Clinical Features, Survival and Prognostic Factors of Glycogen-Rich Clear Cell Carcinoma (GRCC) of the Breast in the U.S. Population
Abstract
:1. Introduction
2. Methods
2.1. Data Source
2.2. Sample Selection and Coding
2.3. Statistical Analysis
3. Results
3.1. Demographics and Clinical Characteristics
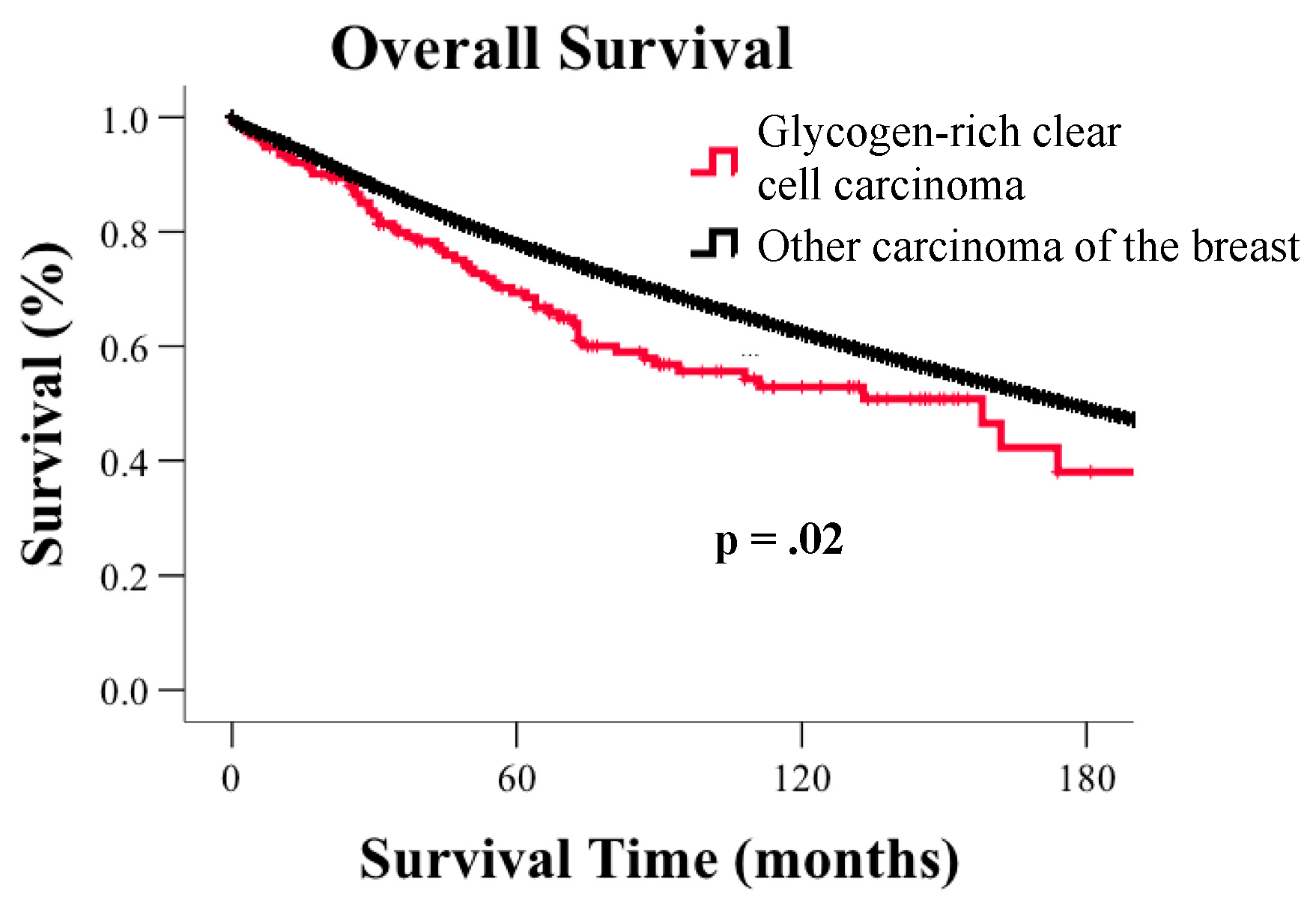
3.2. Survival
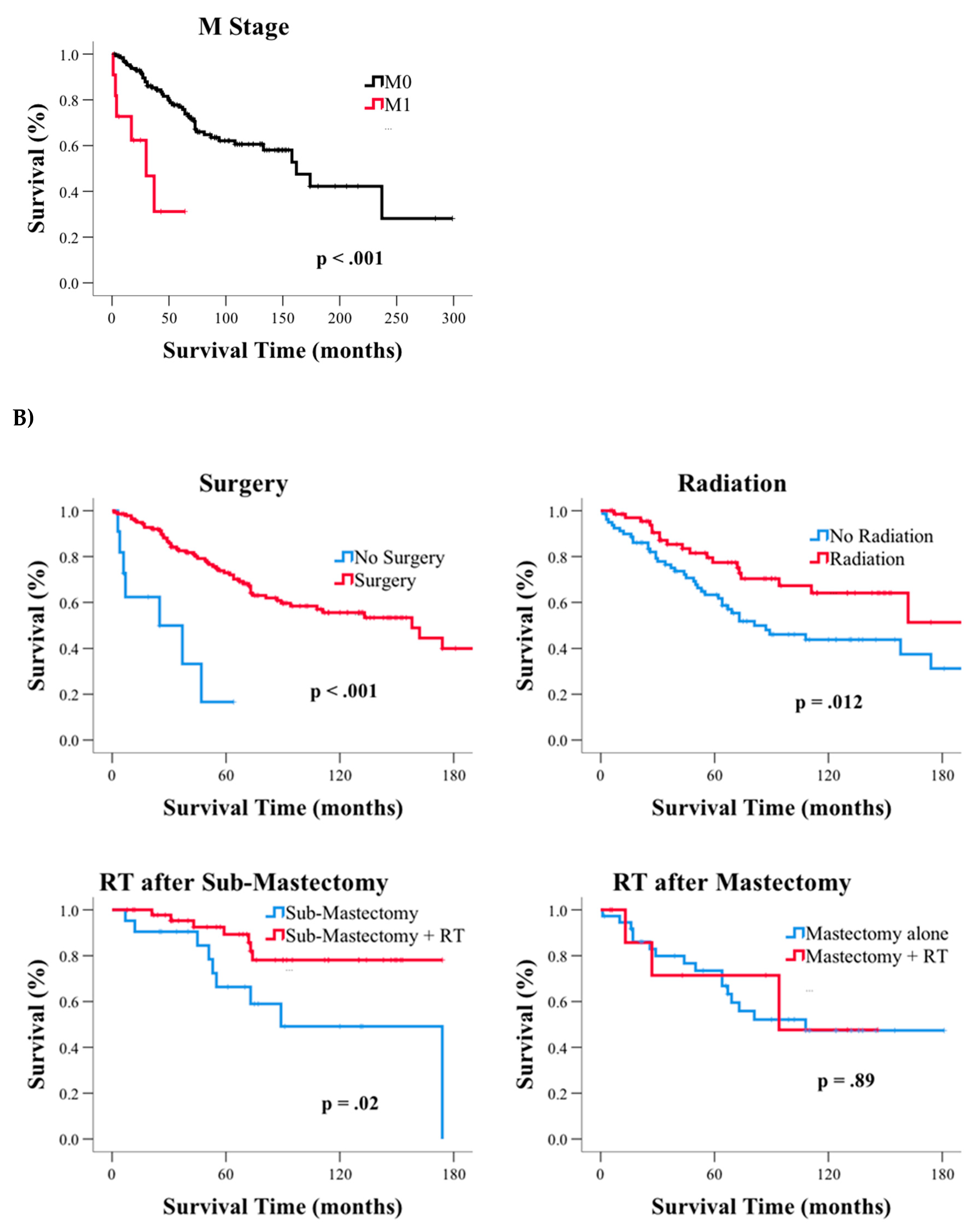
3.3. Subgroup Analysis of GR and CC Carcinoma of the Breast
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Agius, L. Glucokinase and molecular aspects of liver glycogen metabolism. Biochem. J. 2008, 414, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Adeva-Andany, M.M.; Gonzalez-Lucan, M.; Donapetry-Garcia, C.; Fernandez-Fernandez, C.; Ameneiros-Rodriguez, E. Glycogen metabolism in humans. BBA Clin. 2016, 5, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Roach, P.J.; Depaoli-Roach, A.A.; Hurley, T.D.; Tagliabracci, V.S. Glycogen and its metabolism: Some new developments and old themes. Biochem. J. 2012, 441, 763–787. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.D. A microchemical reaction resulting in the staining of polysaccharide structures in fixed tissue preparations. Arch. Biochem. 1948, 16, 131–141. [Google Scholar] [PubMed]
- Crignis, G.S.; Abreu, L.; Bucard, A.M.; Barcaui, C.B. Polarized dermoscopy of mammary Paget disease. An. Bras. Dermatol. 2013, 88, 290–292. [Google Scholar] [CrossRef] [PubMed]
- Warnock, M.L.; Stoloff, A.; Thor, A. Differentiation of adenocarcinoma of the lung from mesothelioma. Periodic acid-Schiff, monoclonal antibodies B72.3, and Leu M1. Am. J. Pathol. 1988, 133, 30–38. [Google Scholar]
- Rousset, M.; Zweibaum, A.; Fogh, J. Presence of glycogen and growth-related variations in 58 cultured human tumor cell lines of various tissue origins. Cancer Res. 1981, 41, 1165–1170. [Google Scholar] [PubMed]
- Rousset, M.; Chevalier, G.; Rousset, J.-P.; Dussaulx, E.; Zweibaum, A. Presence and cell growth-related variations of glycogen in human colorectal adenocarcinoma cell lines in culture. Cancer Res. 1979, 39, 531–534. [Google Scholar]
- Rousset, M.; Chevalier, G.; Rousset, J.-P.; Robine-Leon, S.; Dussaulx, E.; Zweibaum, A. Kinetics of glycogen levels in asynchronous and synchronous cultures of a human colon carcinoma cell line, HT 29. Front. Gastrointest. Res. 1979, 4, 73–79. [Google Scholar]
- Rousset, M.; Robine-Leon, S.; Dussaulx, E.; Chevalier, G.; Zweibaum, A. Glycogen storage in foetal and malignant epithelial cells of the human colon. Front. Gastrointest. Res. 1979, 4, 80–85. [Google Scholar]
- Staedel, C.; Beck, J.-P. Resurgence of glycogen synthesis and storage capacity in cultured hepatoma cells. Cell Differ. 1978, 7, 61–71. [Google Scholar] [CrossRef]
- Sato, A.; Kawasaki, T.; Kashiwaba, M.; Ishida, K.; Nagashima, Y.; Moritani, S.; Ichihara, S.; Sugai, T. Glycogen-rich clear cell carcinoma of the breast showing carcinomatous lymphangiosis and extremely aggressive clinical behavior. Pathol. Int. 2015, 65, 674–676. [Google Scholar] [CrossRef] [PubMed]
- Favaro, E.; Bensaad, K.; Chong, M.G.; Tennant, D.A.; Ferguson, D.J.; Snell, C.; Steers, G.; Turley, H.; Li, J.-L.; Günther, U.L.; et al. Glucose utilization via glycogen phosphorylase sustains proliferation and prevents premature senescence in cancer cells. Cell Metab. 2012, 16, 751–764. [Google Scholar] [CrossRef]
- Altemus, M.A.; Yates, J.A.; Wu, Z.; Bao, L.; Merajver, S.D. Glycogen accumulation in aggressive breast cancers under hypoxia [abstract]. In Proceedings of the American Association for Cancer Research Annual Meeting, Chicago, IL, USA, 14–18 April 2018. [Google Scholar]
- Hochachka, P. Defense strategies against hypoxia and hypothermia. Science 1986, 231, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Crass, M.F.; Pieper, G. Lipid and glycogen metabolism in the hypoxic heart: Effects of epinephrine. Am. J. Physiol. 1975, 229, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Iida, Y.; Aoki, K.; Asakura, T.; Ueda, K.; Yanaihara, N.; Takakura, S.; Yamada, K.; Okamoto, A.; Tanaka, T.; Ohkawa, K. Hypoxia promotes glycogen synthesis and accumulation in human ovarian clear cell carcinoma. Int. J. Oncol. 2012, 40, 2122–2130. [Google Scholar] [CrossRef]
- Pescador, N.; Villar, D.; Cifuentes, D.; Garcia-Rocha, M.; Ortiz-Barahona, A.; Vazquez, S.; Ordonez, A.; Cuevas, Y.; Saez-Morales, D.; Garcia-Bermejo, M.L.; et al. Hypoxia promotes glycogen accumulation through hypoxia inducible factor (HIF)-mediated induction of glycogen synthase 1. PLoS ONE 2010, 5, e9644. [Google Scholar] [CrossRef]
- Shen, G.M.; Zhang, F.L.; Liu, X.L.; Zhang, J.W. Hypoxia-inducible factor 1-mediated regulation of PPP1R3C promotes glycogen accumulation in human MCF-7 cells under hypoxia. FEBS Lett. 2010, 584, 4366–4372. [Google Scholar] [CrossRef]
- Chen, J.; Lee, H.-J.; Wu, X.; Huo, L.; Kim, S.-J.; Xu, L.; Wang, Y.; He, J.; Bollu, L.R.; Gao, G.; et al. Gain of glucose-independent growth upon metastasis of breast cancer cells to the brain. Cancer Res. 2015, 75, 554–565. [Google Scholar] [CrossRef]
- Liu, J.; Gao, L.; Zhang, H.; Wang, D.; Wang, M.; Zhu, J.; Pang, C.; Wang, C. Succinate dehydrogenase 5 (SDH5) regulates glycogen synthase kinase 3β-β-Catenin-mediated lung cancer metastasis. J. Biol. Chem. 2013, 288, 29965–29973. [Google Scholar] [CrossRef]
- Zois, C.E.; Harris, A.L. Glycogen metabolism has a key role in the cancer microenvironment and provides new targets for cancer therapy. J. Mol. Med. 2016, 94, 137–154. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, J.; Bellot, G.; Gounon, P.; Lacas-Gervais, S.; Pouyssegur, J.; Mazure, N.M. Glycogen synthesis is induced in hypoxia by the hypoxia-inducible factor and promotes cancer cell survival. Front. Oncol. 2012, 2, 18. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Shirley, S.E.; Escoffery, C.T.; Titus, I.P.; Williams, E.E.; West, A.B. Clear cell carcinoma of the breast with immunohistochemical evidence of divergent differentiation. Ann. Diagn. Pathol. 2002, 6, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Fisher, E.R.; Tavares, J.; Bulatao, I.S.; Sass, R.; Fisher, B. Glycogen-rich, clear cell breast cancer: With comments concerning other clear cell variants. Hum. Pathol. 1985, 16, 1085–1090. [Google Scholar] [CrossRef]
- Hayes, M.M.; Seidman, J.D.; Ashton, M.A. Glycogen-rich clear cell carcinoma of the breast. A clinicopathologic study of 21 cases. Am. J. Surg. Pathol. 1995, 19, 904–911. [Google Scholar] [CrossRef]
- Lakhani, S.R.; Ellis, I.O.; Schnitt, S.J.; Tan, P.H.; van de Vijver, M.J. World Health Organization Classification of Tumors of the Breast, 4th ed.; IARC Press: Lyon, France, 2012; pp. 74–75. [Google Scholar]
- Kuroda, H.; Sakamoto, G.; Ohnisi, K.; Itoyama, S. Clinical and pathological features of glycogen-rich clear cell carcinoma of the breast. Breast Cancer 2005, 12, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Toikkanen, S.; Joensuu, H. Glycogen-rich clear-cell carcinoma of the breast: A clinicopathologic and flow cytometric study. Hum. Pathol. 1991, 22, 81–83. [Google Scholar] [CrossRef]
- Ma, X.; Han, Y.; Fan, Y.; Cao, X.; Wang, X. Clinicopathologic characteristics and prognosis of glycogen-rich clear cell carcinoma of the breast. Breast J. 2014, 20, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Yerushalmi, R.; Hayes, M.M.; Gelmon, K.A. Breast carcinoma—Rare types: Review of the literature. Ann. Oncol. 2009, 20, 1763–1770. [Google Scholar] [CrossRef]
- Overview of the SEER Program. Available online: https://seer.cancer.gov/about/overview.html (accessed on 30 July 2018).
- Davis, F.G.; McCarthy, B.J.; Berger, M.S. Centralized databases available for describing primary brain tumor incidence, survival, and treatment: Central Brain Tumor Registry of the United States; Surveillance, Epidemiology, and End Results; and National Cancer Data Base. Neuro Oncol. 1999, 1, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Kinslow, C.J.; Bruce, S.S.; Rae, A.I.; Sheth, S.A.; McKhann, G.M.; Sisti, M.B.; Bruce, J.N.; Sonabend, A.M.; Wang, T.J.C. Solitary-fibrous tumor/hemangiopericytoma of the central nervous system: A population-based study. J. Neurooncol. 2018, 138, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database. Incidence—SEER 9 Regs Research Data, Nov 2017 Sub (1973–2015)—Linked To County Attributes—Total U.S., 1969–2016 Counties. released April 2018, based on the November 2017 submission. Available online: https://seer.cancer.gov/data/citation.html (accessed on 1 December 2018).
- Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database. Incidence—SEER 9 Regs Research Data, Nov 2015 Sub (1973–2013)—Linked To County Attributes—Total U.S., 1969–2014 Counties. released April 2016, based on the November 2015 submission. Available online: https://seer.cancer.gov/data/citation.html (accessed on 1 December 2018).
- Collaborative Stage Work Group of the American Joint Committee on Cancer. Part I Section 2: Site-Specific Notes. In Collaborative Stage Data Collection System Coding Manual and Instructions; American Joint Committee on Cancer: Chicago, IL, USA, 2011. [Google Scholar]
- Cheville, J.C.; Lohse, C.M.; Zincke, H.; Weaver, A.L.; Blute, M.L. Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am. J. Surg. Pathol. 2003, 27, 612–624. [Google Scholar] [CrossRef] [PubMed]
- Gadducci, A.; Cosio, S.; Spirito, N.; Cionini, L. Clear cell carcinoma of the endometrium: A biological and clinical enigma. Anticancer Res. 2010, 30, 1327–1334. [Google Scholar] [PubMed]
- Sugiyama, T.; Kamura, T.; Kigawa, J.; Terakawa, N.; Kikuchi, Y.; Kita, T.; Suzuki, M.; Sato, I.; Taguchi, K. Clinical characteristics of clear cell carcinoma of the ovary: A distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy. Cancer 2000, 88, 2584–2589. [Google Scholar] [CrossRef]
- Darby, S.; McGale, P.; Correa, C.; Taylor, C.; Arriagada, R.; Clarke, M.; Cutter, D.; Davies, C.; Ewertz, M.; Godwin, J.; et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011, 378, 1707–1716. [Google Scholar] [CrossRef] [PubMed]
- Curtis, M.; Kenny, H.A.; Ashcroft, B.; Mukherjee, A.; Johnson, A.; Zhang, Y.; Helou, Y.; Batlle, R.; Liu, X.; Gutierrez, N.; et al. Fibroblasts mobilize tumor cell glycogen to promote proliferation and metastasis. Cell Metab. 2019, 29, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Jitariu, A.A.; Cimpean, A.M.; Ribatti, D.; Raica, M. Triple negative breast cancer: The kiss of death. Oncotarget 2017, 8, 46652–46662. [Google Scholar] [CrossRef]
- Elsawaf, Z.; Sinn, H.P. Triple-negative breast cancer: Clinical and histological correlations. Breast Care 2011, 6, 273–278. [Google Scholar] [CrossRef]
- Basu, S.; Chen, W.; Tchou, J.; Mavi, A.; Cermik, T.; Czerniecki, B.; Schnall, M.; Alavi, A. Comparison of triple-negative and estrogen receptor-positive/progesterone receptor-positive/HER2-negative breast carcinoma using quantitative fluorine-18 fluorodeoxyglucose/positron emission tomography imaging parameters: A potentially useful method for disease characterization. Cancer 2008, 112, 995–1000. [Google Scholar] [CrossRef]
- Pelicano, H.; Zhang, W.; Liu, J.; Hammoudi, N.; Dai, J.; Xu, R.H.; Pusztai, L.; Huang, P. Mitochondrial dysfunction in some triple-negative breast cancer cell lines: Role of mTOR pathway and therapeutic potential. Breast Cancer Res. 2014, 16, 434. [Google Scholar] [CrossRef]
- Shen, L.; O’Shea, J.M.; Kaadige, M.R.; Cunha, S.; Wilde, B.R.; Cohen, A.L.; Welm, A.L.; Ayer, D.E. Metabolic reprogramming in triple-negative breast cancer through Myc suppression of TXNIP. Proc. Natl. Acad. Sci. USA 2015, 112, 5425–5430. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.C.; Fadia, M.; Dahlstrom, J.E.; Parish, C.R.; Board, P.G.; Blackburn, A.C. Reversal of the glycolytic phenotype by dichloroacetate inhibits metastatic breast cancer cell growth in vitro and in vivo. Breast Cancer Res. Treat. 2010, 120, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Mc, M.J. Histological and histochemical uses of periodic acid. Stain Technol. 1948, 23, 99–108. [Google Scholar]
- Mc, M.J. Histological demonstration of mucin after periodic acid. Nature 1946, 158, 202. [Google Scholar]
- Hashimoto, K.; Gross, B.G.; Lever, W.F. Angiokeratoma Corporis Diffusum (Fabry). Histochemical and Electron Microscopic Studies of the Skin. J. Investig. Dermatol. 1965, 44, 119–128. [Google Scholar] [CrossRef]
- Dahr, W.; Uhlenbruck, G.; Janssen, E.; Schmalisch, R. Heterogeneity of human cell membrane sialoglycoproteins. Blut 1976, 32, 171–184. [Google Scholar] [CrossRef] [PubMed]

| GRCC | Non-GRCC | |||||
|---|---|---|---|---|---|---|
| Count | % | Count | % | p-Value | ||
| Age | 0–60 years | 78 | 50.3% | 592,544 | 47.3% | 0.46 |
| >60 years | 77 | 49.7% | 659,040 | 52.7% | ||
| Sex | Female | 152 | 98.1% | 1,242,647 | 99.3% | 0.07 |
| Male | 3 | 1.9% | 8937 | 0.7% | ||
| Race | American Indian/Alaska Native | 1 | 0.6% | 6099 | 0.5% | 0.98 |
| Asian or Pacific Islander | 12 | 7.7% | 85,407 | 6.8% | ||
| Black | 16 | 10.3% | 123,081 | 9.8% | ||
| Unknown | 1 | 0.6% | 5967 | 0.5% | ||
| White | 125 | 80.6% | 1,031,030 | 82.4% | ||
| Grade | Well differentiated; Grade I | 5 | 3.2% | 216,525 | 17.3% | <0.001 |
| Moderately differentiated; Grade II | 53 | 34.2% | 446,827 | 35.7% | ||
| Poorly differentiated; Grade III | 64 * | 41.3% | 365,903 | 29.2% | ||
| Anaplastic; Grade IV | 5 * | 3.2% | 16,937 | 1.4% | ||
| Unknown | 28 | 18.1% | 205,392 | 16.4% | ||
| ER Status a | Negative | 58 * | 40.3% | 203,173 | 17.9% | <0.001 |
| Positive | 67 | 46.5% | 807,640 * | 71.1% | ||
| Borderline | 0 | 0.0% | 2837 | 0.2% | ||
| Unknown | 19 | 13.2% | 121,600 | 10.7% | ||
| PR Status a | Negative | 84 * | 58.3% | 310,973 | 27.4% | <0.001 |
| Positive | 40 | 27.8% | 683,610 * | 60.2% | ||
| Borderline | 0 | 0.0% | 5794 | 0.5% | ||
| Unknown | 20 | 13.9% | 134,873 | 11.9% | ||
| HER2 Status c | Negative | 25 | 86.2% | 291,238 | 78.5% | 0.56 |
| Positive | 2 | 6.9% | 52,021 | 14.0% | ||
| Borderline | 0 | 0.0% | 8250 | 2.2% | ||
| Unknown | 2 | 6.9% | 19,359 | 5.2% | ||
| IHC based intrinsic Subtypes c | HER2−/HR+ | 12 | 41.4% | 251,946 * | 67.9% | <0.001 |
| HER2+/HR− | 1 | 3.4% | 15,597 | 4.2% | ||
| HER2+/HR+ | 1 | 3.4% | 36,269 | 9.8% | ||
| Triple Negative | 13 * | 44.8% | 38,750 | 10.4% | ||
| Unknown | 2 | 6.9% | 28,306 | 7.6% | ||
| AJCC 6th Stage | I | 54 | 34.8% | 525,652 | 42.0% | 0.03 |
| II | 59 * | 38.1% | 365,536 | 29.2% | ||
| III | 17 | 11.0% | 139,296 | 11.1% | ||
| IV | 11 | 7.1% | 54,270 | 4.3% | ||
| Unknown | 14 | 9.0% | 166,830 | 13.3% | ||
| T | T0 | 0 | 0.0% | 1110 | 0.1% | 0.01 |
| T1 | 63 | 40.6% | 647,840 * | 51.8% | ||
| T2 | 57 * | 36.8% | 295,198 | 23.6% | ||
| T3 | 7 | 4.5% | 49,233 | 3.9% | ||
| T4 | 3 | 1.9% | 33,931 | 2.7% | ||
| Tis | 0 | 0.0% | 1791 | 0.1% | ||
| Unknown | 25 | 16.1% | 222,481 | 17.8% | ||
| N | N0 | 91 | 58.7% | 718,198 | 57.4% | 0.79 |
| N1 | 32 | 20.6% | 234,449 | 18.7% | ||
| N2 | 9 | 5.8% | 68,563 | 5.5% | ||
| N3 | 6 | 3.9% | 48,862 | 3.9% | ||
| Unknown | 17 | 11.0% | 181,512 | 14.5% | ||
| M | M0 | 132 | 85.2% | 1,083,182 | 86.5% | 0.21 |
| M1 | 11 | 7.1% | 54,270 | 4.3% | ||
| Unknown | 12 | 7.7% | 114,132 | 9.1% | ||
| Bone Metastasis c | No Metastasis | 27 | 93.1% | 351,795 | 94.9% | 0.72 |
| Metastasis | 1 | 3.4% | 13,264 | 3.6% | ||
| Unknown | 1 | 3.4% | 5809 | 1.6% | ||
| Brain Metastasis c | No Metastasis | 27 | 93.1% | 363,212 | 97.9% | 0.03 |
| Metastasis | 1 * | 3.4% | 1501 | 0.4% | ||
| Unknown | 1 | 3.4% | 6155 | 1.7% | ||
| Liver Metastasis c | No Metastasis | 27 | 93.1% | 359,794 | 97.0% | 0.46 |
| Metastasis | 1 | 3.4% | 5079 | 1.4% | ||
| Unknown | 1 | 3.4% | 5995 | 1.6% | ||
| Lung Metastasis c | No Metastasis | 27 | 93.1% | 358,514 | 96.7% | 0.56 |
| Metastasis | 1 | 3.4% | 6,198 | 1.7% | ||
| Unknown | 1 | 3.4% | 6,156 | 1.7% | ||
| Surgery b | No Surgery | 7 | 5.5% | 70,372 | 7.3% | 0.38 |
| Surgery | 118 | 92.9% | 889,255 | 92.0% | ||
| Unknown | 2 | 1.6% | 6822 | 0.7% | ||
| Extent of Surgery b | No Surgery | 8 | 6.3% | 75,877 | 7.9% | 0.51 |
| Sub-Mastectomy # | 70 | 55.1% | 485,610 | 50.2% | ||
| Mastectomy | 48 | 37.8% | 401,380 | 41.5% | ||
| Surgery, Unknown | 0 | 0.0% | 1358 | 0.1% | ||
| Unknown | 1 | 0.8% | 2224 | 0.2% | ||
| Radiation b | No Radiation | 64 | 50.4% | 426,196 | 44.1% | 0.21 |
| Radiation | 58 | 45.7% | 513,445 | 53.1% | ||
| Unknown | 5 | 3.9% | 26,808 | 2.8% | ||
| Median (Months) | 95% CI | p-Value | |||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| All GRCC cases | 158 | 96.5 | 219.5 | NA | |
| Age | 60 years and under | 158 | 96.6 | 219.4 | 0.002 |
| >60 year | 73 | 18.6 | 127.4 | ||
| Sex | Female | - | - | - | 0.56 |
| Male | 81 | 62.5 | 99.5 | ||
| Race | American Indian/Alaska Native | 27 | - | - | 0.16 |
| Asian or Pacific Islander | - | - | - | ||
| Black | - | - | - | ||
| White | 111 | 45.6 | 176.4 | ||
| Grade | Well to Moderately Differentiated | 162 | 105.1 | 218.9 | 0.19 |
| Poorly Differentiated to Anaplastic | 111 | - | - | ||
| ER Status | Negative | 158 | 69.3 | 246.7 | 0.82 |
| Positive | 174 | 61.2 | 286.8 | ||
| PR Status | Negative | 158 | 86.3 | 229.7 | 0.15 |
| Positive | - | - | - | ||
| AJCC Stage | I | 162 | 125.7 | 198.3 | <0.001 |
| II | - | - | - | ||
| III | 69 | 38.4 | 99.6 | ||
| IV | 30 | 7.9 | 52.1 | ||
| T | T1 | 158 | 125.4 | 190.6 | 0.005 |
| T2 | - | - | - | ||
| T3 | 27 | - | - | ||
| T4 | 25 | - | - | ||
| N | N0 | 174 | - | - | 0.001 |
| N1 | 81 | 15.3 | 146.7 | ||
| N2 | 69 | - | - | ||
| N3 | 50 | 12.3 | 87.7 | ||
| M | M0 | 162 | 121.1 | 202.9 | <0.001 |
| M1 | 30 | 7.9 | 52.1 | ||
| Surgery | No Surgery | 25 | 0.0 | 58.1 | <0.001 |
| Surgery | 158 | 100.4 | 215.6 | ||
| Extent of Surgery | No Surgery | 25 | 0.0 | 58.1 | 0.02 |
| Sub-Mastectomy # | 174 | 116.0 | 232.0 | ||
| Mastectomy | 108 | - | - | ||
| Radiation | No Radiation | 81 | 40.4 | 121.6 | 0.012 |
| Radiation | - | - | - | ||
| Variable | Reference | p-Value | HR | 95% CI | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Age | <0.001 | 1.05 | 1.02 | 1.07 | |
| AJCC Stage | I | <0.001 | |||
| II | 0.64 | 1.18 | 0.60 | 2.34 | |
| III | 0.001 | 4.67 | 1.84 | 11.87 | |
| IV | <0.001 | 15.84 | 4.11 | 60.97 | |
| Surgery | No Surgery | 0.39 | 2.05 | 0.40 | 10.45 |
| Radiation | No Radiation | 0.02 | 0.47 | 0.25 | 0.90 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Kinslow, C.J.; Hibshoosh, H.; Guo, H.; Cheng, S.K.; He, C.; Gentry, M.S.; Sun, R.C. Clinical Features, Survival and Prognostic Factors of Glycogen-Rich Clear Cell Carcinoma (GRCC) of the Breast in the U.S. Population. J. Clin. Med. 2019, 8, 246. https://doi.org/10.3390/jcm8020246
Zhou Z, Kinslow CJ, Hibshoosh H, Guo H, Cheng SK, He C, Gentry MS, Sun RC. Clinical Features, Survival and Prognostic Factors of Glycogen-Rich Clear Cell Carcinoma (GRCC) of the Breast in the U.S. Population. Journal of Clinical Medicine. 2019; 8(2):246. https://doi.org/10.3390/jcm8020246
Chicago/Turabian StyleZhou, Zhengqiu, Connor J. Kinslow, Hanina Hibshoosh, Hua Guo, Simon K. Cheng, Chunyan He, Matthew S. Gentry, and Ramon C. Sun. 2019. "Clinical Features, Survival and Prognostic Factors of Glycogen-Rich Clear Cell Carcinoma (GRCC) of the Breast in the U.S. Population" Journal of Clinical Medicine 8, no. 2: 246. https://doi.org/10.3390/jcm8020246
APA StyleZhou, Z., Kinslow, C. J., Hibshoosh, H., Guo, H., Cheng, S. K., He, C., Gentry, M. S., & Sun, R. C. (2019). Clinical Features, Survival and Prognostic Factors of Glycogen-Rich Clear Cell Carcinoma (GRCC) of the Breast in the U.S. Population. Journal of Clinical Medicine, 8(2), 246. https://doi.org/10.3390/jcm8020246





