Biobanking: Objectives, Requirements, and Future Challenges—Experiences from the Munich Vascular Biobank
Abstract
:1. Introduction
2. Experimental Section
2.1. Ethics Approval and Study Population
2.2. Tissue Processing
2.3. RNA Extraction from FFPE Biospecimens by Expression of Housekeeping Genes
2.4. Analysis of RNA Quality from FFPE Biospecimens by RIN and RNA Fragmentation
2.5. Analysis of RNA Quality from FFPE Biospecimens by Expression of Home-Keeping Genes
2.6. Statistical Analysis
3. Results
3.1. Study Population
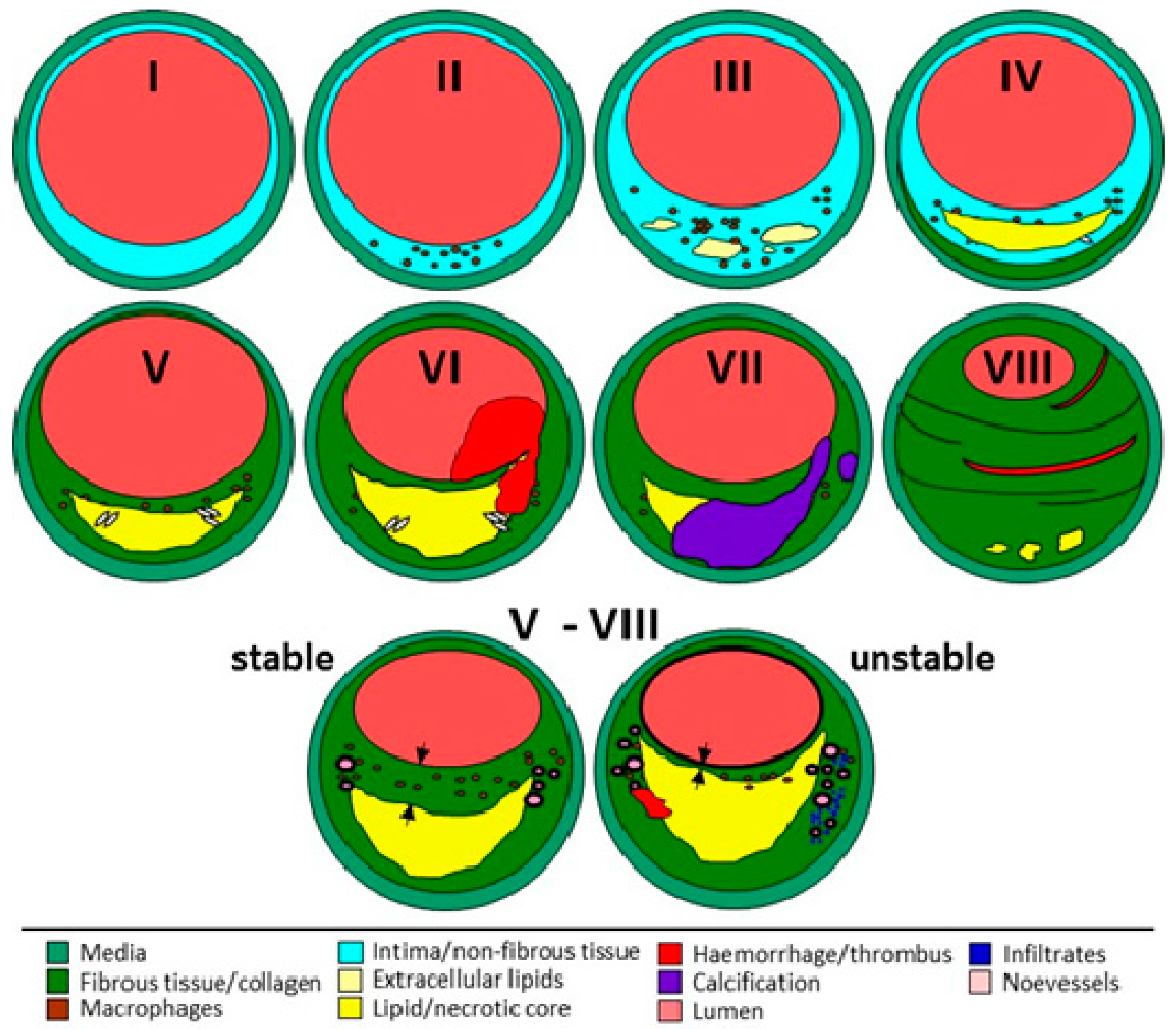
3.2. Tissue Processing and Characterization
3.3. Analysis of RNA Quality from FFPE Biospecimens by RNA Integrity Number
3.4. Analysis of RNA Quality from FFPE Biospecimens Evaluating RNA Fragmentation
3.5. Analysis of RNA Quality from FFPE Biospecimens by Expression of Housekeeping Genes
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- De Souza, Y.G.; Greenspan, J.S. Biobanking past, present and future: Responsibilities and benefits. AIDS 2013, 27, 303. [Google Scholar] [CrossRef] [PubMed]
- Diaz, Z.; Aguilar-Mahecha, A.; Paquet, E.R.; Basik, M.; Orain, M.; Camlioglu, E.; Constantin, A.; Benlimame, N.; Bachvarov, D.; Jannot, G.; et al. Next-generation biobanking of metastases to enable multidimensional molecular profiling in personalized medicine. Mod. Pathol. 2013, 26, 1413–1424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busch, A.; Eken, S.M.; Maegdefessel, L. Prospective and therapeutic screening value of non-coding RNA as biomarkers in cardiovascular disease. Ann. Transl. Med. 2016, 4, 236. [Google Scholar] [CrossRef] [PubMed]
- Kandpal, R.; Saviola, B.; Felton, J. The era of omics unlimited. Biotechniques 2009, 46, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Virchow, R. A more precise account of fatty metamorphosis. In Cellular Pathology as Based Upon Physiological and Pathological History (English Translation of Second German Edition) Lecture XV: JP; Lippincott: Philadelphia, PA, USA, 1971; pp. 350–366. [Google Scholar]
- Ross, R. Atherosclerosis—An inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Falk, E. Morphologic features of unstable atherothrombotic plaques underlying acute coronary syndromes. Am. J. Cardiol. 1989, 63, E114–E120. [Google Scholar] [CrossRef]
- Davies, M.J.; Richardson, P.D.; Woolf, N.; Katz, D.R.; Mann, J. Risk of thrombosis in human atherosclerotic plaques: Role of extracellular lipid, macrophage, and smooth muscle cell content. Br. Heart J. 1993, 69, 377–381. [Google Scholar] [CrossRef]
- Moreno, P.R.; Falk, E.; Palacios, I.F.; Newell, J.B.; Fuster, V.; Fallon, J.T. Macrophage infiltration in acute coronary syndromes. Implications for plaque rupture. Circulation 1994, 90, 775–778. [Google Scholar] [CrossRef]
- Davies, M.J. Stability and instability: Two faces of coronary atherosclerosis. The Paul Dudley White Lecture 1995. Circulation 1996, 94, 2013–2020. [Google Scholar] [CrossRef]
- Virmani, R.; Kolodgie, F.D.; Burke, A.P.; Farb, A.; Schwartz, S.M. Lessons from sudden coronary death: A comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1262–1275. [Google Scholar] [CrossRef]
- Hurks, R.; Hoefer, I.E.; de Kleijn, D.P.; Daemen, M.J.; Moll, F.L.; Pasterkamp, G. Past, current and future concepts in atherosclerotic biobanking. Future Cardiol. 2008, 4, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Hellings, W.E.; Moll, F.L.; de Kleijn, D.P.; Pasterkamp, G. 10-years experience with the Athero-Express study. Cardiovasc. Diagn. Ther. 2012, 2, 63–73. [Google Scholar] [PubMed]
- Redgrave, J.N.; Lovett, J.K.; Rothwell, P.M. Histological features of symptomatic carotid plaques in relation to age and smoking: The Oxford plaque study. Stroke 2010, 41, 2288–2294. [Google Scholar] [CrossRef] [PubMed]
- Vähämurto, L.; Pahkala, K.; Magnussen, C.G.; Hutri-Kähönen, N.; Kähönen, M.; Laitinen, T.; Taittonen, L.; Tossavainen, P.; Lehtimäki, T.; Jokinen, E.; et al. Coronary heart disease risk factor levels in eastern and western Finland from 1980 to 2011 in the cardiovascular risk in Young Finns study. Atherosclerosis 2018, 280, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.L.; Paraskevas, K.I.; Kakkos, S.K.; Golledge, J.; Eckstein, H.H.; Diaz-Sandoval, L.J.; Cao, L.; Fu, Q.; Wijeratne, T.; Leung, T.W.; et al. Systematic Review of Guidelines for the Management of Asymptomatic and Symptomatic Carotid Stenosis. Stroke 2015, 46, 3288–3301. [Google Scholar] [CrossRef] [Green Version]
- Espinola-Klein, C. ESC guidelines 2017 on peripheral arterial diseases: Summary of the most important recommendations and innovations. Herz 2017, 42, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.T.; Ambler, G.K.; Svensjö, S.; Wanhainen, A.; Bown, M.J. Beyond the AAA Guidelines: Core Outcome Sets to Make Life Better for Patients. Eur. J. Vasc. Endovasc. Surg. 2019, 57, 6–7. [Google Scholar] [CrossRef] [PubMed]
- Caixeiro, N.J.; Lai, K.; Lee, C.S. Quality assessment and preservation of RNA from biobank tissue specimens: A systematic review. J. Clin. Pathol. 2016, 69, 260–265. [Google Scholar] [CrossRef]
- Ikeda, K.; Ichihara, K.; Hashiguchi, T.; Hidaka, Y.; Kang, D.; Maekawa, M.; Matsumoto, H.; Matsushita, K.; Okubo, S.; Tsuchiya, T.; et al. Evaluation of the short-term stability of specimens for clinical laboratory testing. Biopreserv. Biobank. 2015, 13, 135–143. [Google Scholar] [CrossRef]
- Hubel, A.; Spindler, R.; Skubitz, A.P. Storage of human biospecimens: Selection of the optimal storage temperature. Biopreserv. Biobank. 2014, 12, 165–175. [Google Scholar] [CrossRef]
- Lee, S.M.; Schelcher, C.; Gashi, S.; Schreiber, S.; Thasler, R.M.; Jauch, K.W.; Thasler, W.E. RNA stability in human liver: Comparison of different processing times, temperatures and methods. Mol. Biotechnol. 2013, 53, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chevyreva, I.; Faull, R.L.; Green, C.R.; Nicholson, L.F. Assessing RNA quality in postmortem human brain tissue. Exp. Mol. Pathol. 2008, 84, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Stary, H.C. Natural history and histological classification of atherosclerotic lesions: An update. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1177–1178. [Google Scholar] [CrossRef] [PubMed]
- Stary, H.C.; Blankenhorn, D.H.; Chandler, A.B.; Glagov, S.; Insull, W., Jr.; Richardson, M.; Rosenfeld, M.E.; Schaffer, S.A.; Schwartz, C.J.; Wagner, W.D.; et al. A definition of the intima of human arteries and of its atherosclerosis-prone regions. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler. Thromb. 1992, 12, 120–134. [Google Scholar] [CrossRef] [PubMed]
- Stary, H.C.; Chandler, A.B.; Dinsmore, R.E.; Fuster, V.; Glagov, S.; Insull, W., Jr.; Rosenfeld, M.E.; Schwartz, C.J.; Wagner, W.D.; Wissler, R.W.; et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 1995, 92, 1355–1374. [Google Scholar] [CrossRef] [PubMed]
- Stary, H.C.; Chandler, A.B.; Glagov, S.; Guyton, J.R.; Insull, W., Jr.; Rosenfeld, M.E.; Schaffer, S.A.; Schwartz, C.J.; Wagner, W.D.; Wissler, R.W. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, AHA. Arterioscler. Thromb. 1994, 14, 840–856. [Google Scholar] [CrossRef] [PubMed]
- Redgrave, J.N.; Gallagher, P.; Lovett, J.K.; Rothwell, P.M. Critical cap thickness and rupture in symptomatic carotid plaques: The oxford plaque study. Stroke 2008, 39, 1722–1729. [Google Scholar] [CrossRef]
- Pelisek, J.; Wendorff, H.; Wendorff, C.; Kuehnl, A.; Eckstein, H.H. Age-associated changes in human carotid atherosclerotic plaques. Ann. Med. 2016, 48, 541–551. [Google Scholar] [CrossRef]
- Zimmermann, A.; Senner, S.; Eckstein, H.H.; Pelisek, J. Histomorphological evaluation of atherosclerotic lesions in patients with peripheral artery occlusive disease. Adv. Med. Sci. 2015, 60, 236–239. [Google Scholar] [CrossRef]
- Gee, M.W.; Reeps, C.; Eckstein, H.H.; Wall, W.A. Prestressing in finite deformation abdominal aortic aneurysm simulation. J. Biomech. 2009, 42, 1732–1739. [Google Scholar] [CrossRef]
- Maier, A.; Gee, M.W.; Reeps, C.; Eckstein, H.H.; Wall, W.A. Impact of calcifications on patient-specific wall stress analysis of abdominal aortic aneurysms. Biomech. Model Mechanobiol. 2010, 9, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Reeps, C.; Gee, M.W.; Maier, A.; Gurdan, M.; Eckstein, H.H.; Wall, W.A. The impact of model assumptions on results of computational mechanics in abdominal aortic aneurysm. J. Vasc. Surg. 2010, 51, 679–688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maier, A.; Essler, M.; Gee, M.W.; Eckstein, H.H.; Wall, W.A.; Reeps, C. Correlation of biomechanics to tissue reaction in aortic aneurysms assessed by finite elements and [18F]-fluorodeoxyglucose-PET/CT. Int. J. Numer. Method Biomed. Eng. 2012, 28, 456–471. [Google Scholar] [CrossRef] [PubMed]
- Reeps, C.; Maier, A.; Pelisek, J.; Härtl, F.; Grabher-Meier, V.; Wall, W.A.; Essler, M.; Eckstein, H.H.; Gee, M.W. Measuring and modeling patient-specific distributions of material properties in abdominal aortic aneurysm wall. Biomech. Model Mechanobiol. 2013, 12, 717–733. [Google Scholar] [CrossRef] [PubMed]
- Evaluating RNA Quality from FFPE Samples. Available online: https://emea.illumina.com/content/dam/illumina-marketing/documents/products/technotes/evaluating-rna-quality-from-ffpe-samples-technical-note-470-2014-001.pdf (accessed on 26 October 2016).
- Moore, H.M.; Compton, C.C.; Alper, J.; Vaught, J.B. International approaches to advancing biospecimen science. Cancer Epidemiol. Biomark. Prev. 2011, 20, 729–732. [Google Scholar] [CrossRef] [PubMed]
- Moore, H.M.; Kelly, A.B.; Jewell, S.D.; McShane, L.M.; Clark, D.P.; Greenspan, R.; Hayes, D.F.; Hainaut, P.; Kim, P.; Mansfield, E.A.; et al. Biospecimen reporting for improved study quality (BRISQ). Cancer Cytopathol. 2011, 119, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Malm, J.; Fehniger, T.E.; Danmyr, P.; Végvári, A.; Welinder, C.; Lindberg, H.; Appelqvist, R.; Sjödin, K.; Wieslander, E.; Laurell, T.; et al. Developments in biobanking workflow standardization providing sample integrity and stability. J. Proteom. 2013, 95, 38–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simeon-Dubach, D.; Perren, A. Better provenance for biobank samples. Nature 2011, 475, 454–455. [Google Scholar] [CrossRef] [Green Version]
- Betsou, F.; Rimm, D.L.; Watson, P.H.; Womack, C.; Hubel, A.; Coleman, R.A.; Horn, L.; Terry, S.F.; Zeps, N.; Clark, B.J.; et al. What are the biggest challenges and opportunities for biorepositories in the next three to five years? Biopreserv. Biobank. 2010, 8, 81–88. [Google Scholar] [CrossRef]
- Karimi-Busheri, F.; Zadorozhny, V.; Shawler, D.L.; Fakhrai, H. The stability of breast cancer progenitor cells during cryopreservation: Maintenance of proliferation, self-renewal, and senescence characteristics. Cryobiology 2010, 60, 308–314. [Google Scholar] [CrossRef]
- Karimi-Busheri, F.; Zadorozhny, V.; Carrier, E.; Fakhrai, H. Molecular integrity and global gene expression of breast and lung cancer stem cells under longterm storage and recovery. Cell Tissue Bank. 2013, 14, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Micke, P.; Ohshima, M.; Tahmasebpoor, S.; Ren, Z.P.; Ostman, A.; Ponten, F.; Botling, J. Biobanking of fresh frozen tissue: RNA is stable in nonfixed surgical specimens. Lab. Investig. 2006, 86, 202–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohashi, Y.; Creek, K.E.; Pirisi, L.; Kalus, R.; Young, S.R. RNA degradation in human breast tissue after surgical removal: A time-course study. Exp. Mol. Pathol. 2004, 77, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Jewell, S.D.; Srinivasan, M.; McCart, L.M.; Williams, N.; Grizzle, W.H.; LiVolsi, V.; MacLennan, G.; Sedak, D.D. Analysis of the molecular quality of human tissues: An experience from the Cooperative Human Tissue Network. Am. J. Clin. Pathol. 2002, 118, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.; Paul Thiery, J.; Magdelenat, H.; Radvanyi, F. Gene expression analysis by real-time reverse transcription polymerase chain reaction: Influence of tissue handling. Anal. Biochem. 2004, 328, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Von Ahlfen, S.; Missel, A.; Bendrat, K.; Schlimpberger, M. Determinants of RNA quality from FFPE samples. PLoS ONE 2007, 2, e1261. [Google Scholar] [CrossRef] [PubMed]
- Penland, S.K.; Keku, T.O.; Torrice, C.; He, X.; Krishnamurthy, J.; Hoadley, K.A.; Woosley, J.T.; Thomas, N.E.; Perou, C.M.; Sandler, R.S.; et al. RNA expression analysis of formalin-fixed paraffin-embedded tumors. Lab. Investig. 2007, 87, 383–391. [Google Scholar] [CrossRef]
- Agilent Technologies. RNA Integrity Number (RIN)—Standardization of RNA Quality Control. Publication PN 5989-1165EN. Available online: https://www.agilent.com (accessed on 21 January 2016).
- Arzt, L.; Kothmaier, H.; Quehenberger, F.; Halbwedl, I.; Wagner, K.; Maierhofer, T.; Popper, H.H. Evaluation of formalin-free tissue fix. for RNA and microRNA studies. Exp. Mol. Pathol. 2011, 91, 490–495. [Google Scholar] [CrossRef]
- Zhang, X.; Han, Q.Y.; Zhao, Z.S.; Zhang, J.G.; Zhou, W.J.; Lin, A. Biobanking of Fresh-Frozen Gastric Cancer Tissues: Impact of Long-Term Storage and Clinicopathological Variables on RNA Quality. Biopreserv Biobank. 2018. [Google Scholar] [CrossRef]
- Olivieri, E.H.; de Andrade Franco, L.; Pereira, R.G.; Mota, L.D.; Campos, A.H.; Carraro, D.M. Biobanking practice: RNA storage at low concentration affects integrity. Biopreserv. Biobank. 2014, 12, 46–52. [Google Scholar] [CrossRef]
- Wendorff, C.; Wendorff, H.; Pelisek, J.; Tsantilas, P.; Zimmermann, A.; Zernecke, A.; Kuehnl, A.; Eckstein, H.H. Carotid plaque morphology is significantly associated with sex, age, and history of neurological symptoms. Stroke 2015, 46, 3213–3219. [Google Scholar] [CrossRef] [PubMed]
- Wendorff, C.; Wendorff, H.; Kuehnl, A.; Tsantilas, P.; Kallmayer, M.; Eckstein, H.H.; Pelisek, J. Impact of sex and age on carotid plaque instability in asymptomatic patients-results from the Munich Vascular Biobank. Vasa 2016, 45, 411–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Year | AAA # | CAROTIS | PAD | |||
|---|---|---|---|---|---|---|
| FFPE Tissue | Serum | FFPE Tissue | Serum | FFPE Tissue | Serum | |
| 2004 | 79 | 47 | ||||
| 2005 | 8 | 17 | 72 | 63 | ||
| 2006 | 36 | 84 | 36 | 84 | ||
| 2007 | 40 | 89 | 79 | 56 | ||
| 2008 | 34 | 84 | 63 | 72 | ||
| 2009 | 40 | 60 | 100 | 62 | 63 | 90 |
| 2010 | 40 | 72 | 94 | 58 | 77 | 78 |
| 2011 | 38 | 91 | 121 | 87 | 51 | 133 |
| 2012 | 33 | 136 | 122 | 111 | 62 | 251 |
| 2013 | 41 | 114 | 126 | 99 | 63 | 228 |
| 2014 | 24 | 131 | 92 | 97 | 63 | 259 |
| 2015 | 26 | 126 | 126 | 131 | 84 | 294 |
| 2016 | 30 | 124 | 124 | 117 | 80/27/35 * | 228 |
| 2017 | 48 | 127 | 173 | 156 | 22/15/23 ** | 116 |
| 2018 | 43 | 125 | 160 | 154 | 16/22 ** | 25 |
| Sum: | 481 | 1380 | 1567 | 1394 | 703 | 1702 |
| Years | AAA | CAROTIS | PAD | ∑ |
|---|---|---|---|---|
| 2004–2008 | 1 | 1 | 1 | 3 |
| 2009–2013 | 17 | 20 | 3 | 40 |
| 2014–2018 | 10 | 23 | 6 | 41 |
| Sum: | 29 | 44 | 11 | 84 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelisek, J.; Hegenloh, R.; Bauer, S.; Metschl, S.; Pauli, J.; Glukha, N.; Busch, A.; Reutersberg, B.; Kallmayer, M.; Trenner, M.; et al. Biobanking: Objectives, Requirements, and Future Challenges—Experiences from the Munich Vascular Biobank. J. Clin. Med. 2019, 8, 251. https://doi.org/10.3390/jcm8020251
Pelisek J, Hegenloh R, Bauer S, Metschl S, Pauli J, Glukha N, Busch A, Reutersberg B, Kallmayer M, Trenner M, et al. Biobanking: Objectives, Requirements, and Future Challenges—Experiences from the Munich Vascular Biobank. Journal of Clinical Medicine. 2019; 8(2):251. https://doi.org/10.3390/jcm8020251
Chicago/Turabian StylePelisek, Jaroslav, Renate Hegenloh, Sabine Bauer, Susanne Metschl, Jessica Pauli, Nadiya Glukha, Albert Busch, Benedikt Reutersberg, Michael Kallmayer, Matthias Trenner, and et al. 2019. "Biobanking: Objectives, Requirements, and Future Challenges—Experiences from the Munich Vascular Biobank" Journal of Clinical Medicine 8, no. 2: 251. https://doi.org/10.3390/jcm8020251
APA StylePelisek, J., Hegenloh, R., Bauer, S., Metschl, S., Pauli, J., Glukha, N., Busch, A., Reutersberg, B., Kallmayer, M., Trenner, M., Wendorff, H., Tsantilas, P., Schmid, S., Knappich, C., Schaeffer, C., Stadlbauer, T., Biro, G., Wertern, U., Meisner, F., ... Eckstein, H. -H. (2019). Biobanking: Objectives, Requirements, and Future Challenges—Experiences from the Munich Vascular Biobank. Journal of Clinical Medicine, 8(2), 251. https://doi.org/10.3390/jcm8020251






