Cell-Free DNA and CXCL10 Derived from Bronchoalveolar Lavage Predict Lung Transplant Survival
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population and Samples
2.3. Biomarker Measurement in BAL Samples
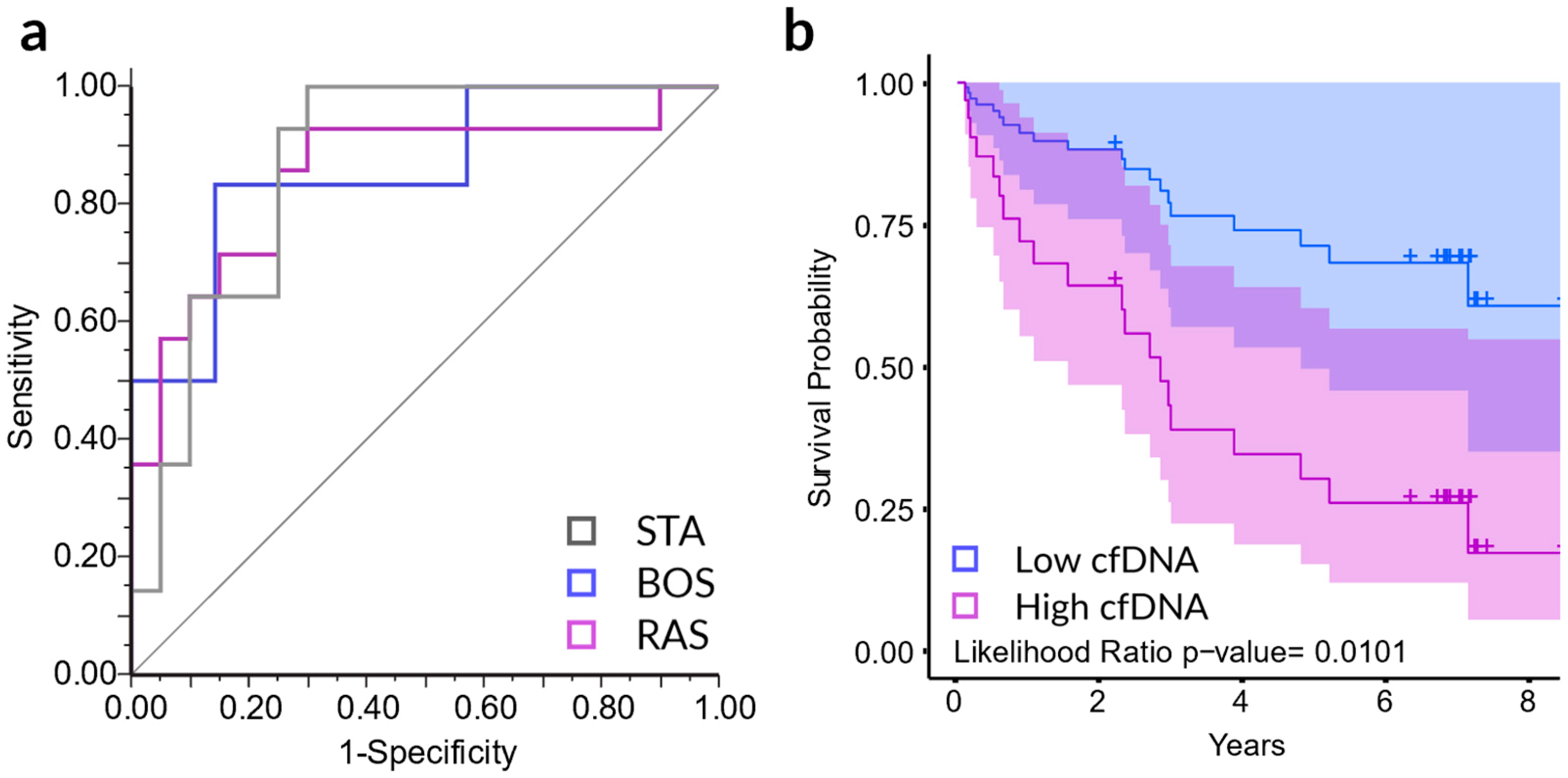
2.4. Statistical Analyses of cfDNA and CXCL10 with CLAD Phenotype and Overall Survival
3. Results
3.1. Patients and BAL Samples
3.2. Traditional Biomarkers in Lung Transplant Recipients
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Verleden, G.M.; Raghu, G.; Meyer, K.C.; Glanville, A.R.; Corris, P. A new classification system for chronic lung allograft dysfunction. J. Heart Lung Transplant. 2014, 33, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Yusen, R.D.; Edwards, L.B.; Dipchand, A.I.; Goldfarb, S.B.; Kucheryavaya, A.Y.; Levvey, B.J.; Lund, L.H.; Meiser, B.; Rossano, J.W.; Stehlik, J. The Registry of the International Society for Heart and Lung Transplantation: Thirty-third adult lung and heart-lung transplant report-2016; Focus theme: Primary diagnostic indications for transplant. J. Heart Lung Transplant. 2016, 35, 1170–1184. [Google Scholar] [CrossRef] [PubMed]
- Kramer, M.R.; Stoehr, C.; Whang, J.L.; Berry, G.J.; Sibley, R.; Marshall, S.E.; Patterson, G.M.; Starnes, V.A.; Theodore, J. The diagnosis of obliterative bronchiolitis after heart-lung and lung transplantation: Low yield of transbronchial lung biopsy. J. Heart Lung Transplant. 1993, 12, 675–681. [Google Scholar] [PubMed]
- Benzimra, M.; Calligaro, G.L.; Glanville, A.R. Acute rejection. J. Thorac. Dis. 2017, 9, 5440–5457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Husain, S.; Resende, M.R.; Rajwans, N.; Zamel, R.; Pilewski, J.M.; Crespo, M.M.; Singer, L.G.; McCurry, K.R.; Kolls, J.K.; Keshavjee, S.; et al. Elevated CXCL10 (IP-10) in bronchoalveolar lavage fluid is associated with acute cellular rejection after human lung transplantation. Transplantation 2014, 97, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Greenland, J.R.; Jewell, N.P.; Gottschall, M.; Trivedi, N.N.; Kukreja, J.; Hays, S.R.; Singer, J.P.; Golden, J.A.; Caughey, G.H. Bronchoalveolar lavage cell immunophenotyping facilitates diagnosis of lung allograft rejection. Am. J. Transplant. 2014, 14, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Sacreas, A.; Yang, J.Y.C.; Vanaudenaerde, B.M.; Sigdel, T.K.; Liberto, J.M.; Damm, I.; Verleden, G.M.; Vos, R.; Verleden, E.; Sarwal, M.M. The common rejection module in chronic rejection post lung transplantation. PLoS ONE 2018, 13, e0205107. [Google Scholar] [CrossRef]
- Verleden, S.E.; Vos, R.; Vanaudenaerde, B.M.; Verleden, G.M. Chronic lung allograft dysfunction phenotypes and treatment. J. Thorac. Dis. 2017, 9, 2650–2659. [Google Scholar] [CrossRef] [Green Version]
- Khatri, P.; Roedder, S.; Kimura, N.; De Vusser, K.; Morgan, A.A.; Gong, Y.; Fischbein, M.P.; Robbins, R.C.; Naesens, M.; Butte, A.J.; et al. A common rejection module (CRM) for acute rejection across multiple organs identifies novel therapeutics for organ transplantation. J. Exp. Med. 2013, 210, 2205–2221. [Google Scholar] [CrossRef] [Green Version]
- Schaub, S.; Nickerson, P.; Rush, D.; Mayr, M.; Hess, C.; Golian, M.; Stefura, W.; Hayglass, K. Urinary CXCL9 and CXCL10 levels correlate with the extent of subclinical tubulitis. Am. J. Transplant. 2009, 9, 1347–1353. [Google Scholar] [CrossRef]
- Yang, J.Y.C.; Sarwal, M.M. Transplant genetics and genomics. Nat. Rev. Genet. 2017, 18, 309–326. [Google Scholar] [CrossRef] [PubMed]
- Snyder, T.M.; Khush, K.K.; Valantine, H.A.; Quake, S.R. Universal noninvasive detection of solid organ transplant rejection. Proc. Natl. Acad. Sci. USA 2011, 108, 6229–6234. [Google Scholar] [CrossRef] [Green Version]
- Sigdel, T.; Archila, F.; Constantin, T.; Prins, S.; Liberto, J.; Damm, I.; Towfighi, P.; Navarro, S.; Kirkizlar, E.; Demko, Z.; et al. Optimizing detection of kidney transplant injury by assessment of donor-derived cell-free DNA via massively multiplex PCR. J. Clin. Med. 2018, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Sigdel, T.K.; Vitalone, M.J.; Tran, T.Q.; Dai, H.; Hsieh, S.-C.; Salvatierra, O.; Sarwal, M.M. A rapid noninvasive assay for the detection of renal transplant injury. Transplantation 2013, 96, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Anker, P.; Mulcahy, H.; Chen, X.Q.; Stroun, M. Detection of circulating tumour DNA in the blood (plasma/serum) of cancer patients. Cancer Metastasis Rev. 1999, 18, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, A.; Zangemeister-Wittke, U.; Stahel, R.A. Circulating DNA: A new diagnostic gold mine? Cancer Treat. Rev. 2002, 28, 255–271. [Google Scholar] [CrossRef]
- De Vlaminck, I.; Martin, L.; Kertesz, M.; Patel, K.; Kowarsky, M.; Strehl, C.; Cohen, G.; Luikart, H.; Neff, N.F.; Okamoto, J.; et al. Noninvasive monitoring of infection and rejection after lung transplantation. Proc. Natl. Acad. Sci. USA 2015, 112, 13336–13341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, J.; Duffy, B.; Slade, M.; Young, A.L.; Steward, N.; Hachem, R.; Mohanakumar, T. Rapid detection of donor cell free DNA in lung transplant recipients with rejections using donor-recipient HLA mismatch. Hum. Immunol. 2017, 78, 342–349. [Google Scholar] [PubMed] [Green Version]
- Agbor-Enoh, S.; Jackson, A.M.; Tunc, I.; Berry, G.J.; Cochrane, A.; Grimm, D.; Davis, A.; Shah, P.; Brown, A.W.; Wang, Y.; et al. Late manifestation of alloantibody-associated injury and clinical pulmonary antibody-mediated rejection: Evidence from cell-free DNA analysis. J. Hear. Lung Transplant. 2018, 37, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.C.; Raghu, G.; Verleden, G.M.; Corris, P.A.; Aurora, P.; Wilson, K.C.; Brozek, J.; Glanville, A.R. An international ISHLT/ATS/ERS clinical practice guideline: Diagnosis and management of bronchiolitis obliterans syndrome. Eur. Respir. J. 2014, 44, 1479–1503. [Google Scholar] [CrossRef]
- Vos, R.; Verleden, S.E.; Verleden, G.M. Chronic lung allograft dysfunction: Evolving practice. Curr. Opin. Organ Transplant. 2015, 20, 483–491. [Google Scholar]
- Tukey, J.W. Comparing individual means in the analysis of variance. Biometric 1949, 5, 99–114. [Google Scholar] [CrossRef]
- Kennedy, V.E.; Todd, J.L.; Palmer, S.M. Bronchoalveolar lavage as a tool to predict, diagnose and understand bronchiolitis obliterans syndrome. Am. J. Transplant 2013, 13, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Thabut, G.; Mal, H. Outcomes after lung transplantation. J. Thorac. Dis. 2017, 9, 2684–2691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hart, A.; Smith, J.M.; Skeans, M.A.; Gustafson, S.K.; Wilk, A.R.; Robinson, A.; Wainright, J.L.; Haynes, C.R.; Snyder, J.J.; Kasiske, B.L.; et al. OPTN/SRTR 2016 annual data report: Kidney. Am. J. Transplant 2018, 18 (Suppl. 1), 18–113. [Google Scholar] [CrossRef]
- Lund, L.H.; Khush, K.K.; Cherikh, W.S.; Goldfarb, S.; Kucheryavaya, A.Y.; Levvey, B.J.; Meiser, B.; Rossano, J.W.; Chambers, D.C.; Yusen, R.D.; et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-fourth adult heart transplantation report-2017; Focus theme: Allograft ischemic time. J. Heart Lung Transplant. 2017, 36, 1037–1046. [Google Scholar] [PubMed]
- Suwara, M.I.; Vanaudenaerde, B.M.; Verleden, S.E.; Vos, R.; Green, N.J.; Ward, C.; Borthwick, L.A.; Vandermeulen, E.; Lordan, J.; van Raemdonck, D.E.; et al. Mechanistic differences between phenotypes of chronic lung allograft dysfunction after lung transplantation. Transpl. Int. 2014, 27, 857–867. [Google Scholar] [CrossRef] [Green Version]
- Verleden, S.E.; Ruttens, D.; Vos, R.; Vandermeulen, E.; Moelants, E.; Mortier, A.; van Raemdonck, D.E.; Proost, P.; Schols, D.; Verleden, G.M.; et al. Differential cytokine, chemokine and growth factor expression in phenotypes of chronic lung allograft dysfunction. Transplantation 2015, 99, 86–93. [Google Scholar] [CrossRef]
- Berastegui, C.; Gómez-Ollés, S.; Sánchez-Vidaurre, S.; Culebras, M.; Monforte, V.; López-Meseguer, M.; Bravo, C.; Ramon, M.-A.; Romero, L.; Sole, J.; et al. BALF cytokines in different phenotypes of chronic lung allograft dysfunction in lung transplant patients. Clin. Transplant. 2017, 31, e12898. [Google Scholar] [CrossRef]
- Shino, M.Y.; Weigt, S.S.; Li, N.; Palchevskiy, V.; Derhovanessian, A.; Saggar, R.; Sayah, D.M.; Gregson, A.L.; Fishbein, M.C.; Ardehali, A.; et al. CXCR3 ligands are associated with the continuum of diffuse alveolar damage to chronic lung allograft dysfunction. Am. J. Respir. Crit. Care Med. 2013, 188, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Shino, M.Y.; Weigt, S.S.; Li, N.; Palchevskiy, V.; Derhovanessian, A.; Saggar, R.; Sayah, D.M.; Huynh, R.H.; Gregson, A.L.; Fishbein, M.C.; et al. The prognostic importance of CXCR3 chemokine during organizing pneumonia on the risk of chronic lung allograft dysfunction after lung transplantation. PLoS ONE 2017, 12, e0180281. [Google Scholar] [CrossRef] [PubMed]
- Schnerch, J.; Prasse, A.; Vlachakis, D.; Schuchardt, K.L.; Pechkovsky, D.V.; Goldmann, T.; Gaede, K.I.; Müller-Quernheim, J.; Zissel, G. Functional Toll-Like Receptor 9 expression and CXCR3 ligand release in pulmonary sarcoidosis. Am. J. Respir. Cell Mol. Biol. 2016, 55, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, A.; Kuba, K.; Morita, M.; Chida, S.; Tezuka, H.; Hara, H.; Sasaki, T.; Ohteki, T.; Ranieri, V.M.; dos Santos, C.C.; et al. CXCL10-CXCR3 enhances the development of neutrophil-mediated fulminant lung injury of viral and nonviral origin. Am. J. Respir. Crit. Care Med. 2013, 187, 65–77. [Google Scholar] [CrossRef] [PubMed]

| Phenotype Characteristic | Stable (20 samples) | BOS (20 samples) | RAS (20 samples) |
|---|---|---|---|
| Recipient | |||
| • Recipient age, year (SD) | 46 (14) | 51 (15) | 44 (16) |
| • Recipient male/female, % | 35 | 40 | 50 |
| Donor | |||
| • Donor age, year (SD) | 39 (15) | 47 (14) | 46 (13) |
| • Donor male/female, % | 40 | 45 | 50 |
| Indication for lung transplantation, % | |||
| • Emphysema | 35 | 35 | 5 |
| • COPD | 0 | 15 | 40 |
| • Cystic fibrosis | 30 | 15 | 25 |
| • Pulmonary Fibrosis | 20 | 5 | 10 |
| • PHT b | 0 | 0 | 10 |
| • PPH b | 5 | 5 | 0 |
| • Other c | 10 | 25 | 10 |
| Spirometry | |||
| • TLCO | 5.25 (1.49) | 5.08 (2.18) | 3.61 (1.56) |
| • PEF | 7.75 (2.15) | 5.51 (2.06) | 5.01 (1.74) |
| • FEF (25–75) | 2.79 (1.36) | 1.04 (0.83) | 1.17 (0.92) |
| • MIF | 4.55 (1.90) | 3.91 (1.08) | 3.60 (1.26) |
| • FEV1/FVC ratio, % | 82 (9) | 58 (13) | 69 (17) |
| Overall survival, days | 2569 (215) | 1009 (526) | 434 (376) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.Y.C.; Verleden, S.E.; Zarinsefat, A.; Vanaudenaerde, B.M.; Vos, R.; Verleden, G.M.; Sarwal, R.D.; Sigdel, T.K.; Liberto, J.M.; Damm, I.; et al. Cell-Free DNA and CXCL10 Derived from Bronchoalveolar Lavage Predict Lung Transplant Survival. J. Clin. Med. 2019, 8, 241. https://doi.org/10.3390/jcm8020241
Yang JYC, Verleden SE, Zarinsefat A, Vanaudenaerde BM, Vos R, Verleden GM, Sarwal RD, Sigdel TK, Liberto JM, Damm I, et al. Cell-Free DNA and CXCL10 Derived from Bronchoalveolar Lavage Predict Lung Transplant Survival. Journal of Clinical Medicine. 2019; 8(2):241. https://doi.org/10.3390/jcm8020241
Chicago/Turabian StyleYang, Joshua Y.C., Stijn E. Verleden, Arya Zarinsefat, Bart M. Vanaudenaerde, Robin Vos, Geert M. Verleden, Reuben D. Sarwal, Tara K. Sigdel, Juliane M. Liberto, Izabella Damm, and et al. 2019. "Cell-Free DNA and CXCL10 Derived from Bronchoalveolar Lavage Predict Lung Transplant Survival" Journal of Clinical Medicine 8, no. 2: 241. https://doi.org/10.3390/jcm8020241
APA StyleYang, J. Y. C., Verleden, S. E., Zarinsefat, A., Vanaudenaerde, B. M., Vos, R., Verleden, G. M., Sarwal, R. D., Sigdel, T. K., Liberto, J. M., Damm, I., Watson, D., & Sarwal, M. M. (2019). Cell-Free DNA and CXCL10 Derived from Bronchoalveolar Lavage Predict Lung Transplant Survival. Journal of Clinical Medicine, 8(2), 241. https://doi.org/10.3390/jcm8020241






