.29 ± 0.13 | 0.733 |
| Inflection Count Metric ± SD | 0.25 ± 0.13 | 0.26 ± 0.15 | 0.828 |
| Variable | RL | SOAM | PAD | TI | ICM |
|---|---|---|---|---|---|
| Age (years) | 0.053 | 0.016 | 0.002 | 0.089 | 0.024 |
| p-Value | 0.371 | 0.791 | 0.973 | 0.131 | 0.682 |
| C6 segment diameter (mm) | −0.040 | −0.070 | −0.084 | −0.082 | −0.016 |
| p-Value | 0.498 | 0.232 | 0.155 | 0.162 | 0.787 |
| C7 segment diameter (mm) | −0.026 | 0.024 | 0.025 | 0.045 | 0.068 |
| p-Value | 0.659 | 0.683 | 0.668 | 0.444 | 0.245 |
| Mean ICA diameter (mm) | −0.187 | −0.034 | −0.057 | 0.072 | 0.091 |
| p-Value | 0.001 | 0.562 | 0.335 | 0.219 | 0.123 |
| MCA diameter (mm) | 0.082 | −0.003 | 0.024 | −0.031 | −0.013 |
| p-Value | 0.162 | 0.965 | 0.680 | 0.596 | 0.827 |
| ACA diameter (mm) | 0.024 | 0.026 | 0.060 | −0.028 | 0.034 |
| p-Value | 0.681 | 0.663 | 0.309 | 0.635 | 0.568 |
| Time after SAH (months) | −0.437 | 0.447 | 0.229 | 0.534 | 0.773 |
| p-Value | 0.118 | 0.109 | 0.432 | 0.049 | 0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kliś, K.M.; Krzyżewski, R.M.; Kwinta, B.M.; Stachura, K.; Gąsowski, J. Tortuosity of the Internal Carotid Artery and Its Clinical Significance in the Development of Aneurysms. J. Clin. Med. 2019, 8, 237. https://doi.org/10.3390/jcm8020237
Kliś KM, Krzyżewski RM, Kwinta BM, Stachura K, Gąsowski J. Tortuosity of the Internal Carotid Artery and Its Clinical Significance in the Development of Aneurysms. Journal of Clinical Medicine. 2019; 8(2):237. https://doi.org/10.3390/jcm8020237
Chicago/Turabian StyleKliś, Kornelia M., Roger M. Krzyżewski, Borys M. Kwinta, Krzysztof Stachura, and Jerzy Gąsowski. 2019. "Tortuosity of the Internal Carotid Artery and Its Clinical Significance in the Development of Aneurysms" Journal of Clinical Medicine 8, no. 2: 237. https://doi.org/10.3390/jcm8020237
APA StyleKliś, K. M., Krzyżewski, R. M., Kwinta, B. M., Stachura, K., & Gąsowski, J. (2019). Tortuosity of the Internal Carotid Artery and Its Clinical Significance in the Development of Aneurysms. Journal of Clinical Medicine, 8(2), 237. https://doi.org/10.3390/jcm8020237





